Abstract
Recent studies suggest that hippocampal function is partially dissociable along its septo-temporal axis: the septal hippocampus is more critical for spatial processing, while the temporal hippocampus may be more important for non-spatial-related behavior. In young adults, water maze training specifically activates new neurons in the temporal hippocampus but it is unknown whether subregional differences are maintained in older animals, which have reduced neurogenesis levels. We therefore examined gradients of activity-related Fos expression and neurogenesis in 13-month-old rats and found that neurogenesis occurs relatively evenly throughout the dentate gyrus. Water maze experience significantly increased Fos expression in the suprapyramidal blade and Fos was highest in the septal pole of the dentate gyrus whether the animal learned a platform location, swam in the absence of a platform or remained in their cage. No Fos+ young neurons were found using typical markers of immature neurons. However, Fos expression in the subgranular zone, where adult-born neurons predominate, was disproportionally high in the temporal dentate gyrus. These findings indicate that adult-born neurons in the temporal hippocampus are preferentially activated compared with older neurons.
Keywords: adult neurogenesis, dentate gyrus, septal, temporal, dorsal, ventral, fos, immediate-early gene, aging, spatial, water maze, stress, anxiety
1. Introduction
While a role for the hippocampus in spatial memory is well established (Frankland and Bontempi, 2005), more recent evidence also points to a role for the hippocampus in regulating anxiety-related behavior. There is substantial evidence suggesting that these two functions of the hippocampus are at least partially subserved by different anatomical subregions: the septal hippocampus is particularly important for spatial learning and the temporal hippocampus regulates defensive, anxiety-related, and odor-mediated behaviors (Bannerman et al., 2004; Pentkowski et al., 2006; Hunsaker et al., 2008). Other studies suggest that the temporal hippocampus contributes to spatial learning as well, but to different aspects than the septal hippocampus, including processing larger spatial environments (Jung et al., 1994; Kjelstrup et al., 2008), learning over longer timescales (de Hoz et al., 2003), and mediating context-based inhibitory associations (McDonald et al., 2006). Thus, the function of the temporal hippocampus in regulating anxiety and spatial processing appears to differ from that of the septal hippocampus.
Given the septo-temporal functional dissociation, and findings suggesting that adult neurogenesis in the hippocampus is involved in both spatial learning and anxiety-related behavior (Leuner et al., 2006; Drew and Hen, 2007), we previously examined septo-temporal gradients (there referred to as “dorso-ventral” gradients) of neurogenesis and activity (i.e. Fos+ cells) in young neurons in young adult rats after learning in a spatial water maze task (Snyder et al., 2009). In that study, both neurogenesis and granule neuron Fos expression were higher in the septal dentate gyrus of the hippocampus. However, expression of Fos by young neurons after water maze training was specific for the temporal dentate gyrus, suggesting that young granule neurons may play a different role than older granule neurons in water maze learning.
It is well documented that neurogenesis declines with age (Altman and Das, 1965; Seki and Arai, 1995; Kuhn et al., 1996; McDonald and Wojtowicz, 2005), and there are also reports that the expression of activity-dependent immediate early genes (IEGs) is reduced with age (Small et al., 2004). However, it is unclear whether there are changes in septo-temporal gradients of neurogenesis and activity with aging, which could alter distinct aspects of hippocampus-dependent learning in old age. Therefore, in the current study, we examined neurogenesis and water maze-induced Fos expression in septo-temporal and infrapyramidal-suprapyramidal axes of the dentate gyrus in 13-month-old rats. We found weak subregional differences in levels of neurogenesis but strong biases for Fos expression in the septal dentate gyrus and in the suprapyramidal blade after water maze experience. In the temporal dentate gyrus, but not septal dentate gyrus, water maze training activated adult-born neurons (in the subgranular zone) more than older neurons (in the outer rows).
2. Methods
2.1 Animals and treatments
Fourteen 13-month-old male Long Evans rats (Charles River, Quebec) were used in the following experiments. All animals were individually housed, and all treatments conformed to animal health and welfare guidelines of the University of Toronto. To label adult-born DG granule cells, all rats were given 2 intraperitoneal injections of 5-bromo-2′-deoxyuridine (BrdU; Sigma, 50 mg/kg/injection, dissolved at 20 mg/ml in saline, 0.007 N NaOH) spaced 10 hours apart, for 5 consecutive days. Beginning three weeks after BrdU injections, all 14 rats were handled 5 minutes per day for 5 days to minimize stress associated with behavioral procedures. Four weeks after BrdU injections, rats were divided into three groups that were either trained in the Morris water maze (as described below), put in the water maze with no platform (swim controls), or left untouched (cage controls). All rats were perfused exactly 2 hours after their first water maze trial (~90 min after their last trial) or at the same time of day (cage controls) to assess activity-dependent Fos expression.
2.2 Water maze
On the final day of the experiment, 8 rats were trained in the Morris water maze, a hippocampus-dependent task (Morris et al., 1982) known to induce Fos expression in adult-born granule neurons (Jessberger and Kempermann, 2003; Kee et al., 2007; Tashiro et al., 2007). The testing apparatus was as previously described (Snyder et al., 2005). Briefly, the pool was 180 cm in diameter and filled with water. Water was kept at 25°C and non-toxic white paint was added to hide a 10 cm wide platform present in the SE quadrant. Distal cues were present on the walls of the room to allow rats to develop a spatial strategy for escaping the water by mounting the platform. Rats were allowed to remain on the platform for 10 sec after finding it. If a rat failed to locate the platform within 60 seconds, it was guided to the correct location by the experimenter. Rats were trained in pairs for 16 trials, 1–2 min apart, allowing initially-naïve rats to quickly develop a spatial memory for the platform location. Latency to find the platform and mean proximity to the platform were calculated for each trial using HVS Image software (Buckingham, UK). For the proximity measure (Gallagher et al., 1993), the distance of the rat from the platform location was calculated at 0.1 sec intervals. The mean of these values was then calculated for each trial. A rat that searches in the correct area of the pool will have a low mean distance value, providing a measure of spatial bias for the platform location that should decrease across trials if animals learn the location of the platform. In addition, if the search pattern is spatially selective, the difference between the mean distance from the platform location and from a similarly-sized location at the opposite side of the pool should increase across blocks. Following training, rats were returned to their home cages until being perfused exactly 2 hours after their first training trial. Swim control rats (n=3) were placed in the pool in the absence of a platform and allowed to swim for 60 sec, 45 sec, 30 sec and 15 sec (4 trials of each) to approximate the swim times of the trained rats. They were perfused 2 hours after the first swim trial. Cage control rats (n=3) were perfused directly from their home cages with no behavioral manipulation.
2.3 Immunohistochemistry
Animals were perfused with phosphate-buffered saline followed by 8% paraformaldehyde. Brains were fixed in paraformaldehyde for an additional 24 hours. The right hippocampus was extracted, cut into 4 approximately equal-length pieces and sectioned essentially perpendicular to its long (septo-temporal) axis. Since hippocampi were not forcibly straightened some curvature remained, but this procedure generally enabled comparable analyses along the entire axis (Gaarskjaer, 1978; Rapp and Amaral, 1988) (Fig. 3a). Sections were cut at 40 μm using a vibratome for a total of ~200 sections.
Figure 3.
Neurogenesis in septo-temporal and infrapyramidal-suprapyramidal blade subregions. A) A schematic of the hippocampus illustrating how septo-temporal subregions were defined. Solid lines divide septo-temporal quartiles; dashed lines approximate a 1 in 10 section sampling scheme. B) Two-way ANOVA found a significant effect of septo-temporal position on BrdU+ cell density, due to higher BrdU+ cell density in region S2 relative to region V4 (post hoc, *p=0.01). There was no significant effect of blade on the density of BrdU+ cells. Densities presented as “both blades” reflects the total (i.e. infra + supra) number of BrdU+ cells divided by the total granule cell layer volume, not the average of the separately-calculated blade values, and was not included in statistical analyses.
Sequential fluorescent double labeling was performed for Fos, followed by BrdU, on free floating sections. For BrdU/Fos double-immunolabeling, sections were first incubated with rabbit anti-Fos antibody (1:10,000; Calbiochem #PC38; 3 days at 4°C) followed by Alexa568 goat anti-rabbit secondary antibody (1:200; Molecular Probes; 2 hours at room temperature). Sections were then treated with 1N HCl at 45°C for 40 min to denature DNA and expose BrdU. Sections were then incubated with rat anti-BrdU antibody (1:200; Accurate #OBT0030; 1 day at 4°C) followed by Alexa488 goat anti-rat secondary antibody (1:200; Molecular Probes; 2 hours at room temperature). PSA-NCAM/Fos staining was done similarly, using rabbit anti-Fos (1:10,000; Calbiochem #PC38; 3 days at 4°C) then Alexa568 goat anti-rabbit followed by mouse anti-PSA-NCAM (1:200, Chemicon #MAB3524; 3 days at 4°C) then Alexa488 goat anti-mouse. All antibodies were diluted in phosphate-buffered saline containing 0.03% Triton X-100. One rat in the water maze-trained group had poor immunolabeling and was excluded from histological analysis.
2.4 Cell quantification
For regional comparison, sections were assigned to one of four equal-length bins along the septo-temporal axis of the DG (S1=septal, S2=mid-septal, T3=mid-temporal, T4=temporal; Fig. 3a). Within each section, attention was paid to whether cells were located within the infrapyramidal or suprapyramidal blade of the DG.
Quantification of BrdU+ cells was performed on every 5th section throughout the septo-temporal extent of the DG in the water maze trained rats (40–46 sections per rat). Quantification of Fos+ cells was performed on every 10th section in the water maze trained, swim control and cage control rats. Nuclei were counted as positive for Fos expression if the Fos signal stood out clearly compared to the surrounding tissue and if the staining evenly filled the entire nucleus. Analysis of potential Fos staining in PSA-NCAM+ cells was performed on every 5th section in the S1 and T4 quartiles. Every BrdU+ cell (mean = 124 per animal) and PSA-NCAM+ cell (approximately 75 cells per quartile, 150 per animal) was examined for Fos expression with an epifluorescence microscope (Olympus BX51) and a 60x oil immersion lens (N.A. 1.25). For all analyzed sections, cross-sectional area of the granule cell layer was measured at 4x and multiplied by the section thickness (40 μm) to yield region volumes. Cell densities were then determined by dividing the number of cells in each region of the granule cell layer by its respective volume. Since cells present at both the top and bottom-most focal planes were counted, these measurements likely overestimate true values (West et al., 1991). To assess whether septo-temporal differences in cell size exist, and might therefore bias density calculations, Fos+ cell size was measured in a single section from the middle of both the septal and temporal poles of 6 animals; the cross sectional area of 6 randomly chosen Fos+ cells, spanning both blades, was measured. Measurements were made at the focal plane where the cell was largest (roughly the middle of the cell) using a 60x objective and confocal microscopy.
To characterize activity in a broad population of adult-born cells, we calculated the proportion of Fos+ cells that were located in the subgranular zone (SGZ), defined for this purpose as the deepest row of granule cells in the granule cell layer, bordering the hilus. The granule cell layer follows an outside-in gradient of formation that does not produce strict layering of granule cells by age but nonetheless results in the deepest portion of the granule cell layer containing a high proportion of granule cells that are young (Crespo et al., 1986; Dayer et al., 2003; Kempermann et al., 2003; Seri et al., 2004) and possess immature electrophysiological properties (Wang et al., 2000). The number of Fos+ cells in the SGZ and the total number of Fos+ cells in the entire granule cell layer (SGZ plus other rows) were counted in two sections per animal, one from the middle of S1 and one from the middle of T4 and the percentage of Fos+ cells in the SGZ was calculated (100 × FosSGZ/Fostotal). The percentage of Fos+ cells in the SGZ was compared across subregions (S1 vs. T4) and was also compared, within each subregion, to the chance value expected if Fos+ cells were distributed equally in all rows, where chance = (100%)/(# of rows).
2.5 Statistical analysis
Statistical analyses were performed using Systat 12 (http://www.systat.com) and Prism 5.0 (Graphpad) software. Comparisons were made using 1-way or 2-way ANOVA and Tukey’s HSD for post hoc analyses, with significance set at p<0.05. A one-sample t-test was used to compare the percentages of Fos+ cells in the SGZ to chance values.
3. Results
3.1 Water maze behavior
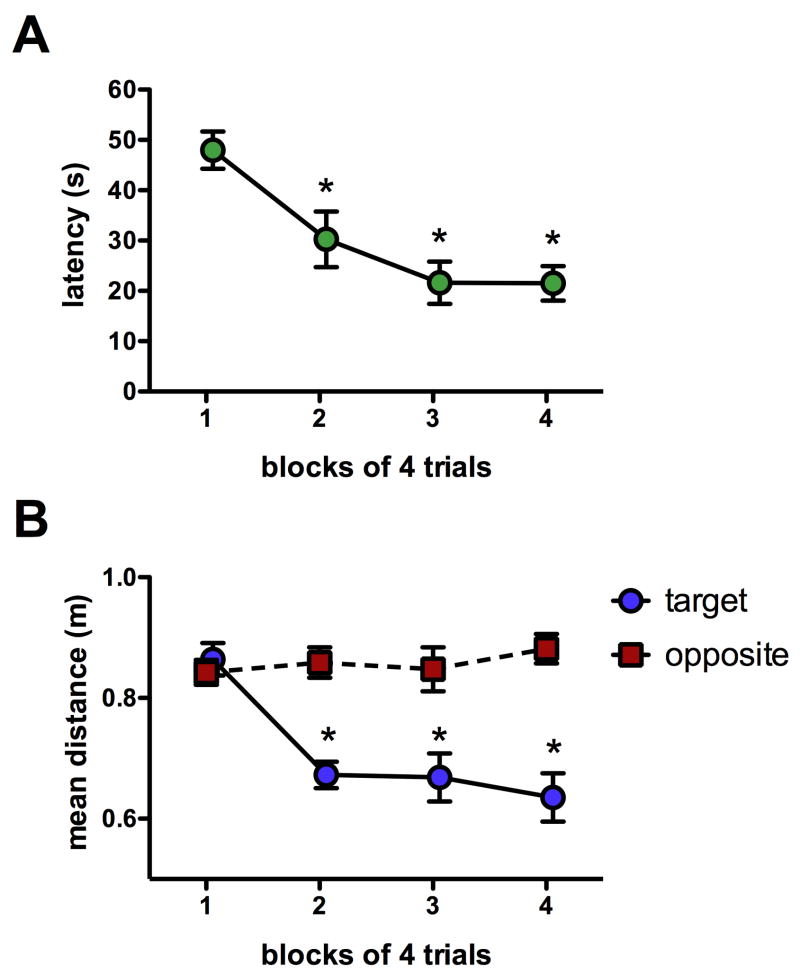
Rats showed a decreased latency to find the hidden platform over 4 blocks of trials in the water maze, indicating rapid acquisition of spatial information (repeated measures ANOVA, main effect of block, F3,21=9.9, p<0.001; Fig. 1a). The latency for block 1 was significantly higher than all other blocks (post hoc, p<0.05 for all) and blocks 2, 3 and 4 were not different from each other (p>0.05). To verify that decreases in latency reflected spatial search strategies, the mean distance (proximity) to the platform location was also examined and compared to the mean distance to a hypothetical platform located in the opposite quadrant of the pool (Gallagher et al., 1993). Over successive blocks there was a decrease in mean distance to the target platform location but no change in the mean distance to the equivalent location in the opposite quadrant of the pool (Fig. 1b), suggesting an increased preference for the platform location indicative of spatial learning. Two-way repeated measure ANOVAs showed significant main effects of block (F3,42=5.1, p=0.0044), platform location (F1,42=39.0, p<0.0001) and a significant block × platform location interaction (F3,42=8.0, p=0.0002). Post hoc tests found that the mean distance to the correct platform location was less than the mean distance to the opposite location for blocks 2, 3 and 4 (p<0.001 for all).
Figure 1.
Spatial water maze acquisition. A) The latency required for rats to find the hidden platform decreased across blocks within a single training session (ANOVA, effect of block, p<0.001; post hoc, *p<0.05 relative to first block). B) The mean distance from the target (correct) platform location decreased across blocks whereas the mean distance from a hypothetical, equivalent platform located in the opposite quadrant of the pool did not change across blocks (post hoc, *p<0.001 relative to opposite location).
3.2 Regional differences in neurogenesis
BrdU+ cell density was compared across the septo-temporal axis and infrapyramidal-suprapyramidal blades with a 2-way ANOVA. There was a significant main effect of septo-temporal position on BrdU+ cell density (F3,48=4.2, p<0.05), with post hoc tests showing that S2 had a significantly higher cell density than T4 (p=0.010; Fig. 2, 3b). There was no significant main effect of blade on BrdU+ cell density (F1,48=1.2, p=0.284) or interaction between blade and septo-temporal position (F3,48=0.1, p=0.796).
Figure 2.
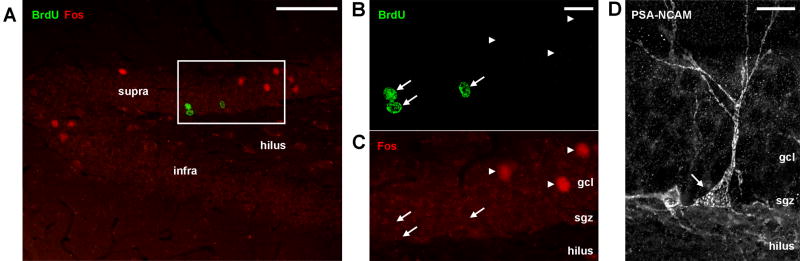
Confocal images of markers used to measure neurogenesis and neuronal activity. A) Low magnification photograph of BrdU and Fos immunostaining in the septal dentate gyrus of a swim control rat; Fos+ cells primarily located in the suprapyramidal (supra) blade of the granule cell layer and the crest where the suprapyramidal and infrapyramidal (infra) blades meet. B, C) High magnification images of BrdU (B) and Fos (C) staining from boxed region in A. Arrows indicate 3 BrdU+ cells and arrowheads indicate 3 Fos+ cells. No BrdU+/Fos+ cells were found. D) A representative cell immunostained for the endogenous young neuronal marker PSA-NCAM (arrow). gcl, granule cell layer; sgz, subgranular zone. Scale bars 200 μm (A), 20 μm (B-D).
3.3 Fos expression in the entire granule cell population
We next examined Fos expression in the granule cell layer of water maze-trained, swim control and cage control rats (Fig. 2, 4). A significant main effect of experience on Fos+ cell density was found by 3-way ANOVA (with experience, septo-temporal position, and blade as factors, F2,80=27, p<0.001). The overall Fos+ cell densities in swim control and water maze trained rats were 295% and 177% higher, respectively, than in cage controls – differences that were both significant (post hoc, p<0.001). Swim control rats had significantly greater Fos expression than water maze trained rats (p=0.003). There was a significant main effect of blade, with higher Fos+ cell density in the suprapyramidal blade than in the infrapyramidal blade (F1,80=48, p<0.001). The only significant interaction was between experience and blade (F2,80=6, p=0.005), with post hoc analyses showing that both water maze and swim control animals had increased Fos+ cell density specific to the suprapyramidal blade (p<0.05 compared to infrapyramidal blades and both blades of cage controls). A significant main effect of septo-temporal position was seen (F3,80=26, p<0.001), with post hoc analysis showing higher Fos+ cell density in the first quartile than all other quartiles (p<0.001). There was no interaction between experience and septo-temporal subregion, indicating that changes in activation across treatment did not occur preferentially in any septo-temporal region. To assess whether subregion-dependent differences in cell size might contribute to these findings the size of Fos+ cells was measured in septal and temporal poles. The cross sectional area of Fos+ cells was not significantly different between the septal and temporal poles, and in any case quite small relative to observed gradients, indicating that cell size is not a significant factor underlying subregional differences in Fos+ cell density (septal: 198±6μm2, temporal 219±18μm2, mean±se; T10=1.1, p=0.3).
Figure 4.
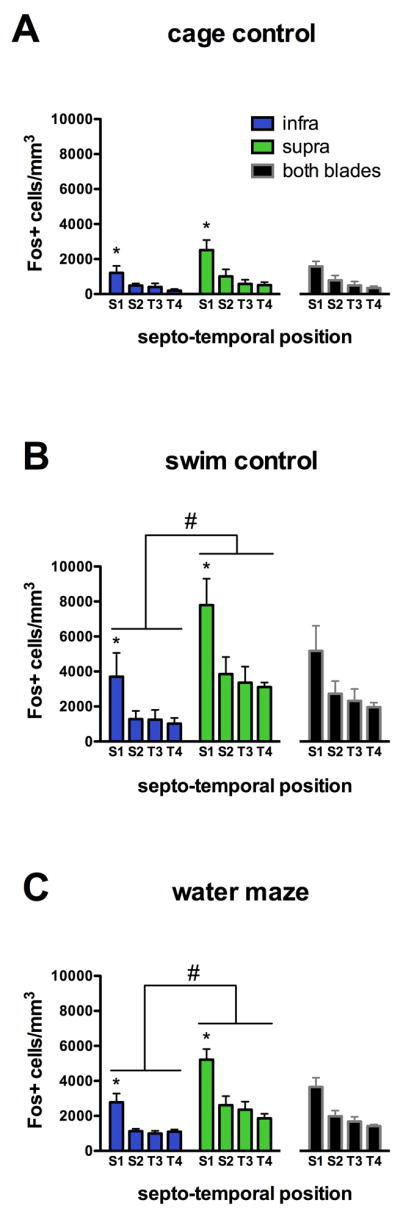
Fos expression in septo-temporal and infrapyramidal-suprapyramidal blade subregions in cage control (A), swim control (B) and water maze-trained (C) rats. A 3-way ANOVA found significant main effects of treatment, septo-temporal position and blade on Fos+ cell density (p<0.001 for all main effects). Both swim control and water maze-trained rats had greater Fos+ cell density than cage controls, and swim controls had greater Fos+ cell density than water maze-trained rats (post hoc, p<0.01 for all). The effect of septo-temporal position was due to greater Fos+ cell density in region S1 compared to all other quartiles (post hoc, *p<0.001). A blade × treatment interaction was also found, such that Fos+ cell density in the suprapyramidal blade was elevated in swim control and water maze-trained rats relative to the infrapyramidal blade and both blades of cage controls (post hoc, #p<0.05 for all).
3.4 Fos expression in adult-born granule cells
No BrdU+/Fos+ or PSA-NCAM+/Fos+ cells were found in trained or control groups (average 124 BrdU+ cells examined per rat, range = 55–210; approximately 140 PSA-NCAM+ cells examined per rat, 70 per quartile). To obtain a measure of regional activity in a broader population of adult-born neurons, we analyzed the proportion of Fos+ cells that were located in the SGZ in water maze-trained rats (Fig. 5). The granule cell layer follows an outside-in gradient of formation that continues through adulthood, resulting in a high proportion of young neurons in the SGZ (Crespo et al., 1986; Dayer et al., 2003; Kempermann et al., 2003). By 2-way ANOVA we found no main effect of blade but did see a significant main effect of septo-temporal location: Fos+ cells in the SGZ made up a significantly larger proportion of the activated population in the temporal DG than in the septal DG (F1,28=37, p<0.001). The proportion of Fos+ cells in the SGZ was not different from chance in the septal DG (both blades pooled: 11.0% versus an expected value of 13.2% based on an average of 7.6 rows; one sample t-test, T7=1.3, p=0.2). However, in the temporal DG, the proportion of Fos+ cells in the SGZ was significantly greater than chance (35.6% versus the expected 17.4% based on 5.8 rows; T7=3.7, p<0.01).
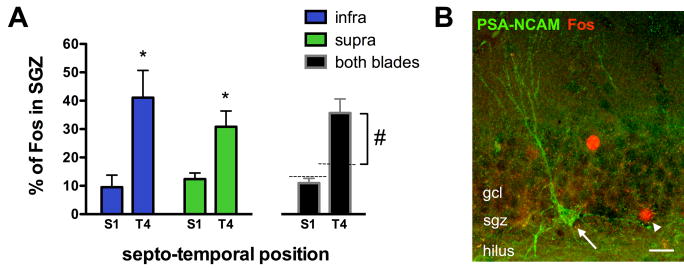
Figure 5.
Water maze training preferentially induces Fos expression in the subgranular zone (SGZ) of the temporal dentate gyrus, where adult-born neurons reside. A) The proportion of Fos+ cells located in the SGZ was examined in the septal and temporal quartiles of the dentate gyrus, using a 2-way ANOVA. There was no difference between blades but there was an effect of septo-temporal position: a significantly greater proportion of Fos+ cells were located in the SGZ in the temporal dentate gyrus compared to the septal dentate gyrus (*p<0.001). Furthermore, in the septal dentate gyrus, the proportion of Fos+ cells that were located in the SGZ was not different from chance. In contrast, in the temporal dentate gyrus, the proportion of Fos-expressing cells located in the SGZ was significantly greater than chance (one sample t-test, #p<0.01). B) Confocal image of PSA-NCAM and Fos immunostaining in the temporal dentate gyrus. A PSA-NCAM+ cell (arrow) and a Fos+ cell (arrowhead) are both located in the deepest row of cells in the granule cell layer, the SGZ. Scale bar 20 μm, gcl = granule cell layer.
4. Discussion
4.1 Neurogenesis gradients and aging
No suprapyramidal-infrapyramidal gradient in neurogenesis was observed in these 13-month-old rats. A significant septo-temporal gradient was observed but only in the mid-septal quadrant; the septal pole showed levels of neurogenesis identical to that in the temporal pole. This contrasts with young adults, which have higher neurogenesis in the infrapyramidal blade than in the suprapyramidal blade and twice as much neurogenesis in the septal pole than in the temporal pole (Snyder et al., 2009), suggesting a flattening of neurogenesis gradients with age. The septal pole and infrapyramidal blade of the dentate gyrus are the last to be formed during early postnatal development (Schlessinger et al., 1975; Piatti et al., 2006). The higher levels of neurogenesis in these regions in young adults (Snyder et al., 2009) therefore appear to reflect continued developmental gradients. The current data suggests that developmental neurogenesis gradients continue to diminish into middle age, leaving residual low levels of neurogenesis that occur roughly equally in all subregions. It is possible that subregional differences in BrdU+ cell density reflect more general differences in granule cell density or cell size. However, we feel these explanations are unlikely since we and others have reported no difference in granule cell density between the septal and temporal poles (West et al., 1988; Snyder et al., 2009) and our analysis of Fos+ granule cells showed that cell size was not significantly different along the septo-temporal axis.
4.2 Gradients of activation in 13-month-old rats
An activity-induced increase in the density of Fos+ cells occurred only in the suprapyramidal blade, consistent with what has been seen in young adult rats (Chawla et al., 2005; Snyder et al., 2009). The septal pole of the dentate gyrus consistently had the highest levels of Fos expression, after water maze experience as well as in control animals. These anatomical gradients of Fos expression mirror those recently found in young adults (Snyder et al., 2009), suggesting that functional subdivisions of the DG are conserved with age.
While the gradients of Fos expression are preserved with age, the overall density of Fos+ cells in 13-month-old rats was reduced by approximately 50% (ANOVA, p < 0.001) relative to what was previously observed in young adults (Snyder et al., 2009). The decrease in Fos expression in 13-month-old rats relative to young adults extends findings that Arc expression is reduced with age, specifically in the DG subfield of the hippocampus (Small et al., 2004). Given the role IEGs have in memory consolidation (Kubik et al., 2007), it is possible that decreased DG Fos expression is a contributing factor in the age-related decline in hippocampal function (Driscoll and Sutherland, 2005). Another distinction from previous findings in young adults was the increased number of Fos+ cells in swim controls relative to water maze-trained rats. The reason for this change with aging is not clear, but a recent study has shown that water maze IEG expression varies according to task demands, and can be particularly elevated by unreinforced swimming (Shires and Aggleton, 2008). The increased Fos+ cell density in both maze-trained and swim control rats relative to cage controls is consistent with previous IEG data showing that the hippocampus processes spatial information regardless of whether or not this information signals reinforcement (Guzowski et al., 1999).
4.3 Implications for young neurons in learning and behavior
Sparse Fos expression (~2% of granule cells in young adults (Kee et al., 2007)) can make it difficult to assess activity in adult-born neurons using BrdU or PSA-NCAM. However, given a 50% reduction in Fos+ cell density compared to young adults and the fact that we examined 871 BrdU+ cells, we would still expect to have found ~9 BrdU+/Fos+ cells (1% of 871). That we found no BrdU+/Fos+ cells suggests the rate of colocalization is ≤ 0.1%. The SGZ provides a much larger population of granule cells that are nearly all born during adulthood and are on average much younger than those found in the more superficial rows (Crespo et al., 1986; Dayer et al., 2003; Kempermann et al., 2003). of After water maze training, the density of Fos+ young neurons in 13-month-old rats was higher in the temporal DG than the septal DG, in contrast to the granule cell population as a whole, which was more active in the septal DG. Young neurons in the temporal region were actually more likely to be activated than older neurons, as indicated by the proportion of Fos+ cells in the SGZ being significantly higher than chance levels. This result matches what was seen in young adults and confirmed in those animals by the additional finding that PSA-NCAM+ young neurons were also more likely to be activated in the temporal DG than in the septal DG (Snyder et al., 2009). The mechanisms underlying differential activation of young and mature neurons is currently unknown. Young neurons possess a lower threshold for synaptic plasticity in vitro and therefore could be more easily recruited to participate in memory formation than mature neurons (Wang et al., 2000; Snyder et al., 2001; Schmidt-Hieber et al., 2004; Ge et al., 2007). The temporal DG-specific enhancement of activity in young neurons may be due to septo-temporal differences in cortical or subcortical inputs (Risold and Swanson, 1996; Dolorfo and Amaral, 1998; Pitkanen et al., 2000; Petrovich et al., 2001) that are especially capable of activating young neurons.
The lack of PSA-NCAM+ cells expressing Fos suggests that activated SGZ cells are more mature than PSA-NCAM+ cells, in line with data from young adult rats [Snyder 2009] indicating that the SGZ population is 5–10 times more likely to be activated than the PSA-NCAM+ population. Since neurogenesis is significantly reduced in 13-month-old rats, and the average age of neurons in the SGZ is therefore much greater, it is somewhat surprising that SGZ cells are still preferentially activated at this age. This leads to two intriguing possibilities: 1) The behavioral critical period, during which adult-born neurons show enhanced behavior-related activity, might outlast the physiological critical period for synaptic plasticity, which lasts only 6 weeks and ends with adult-born granule cells having essentially identical physiological properties to granule neurons born in development, at least in mice (Laplagne et al., 2006; Ge et al., 2007). 2) The behavioral critical period may be prolonged in 13-month-old rats, perhaps due to the low number of new neurons or the absence of novel stimuli.
Acknowledgments
The authors would like to thank Preethi Ramchand and Sarah Rabbett for assistance with histological procedures. This work was funded by an Ontario Graduate Scholarship (JSS), the Canadian Institutes of Health Research (JMW) and the National Institute of Mental Health (HAC).
Footnotes
Disclosure statement
The authors declare no actual or potential conflicts of interest regarding this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Chawla MK, Guzowski JF, Ramirez-Amaya V, Lipa P, Hoffman KL, Marriott LK, Worley PF, McNaughton BL, Barnes CA. Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus. 2005;15:579–586. doi: 10.1002/hipo.20091. [DOI] [PubMed] [Google Scholar]
- Crespo D, Stanfield BB, Cowan WM. Evidence that late-generated granule cells do not simply replace earlier formed neurons in the rat dentate gyrus. Exp Brain Res. 1986;62:541–548. doi: 10.1007/BF00236032. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- de Hoz L, Knox J, Morris RG. Longitudinal axis of the hippocampus: both septal and temporal poles of the hippocampus support water maze spatial learning depending on the training protocol. Hippocampus. 2003;13:587–603. doi: 10.1002/hipo.10079. [DOI] [PubMed] [Google Scholar]
- Dolorfo CL, Amaral DG. Entorhinal cortex of the rat: topographic organization of the cells of origin of the perforant path projection to the dentate gyrus. J Comp Neurol. 1998;398:25–48. [PubMed] [Google Scholar]
- Drew MR, Hen R. Adult hippocampal neurogenesis as target for the treatment of depression. CNS & neurological disorders drug targets. 2007;6:205–218. doi: 10.2174/187152707780619353. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Sutherland RJ. The aging hippocampus: navigating between rat and human experiments. Rev Neurosci. 2005;16:87–121. doi: 10.1515/revneuro.2005.16.2.87. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Gaarskjaer FB. Organization of the mossy fiber system of the rat studied in extended hippocampi. I. Terminal area related to number of granule and pyramidal cells. J Comp Neurol. 1978;178:49–72. doi: 10.1002/cne.901780104. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Fieldsted PM, Rosenberg JS, Kesner RP. Dissociating the roles of dorsal and ventral CA1 for the temporal processing of spatial locations, visual objects, and odors. Behav Neurosci. 2008;122:643–650. doi: 10.1037/0735-7044.122.3.643. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur J Neurosci. 2003;18:2707–2712. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- Jung MW, Wiener SI, McNaughton BL. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J Neurosci. 1994;14:7347–7356. doi: 10.1523/JNEUROSCI.14-12-07347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KB, Solstad T, Brun VH, Hafting T, Leutgeb S, Witter MP, Moser EI, Moser MB. Finite scale of spatial representation in the hippocampus. Science. 2008;321:140–143. doi: 10.1126/science.1157086. [DOI] [PubMed] [Google Scholar]
- Kubik S, Miyashita T, Guzowski JF. Using immediate-early genes to map hippocampal subregional functions. Learn Mem. 2007;14:758–770. doi: 10.1101/lm.698107. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplagne DA, Esposito MS, Piatti VC, Morgenstern NA, Zhao C, van Praag H, Gage FH, Schinder AF. Functional convergence of neurons generated in the developing and adult hippocampus. PLoS biology. 2006;4:e409. doi: 10.1371/journal.pbio.0040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- McDonald HY, Wojtowicz JM. Dynamics of neurogenesis in the dentate gyrus of adult rats. Neurosci Lett. 2005;385:70–75. doi: 10.1016/j.neulet.2005.05.022. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, Jones J, Richards B, Hong NS. A double dissociation of dorsal and ventral hippocampal function on a learning and memory task mediated by the dorso-lateral striatum. Eur J Neurosci. 2006;24:1789–1801. doi: 10.1111/j.1460-9568.2006.05064.x. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Pentkowski NS, Blanchard DC, Lever C, Litvin Y, Blanchard RJ. Effects of lesions to the dorsal and ventral hippocampus on defensive behaviors in rats. Eur J Neurosci. 2006;23:2185–2196. doi: 10.1111/j.1460-9568.2006.04754.x. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Piatti VC, Esposito MS, Schinder AF. The timing of neuronal development in adult hippocampal neurogenesis. Neuroscientist. 2006;12:463–468. doi: 10.1177/1073858406293538. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Amaral DG. The time of origin of somatostatin-immunoreactive neurons in the rat hippocampal formation. Brain Res. 1988;469:231–239. doi: 10.1016/0165-3806(88)90185-x. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Structural evidence for functional domains in the rat hippocampus. Science. 1996;272:1484–1486. doi: 10.1126/science.272.5267.1484. [DOI] [PubMed] [Google Scholar]
- Schlessinger AR, Cowan WM, Gottlieb DI. An autoradiographic study of the time of origin and the pattern of granule cell migration in the dentate gyrus of the rat. J Comp Neurol. 1975;159:149–175. doi: 10.1002/cne.901590202. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Seki T, Arai Y. Age-related production of new granule cells in the adult dentate gyrus. Neuroreport. 1995;6:2479–2482. doi: 10.1097/00001756-199512150-00010. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478:359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- Shires KL, Aggleton JP. Mapping immediate-early gene activity in the rat after place learning in a water-maze: the importance of matched control conditions. Eur J Neurosci. 2008;28:982–996. doi: 10.1111/j.1460-9568.2008.06402.x. [DOI] [PubMed] [Google Scholar]
- Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc Natl Acad Sci U S A. 2004;101:7181–7186. doi: 10.1073/pnas.0400285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Radik R, Wojtowicz JM, Cameron HA. Anatomical gradients of adult neurogenesis and activity: young neurons in the ventral dentate gyrus are activated by water maze training. Hippocampus. 2009;19:360–370. doi: 10.1002/hipo.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Scott BW, Wojtowicz JM. Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol. 2000;42:248–257. [PubMed] [Google Scholar]
- West MJ, Coleman PD, Flood DG. Estimating the number of granule cells in the dentate gyrus with the disector. Brain Res. 1988;448:167–172. doi: 10.1016/0006-8993(88)91114-6. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]