Abstract
Objective
Previous functional magnetic resonance imaging(fMRI) studies examined neural activity responses to emotive stimuli in healthy individuals after acute/subacute administration of antidepressants. We now report the effects of repeated use of the antidepressant clomipramine on fMRI data acquired during presentation of emotion-provoking and neutral stimuli on healthy volunteers.
Methods
Twelve volunteers were evaluated with fMRI after receiving low doses of clomipramine for four weeks, and again after four weeks of washout. Fear-, happiness-, anger-provoking and neutral pictures from the IAPS were used. Data analysis was conducted with statistical parametric mapping(p<0.05).
Results
Paired t-test comparisons for each condition between medicated and unmedicated states showed, to negative valence paradigms, decrease brain activity in the amygdala when participants were medicated. We also demonstrated, across both positive and negative valence paradigms, consistent brain activity decreases in the medicated state in the anterior cingulate gyrus and insula.
Discussion
This is the first report of modulatory effects of repeated antidepressant use on the central representation of somatic states in response to emotions of both negative and positive valence in healthy individuals. Also, our results corroborate findings of antidepressant-induced temporolimbic activity changes to emotion-provoking stimuli obtained in studies of subjects treated acutely with such agents.
Keywords: MRI, Functional, Antidepressive Agents, Tricyclic, Clomipramine, Emotions, healthy subjects
INTRODUCTION
The serotonin (5-HT) and noradrenaline (NA) transmitter systems are critical for the development of normal social behavior. Their efferent neurons project to several brain regions involved in emotional processing in healthy individuals: the amygdala, the ventral striatum, the orbitofrontal and visual cortical areas (implicated in the evaluation of emotionally-salient stimuli and generation of emotional states), the ventral anterior cingulate cortex and the insula (involved in the central mapping of autonomic and visceral reactions associated with emotions), the hippocampus, the dorsal anterior cingulate, and the dorsolateral prefrontal cortices (regulating emotional behaviors)(Phillips et al. 2003; Critchley 2005; Phillips et al. 2008).
To date, many studies have shown functional abnormalities in the brain regions involved in emotional processing of negative and positive emotionally-salient stimuli in unipolar depression (Siegle 1999; Sheline et al. 2001; Fu et al. 2004; Keedwell et al. 2005; Keedwell et al. 2005; Surguladze et al. 2005; Schaefer et al. 2006; Siegle et al. 2006). Subjects with unipolar depression have shown normalization of neural activity after response to antidepressant drugs acting as 5-HT reuptake inhibitors (SRIs) and/or NA reuptake inhibitors (NRIs)(Mayberg et al. 2000; Kennedy et al. 2001; Fu et al. 2004; Canli et al. 2005; Schaefer et al. 2006). In such studies, however, the detection of the direct neural mechanism by which SRIs/NRIs mediate their antidepressant effects was confounded by the significant changes in mood which might influence activity of brain regions involved in emotional processing.
Understanding the effects of 5-HT/NA augmentation upon activity in the above neural system can be facilitated by examining the effect of such medication in healthy individuals without family history of psychiatric disorders. Acute, intravenous administration of a SRI (citalopram) to healthy individuals has been associated with increased recognition of fear and happy facial expressions(Harmer et al. 2002), whereas sub-acute (seven-day) oral administration of citalopram or a NRI (reboxetine) has been associated with reduced fear and anger recognition and enhanced positive stimuli recall(Harmer et al. 2004). Regarding direct effects upon neural activity, acute administration of a SRI (citalopram) has been associated with increased amplitude of visual evoked potentials in parieto-occipital areas to pleasant scenes and suppressed responses in frontal and occipital regions to unpleasant scenes(Kemp et al. 2004). In addition, in recent functional magnetic resonance imaging (fMRI) studies acute administration of a SRI (citalopram) has been associated with increased resting activity in amygdala, striatum, thalamus and hippocampus(McKie et al. 2005); with attenuated ventrolateral prefrontal cortical and amygdalar responses to aversive faces(Del-Ben et al. 2005), and, in one study, with increased amygdala activity when processing faces (Bigos et al. 2008). Acute administration of a SRI (fluvoxamine) has been associated with decreased amygdalar response to aversive compared with neutral stimuli(Takahashi et al. 2005). Also, seven-day administration of citalopram has been associated with decreased amygdalar, hippocampal and medial frontal activity to fearful faces(Harmer et al. 2006). Similarly, seven-day administration of reboxetine has been shown to provoke reduced amygdalar responses to fear-provoking faces(Norbury et al. 2007). These findings suggest that 5-HT/NA reuptake inhibiting antidepressants may attenuate the activity in subcortical limbic neural regions to a higher degree when in presence of negative emotional stimuli compared to positive emotional stimuli.
The above findings need to be interpreted in the light of some limitations. First, the studies have not examined neural activity responses to emotive stimuli in healthy individuals after several weeks of sustained 5-HT and/or NA re-uptake inhibition, which more closely parallels the clinical uses of antidepressants in unipolar depression and anxiety disorders. Second, previous fMRI studies used paradigms requiring passive viewing or identification of emotion in emotional displays, rather than paradigms that more closely resemble the emotion and cognitive processes involved in social interactions, namely, the appraisal of, and empathic response to, more complex emotionally-salient stimuli. The latter paradigms may be more appropriate to examine the putative neural mechanisms mediating antidepressant response.
The present fMRI study sought to compare fMRI data acquired during presentation of emotion-provoking (happiness, fear and anger) and neutral stimuli among healthy volunteers during 4-week administration of the 5HT/NE re-uptake inhibitor clomipramine (medicated status) and after 4-week of clomipramine washout (unmedicated status). We hypothesized that:
There would be decrease amygdala activity after repeated clomipramine use in response to negative stimuli;
There would be no change or increased neural response in the amygdala after repeated clomipramine use in response to positive stimuli;
Repeated clomipramine use would engage other brain regions involved in emotion processing in response to positive and negative stimuli.
METHODS
Subjects
Twelve healthy individuals [3 males and 9 females, mean age = 33.5 years (SD=6.9), mean education = 11.1 years (SD=0.7)] were selected from a larger sample included in a controlled drug trial conducted at the Institute of Psychiatry of São Paulo, Brazil. This study aimed at investigating whether low doses of clomipramine had an effect on mood improvement in healthy subjects (Gentil et al. 2007). Healthy subjects were recruited through newspaper and radio advertisements, and were screened by a team of experienced psychiatrists using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (First et al. 1995). Subjects did not have either first-degree family history of psychiatric disorders including psychosis, recurrent mood disorders and substance dependence, as assessed by the Family History Screen (Weissman et al. 2000). As part of the drug trial, clomipramine was gradually increased according to subject tolerance to up 40Mg/day over 2 weeks and maintained thereafter for an additional period of 2 weeks (mean clomipramine dose 37.5Mg/day – SD=4.5). Subjects were followed weekly for subjective changes in emotional response to every day stimuli. Side-effects (mainly constipation, dry mouth and sexual dysfunction) were present in few subjects and considered mild. No volunteer dropped-out due to any of these complains. Only subjects showing no subjective mood change to clomipramine (as assessed every week blindly by two psychiatrists) (Gentil et al. 2007), and who were right-handed according to the Edinburgh Handedness Inventory were included in this study. Exclusion criteria were: (a) current age greater than 50 or lower than 21 years; (b) current or previous history of neurological and/or general medical conditions, as assessed by non-structured clinical interviewing, physical examination, electrocardiogram and blood/urinary work-up; (c) current use of other drugs psychoactive effects; (d) and for female subjects, history of pregnancy or lactation during the past six months.
Local ethical committees approved the study and all participants signed a written informed consent forms.
Experimental Paradigm
We used the following stimuli: 45 pictures from the International Affective Picture System (IAPS)(Lang et al. 1997) added by 3 pictures obtained from internet versions of local newspapers, divided in three sets with different emotional content (fear, happiness or anger); and 27 pictures of neutral scenes from the IAPS. The selection of happiness-inducing pictures was restricted to the excited (rather than relaxed) kind, in order to minimize the possibility of major decrements in the level of arousal elicited by happiness-provoking stimuli compared to the fear- and anger-inducing pictures. All pictures were presented via a mirror mounted on the head coil of the fMRI scanner.
Three, 5-minute experiments (order counterbalanced across subjects) were carried out during fMRI acquisition, each consisting of a block design paradigm, with five blocks of one type of emotion (fear, anger or happiness) alternated with three blocks of neutral pictures. Before the experiments, subjects were instructed to imagine themselves in the situations while looking at the pictures. Each block comprised three pictures (each presented for 7.5s, with no inter-stimuli interval), all with the same emotional content (fear, anger, happiness or neutral). The themes for each picture set were the same, involving respectively: accidents, natural phenomena, dangerous animals and dentist for the fear blocks; adventure scenes and soccer for the happiness-aroused blocks; observational view of threatening situations or revolting situations for the anger blocks; and personal objects and domestic utilities for the neutral blocks. Stimuli presentation was immediately preceded by a statement shown visually on a black background (for 5s); this strategy was aimed at directing the subject toward experiencing one of the three specific emotions, thus enhancing the desired emotional response. For example, for a block of three fear stimuli including a gun, a thief and a robbery, the instruction was “you are being robbed”.
After each 3-picture set, subjective self-ratings (for fear, anger, happiness and arousal) were obtained over a period of 20s using discretized scales presented visually, in pseudo-randomized order. For each scale, individuals quantified the intensity of the emotional experience as follows: 1. almost nothing, 2. a little, 3. moderate, 4.intense.
In order to confirm that each set of pictures and its associated cue sentence would elicit the desired emotion in a specific fashion, an off-scanner validation study was carried out previously with 156 undergraduate students (91 men and 65 women) at the same university where the current imaging study was conducted (Tavares et al, submitted). A larger pool of pictures were rated using the same four scales cited above. The final sets of three pictures selected for the fMRI study were matched across emotional conditions (happiness, fear or anger) with regard to the mean arousal scores and mean intensity of the key emotion obtained in the off-scanner study. Also, the emotion-provoking sets of pictures were matched to the neutral pictures in regard to their degree of visual complexity.
Occasions of Data Acquisition
Each subject participated in the above imaging experiment on two occasions: (1 - medicated) after a 4-week repeated use of low doses of clomipramine (mean dose 37.5Mg/day) and (2 - unmedicated) after a 4-week period of washout. A simulated session was performed in the week prior to the medicated state scan using a mock scanner that replicated the MRI environment, in order to habituate each subject to the fMRI procedure. In this sham session, each subject was trained to use the response glove, and became used to the paradigm projection, gradient noise and room temperature. During the fMRI session, no subject presented side effects impeditive of successful realization of the scan.
Image Acquisition
Gradient-echo imaging was used to acquire T2*-weighted image volumes using a 1.5 Tesla GE (Milkwalkee, USA) signa LX v8.3 gradient of 23mT/m, in the Institute of Radiology at the FMUSP. For each of the 152 brain volumes, twenty-five axial planes parallel to the AC-PC plane were collected with the following parameters: TR 2500 ms, TE 40ms, slice thickness: 5 mm, 0.7 mm gap, FOV 240 mm and matrix 64×64. In addition, a high-resolution three-dimensional structural axial SPGR sequence was acquired (TE: 4.2ms, TI: 400ms, TR: 10.5ms, flip angle 15°, FOV: 240mm, slice thickness: 1.6mm - zero filled to 0.8mm, matrix: 256×256, 232 slices). Image acquisition, stimulus presentation and subjects’ response were synchronized via an optical-electrical trigger based on the RF signal used to excite each image slice.
Behavioral Data Analysis
Planned comparison using paired t-tests were calculated contrasting scale scores between the medicated and unmedicated states for each condition (emotional or neutral pictures) in each of the three paradigms.
Image Analysis
Data were pre-processed and analyzed using statistical parametric mapping software (SPM5; www.fil.ion.ucl.ac.uk/spm). Data for each participant were initially realigned using the first brain volume acquired as a reference, and unwarped to correct for static inhomogeneity of the magnetic field and movement by inhomogeneity interactions. They were then co-registered with the subjects structural brain dataset, segmented/normalized to the standard Montreal Neurological Institute template (Ashburner and Friston 2005), resampled to 2×2×2 mm3 voxels, and spatially smoothed with a Gaussian kernel of 6-mm full-width at half-maximum. The time-series in each voxel were high pass-filtered to 1/128 Hz. Images from one subject during the Fear paradigm were not available due to technical problems during data acquisition. The structural dataset was always co-registered with the functional images acquired during the same session.
A single-subject, first-level fixed effect model was constructed with emotion and neutral pictures as separate conditions in a block design, using the period of visual scale presentation and responses as baseline in the design matrix. Movement parameters from the realignment stage were entered as a covariate of no interest to further control for subject movement. Trials were modeled using the Canonical Haemodynamic Response Function (Friston 2007). The two conditions were then entered into the second-level analyses using the relevant t-contrast images (emotional pictures > baseline and neutral pictures > baseline).
A group analysis was conducted on the t-contrasts generated in the previous single-subject analyses using planned comparisons (paired t-test) to compare each condition (either emotional pictures or neutral pictures) at time 1 (medicated state) with the analogous condition in time 2 (unmedicated state). Paired t-testing has been used in previous neuroimaging studies investigating the effect of psychotropic medications on brain functioning (Hariri et al. 2002; Aizenstein et al. 2008).
Region of Interest (ROI) Analyses
A priori regions of interest were targeted using anatomical masks created with the Wake Forest University (WFU) Pick Atlas (Maldjian et al. 2003). To control for multiple statistical testing we maintained conservative threshold with a cluster-level false positive detection rate at p < 0.05 by using a voxel threshold of p < 0.05 with a cluster (k) extent empirically determined by Monte Carlo simulations implemented in AlphaSim, which accounted for spatial correlations between BOLD signal changes in neighboring voxels (Forman et al. 1995; Gianaros et al. 2008). We first accessed neural response in the amygdala. Sequentially, we explored regions involved in emotion processing as follows: ventrolateral prefrontal cortex (vlPFC - BA47), dorsolateral prefrontal cortex (DLPFC – BA9 and 46), ventromedial prefrontal cortex (vmPFC - BA11), dorsomedial prefrontal cortex (mPFC - medial region of BA10), anterior cingulate gyrus (ACG - BA24, 25 and 32), hippocampus, parahippocampal gyrus, insula, caudate, thalamus and putamen.
RESULTS
Behavioral Data – Subjective State Ratings
There was no significant difference in the mean scores for each scale between medicated and unmedicated states, with the exception of trends toward greater arousal scores in the medicated state during the presentation of neutral pictures in the Happiness and Fear paradigms (p=0.081 and p=0.095 respectively), and greater anger scale scores in the medicated state during the presentation of anger-provoking pictures (p=0.065) (Table 1).
Table 1.
Subjective Self-rating Scores after Presentation of Emotional and Neutral Blocks of Pictures from the International Affective Picture System
| Medicated status | Unmedicated status | Planned comparison | ||||||
|---|---|---|---|---|---|---|---|---|
| emotion | neutral | |||||||
| Scales | emotion (SD) | neutral (SD) | emotion (SD) | neutral (SD) | t | p | t | p |
| Happiness Paradigm | ||||||||
| Anger | 1.05 (0.12) | 1.03 (0.09) | 1.07 (0.18) | 1.11 (0.36) | −0.27 | 0.79 | −0.90 | 0.39 |
| Arousal | 2.83 (0.82) | 1.97 (0.85) | 2.77 (0.73) | 1.64 (0.95) | 0.37 | 0.72 | 1.91 | 0.08 |
| Fear | 1.82 (0.49) | 1.03 (0.09) | 1.82 (0.7) | 1.05 (0.13) | −0.05 | 0.96 | −1.00 | 0.34 |
| Happiness | 2.72 (0.86) | 1.75 (0.79) | 2.87 (0.87) | 1.75 (0.96) | −1.06 | 0.31 | <0.01 | 1.00 |
| Fear Paradigm | ||||||||
| Anger | 2.08 (0.58) | 1.05 (0.13) | 1.97 (0.65) | 1.17 (0.3) | 0.54 | 0.60 | −1.48 | 0.17 |
| Arousal | 3.12 (0.78) | 1.86 (0.96) | 3.17 (0.82) | 1.58 (0.83) | −0.37 | 0.72 | 1.82 | 0.10 |
| Fear | 3.05 (0.8) | 1.03 (0.09) | 3.08 (0.75) | 1.03 (0.09) | −0.30 | 0.77 | <0.01 | 1.00 |
| Happiness | 1.13 (0.27) | 1.44 (0.64) | 1.15 (0.23) | 1.47 (0.72) | −0.17 | 0.87 | −0.13 | 0.90 |
| Anger Paradigm | ||||||||
| Anger | 2.72 (0.48) | 1.36 (0.39) | 2.68 (0.76) | 1.08 (0.21) | 0.16 | 0.87 | 2.06 | 0.06 |
| Arousal | 3.23 (0.73) | 2.11 (0.88) | 3.23 (0.85) | 1.92 (0.88) | −0.03 | 0.98 | 0.69 | 0.51 |
| Fear | 2.66 (0.83) | 1.28 (0.4) | 2.61 (0.74) | 1.22 (0.29) | 0.44 | 0.67 | 1.00 | 0.34 |
| Happiness | 1.02 (0.06) | 1.53 (0.48) | 1.08 (0.2) | 1.64 (0.63) | −1.08 | 0.30 | −0.57 | 0.58 |
Values are means scores; SD = standard deviation;
Functional Neuroimaging Results
During emotion induction in the Happiness paradigm, ROI paired t-tests did not detect significant activity changes in the right or left amygdala. Exploratory analyses revealed significant reductions in brain activity in the right insula, right and left caudate body, right and left ACG (BA24 and 32), right vmPFC (BA11), left mPFC (BA10) and left putamen when subjects were medicated relative to not medicated (Table 2a; Figure 1). There were no statistical increases in the BOLD effect during the Happiness paradigm when we compared the medicated state (clomipramine) with the unmedicated state (washout) using ROI approach. There were no differences in brain activity (at p<0.05) between the medicated relative to not medicated state during presentation of the neutral pictures.
Table 2.
| Table 2a: Anatomical Locations of the Planned Comparisons during Happiness Paradigm | ||||||||
|---|---|---|---|---|---|---|---|---|
| MNI coordinates |
||||||||
| Region | Side | X | Y | Z | K | T | z | p |
| Happy pictures | ||||||||
| Clomipramine < Washout | ||||||||
| vmPFC (BA11) | R | 28 | 42 | −8 | 26 | 3.38 | 2.74 | 0.003 |
| Insula | R | 40 | 14 | 8 | 77 | 3.43 | 2.77 | 0.003 |
| R | 32 | −26 | 10 | 67 | 2.76 | 2.35 | 0.009 | |
| Anterior Cingulate (BA24) | R | 4 | 28 | 22 | 29 | 3.05 | 2.54 | 0.005 |
| Anterior Cingulate (BA32) | L | −8 | 42 | 14 | 80 | 3.85 | 3 | 0.001 |
| Caudate body | L | −16 | −14 | 26 | 157 | 4.11 | 3.13 | 0.001 |
| R | 16 | −14 | 24 | 132 | 5.5 | 3.74 | <0.001 | |
| medial PFC (BA10) | L | −16 | 42 | 14 | 32 | 3.19 | 2.63 | 0.004 |
| Putamen | L | −14 | 2 | 10 | 66 | 2.34 | 2.07 | 0.019 |
| Table 2b: Anatomical Locations of the Planned Comparisons during Fear Paradigm | ||||||||
|---|---|---|---|---|---|---|---|---|
| MNI coordinates |
||||||||
| Region | Side | X | Y | Z | K | T | z | p |
| Fear pictures | ||||||||
| Clomipramine < Washout | ||||||||
| vLPFC (BA47) | L | −32 | 16 | −2 | 24 | 3.22 | 2.61 | 0.005 |
| DLPFC (BA46) | L | −46 | 36 | 16 | 30 | 3.25 | 2.62 | 0.004 |
| Amygdala | L | −28 | −8 | −12 | 12 | 2.83 | 2.37 | 0.009 |
| Insula | L | −44 | −16 | 18 | 97 | 5.06 | 3.49 | <0.001 |
| R | 38 | 0 | 14 | 186 | 6.46 | 3.97 | <0.001 | |
| Parahippocampal Gyrus | R | 20 | −34 | −14 | 184 | 6.2 | 3.89 | <0.001 |
| DLPFC (BA9) | R | 42 | 22 | 36 | 47 | 2.69 | 2.28 | 0.011 |
| L | −52 | 2 | 36 | 24 | 3.62 | 2.83 | 0.002 | |
| Thalamus | R | 20 | −14 | 6 | 253 | 4.31 | 3.17 | <0.001 |
| Putamen | R | 28 | −16 | −4 | 114 | 4.65 | 3.32 | <0.001 |
| Neutral pictures | ||||||||
| Clomipramine > Washout | ||||||||
| vmPFC (BA11) | L | −24 | 44 | −6 | 8 | 2.22 | 1.96 | 0.025 |
| Clomipramine < Washout | ||||||||
| Anterior Cingulate (BA24) | L | −4 | 22 | 22 | 24 | 2.55 | 2.19 | 0.014 |
| Amygdala | R | 28 | −8 | −14 | 65 | 3.58 | 2.81 | 0.002 |
| Table 2c: Anatomical Locations of the Planned Comparisons during Anger Paradigm | ||||||||
|---|---|---|---|---|---|---|---|---|
| MNI coordinates |
||||||||
| Region | Side | X | Y | Z | K | T | z | p |
| Anger pictures | ||||||||
| Clomipramine < Washout | ||||||||
| vLPFC (BA47) | R | 36 | 30 | −2 | 22 | 3.49 | 2.8 | 0.003 |
| R | 26 | 16 | −12 | 42 | 3.29 | 2.7 | 0.004 | |
| R | 44 | 18 | 2 | 47 | 3.03 | 2.5 | 0.006 | |
| L | −32 | 16 | −12 | 72 | 8.49 | 4.6 | <0.001 | |
| DLPFC (BA46) | R | 42 | 36 | 16 | 53 | 4.15 | 3.2 | 0.001 |
| Amygdala | R | 28 | −4 | −16 | 61 | 3.02 | 2.5 | 0.006 |
| Insula | L | −32 | 16 | −10 | 529 | 6.62 | 4.1 | <0.001 |
| L | −50 | −38 | 20 | 66 | 2.73 | 2.3 | 0.01 | |
| R | 38 | 12 | −10 | 82 | 4.78 | 3.4 | <0.001 | |
| R | 32 | 14 | 12 | 524 | 4.34 | 3.3 | <0.001 | |
| R | 42 | −40 | 18 | 86 | 3.82 | 3 | 0.001 | |
| Anterior Cingulate (BA24) | R | 4 | 30 | 24 | 35 | 3.61 | 2.9 | 0.002 |
| L | −2 | 32 | 18 | 41 | 2.82 | 2.4 | 0.008 | |
| Anterior Cingulate (BA32) | R | 6 | 32 | 26 | 35 | 4.37 | 3.3 | 0.001 |
| L | −2 | 32 | 22 | 35 | 2.44 | 2.1 | 0.016 | |
| Parahippocampal Gyrus | R | 16 | −48 | 2 | 143 | 3.09 | 2.6 | 0.005 |
| L | −20 | −34 | −8 | 270 | 3 | 2.5 | 0.006 | |
| Hippocampus | L | −26 | −36 | −4 | 9 | 2.73 | 2.3 | 0.01 |
| DLPFC (BA9) | R | 4 | 46 | 28 | 37 | 3.45 | 2. 8 | 0.003 |
| R | 34 | 20 | 34 | 129 | 3.31 | 2.7 | 0.003 | |
| Thalamus | R | 8 | −22 | −2 | 621 | 5.02 | 3.5 | <0.001 |
| L | −18 | −22 | 0 | 282 | 6.72 | 4.1 | <0.001 | |
| Caudate body | R | 8 | 12 | 8 | 173 | 6.02 | 3.9 | <0.001 |
| Caudate head | R | 8 | 18 | 6 | 169 | 6.31 | 4.0 | <0.001 |
| L | −8 | 6 | 0 | 64 | 2.65 | 2.3 | 0.011 | |
| Putamen | R | 22 | −2 | 0 | 552 | 5.4 | 3.7 | <0.001 |
| L | −18 | 4 | −8 | 256 | 4.33 | 3.2 | 0.001 | |
| Neutral pictures | ||||||||
| Clomipramine < Washout | ||||||||
| Insula | L | −40 | −12 | 6 | 122 | 4.72 | 3.4 | <0.001 |
| R | 40 | −20 | 6 | 72 | 4.56 | 3.3 | <0.001 | |
vmPFC: ventromedial prefrontal cortex; R, right; L, left; BA, Brodmann area; Coordinates correspond to the stereotaxic array of Montreal Neurologic Institute; K: cluster size; PFC, prefrontal cortex
vLPFC: ventrolateral prefrontal cortex DLPFC: dorsolateral prefrontal cortex; R, right; L, left; BA, Brodmann area; Coordinates correspond to the stereotaxic array of Montreal Neurologic Institute; K: cluster size; vmPFC, ventromedial prefrontal cortex
vLPFC: ventrolateral prefrontal cortex DLPFC: dorsolateral prefrontal cortex; Coordinates correspond to the stereotaxic array of Montreal Neurologic Institute; BA, Brodmann area; R, right; L, left; K: cluster size
Fig. 1.
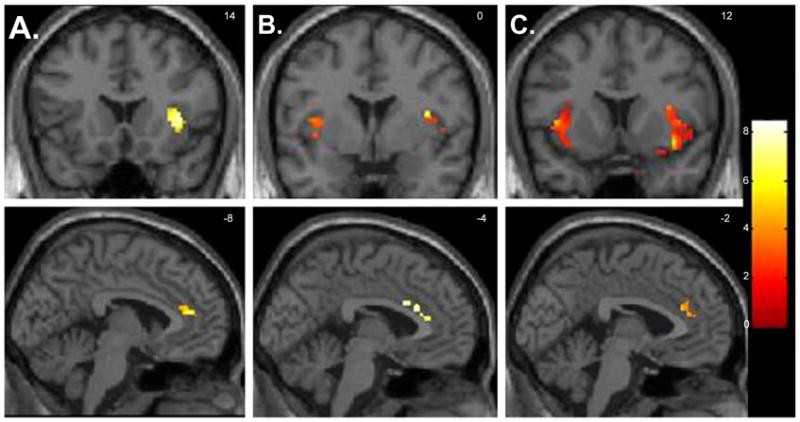
Selected regions of interest where there were foci of significantly decreased neural activity when healthy volunteers (n=12) were medicated versus unmedicated while viewing emotion-provoking paradigm (paired t-tests, p<0.05 corrected for multiple comparisons). Foci of significance were overlaid on coronal and sagittal brain slices spatially normalized into the Montreal Neurologic Institute coordinates system. A. Happiness paradigm; B. Fear paradigm and C. Anger paradigm. Significant differences were detected across the three emotion-provoking paradigms in the insula (shown on the sections on the superior side of the illustration), and in the anterior cingulate gyrus (shown on the inferior slices). Brain slices correspond to coronal view (superior panel) an sagittal view (inferior panel). Left side of the brain corresponds to the left side of the picture. The numbers at the right upper corner at each frame represent standard coordinates in the y (coronal view) and x (sagittal view) axis.
Emotion induction in the Fear Paradigm, using paired t-test comparisons, revealed decreased activity in the left amygdala when subjects were medicated. We also observed decreased activity in the right amygdala during visualization of neutral pictures when subjects were medicated. Exploratory analyses revealed significant reduction in brain activity in the right and left insula, right putamen, right parahippocampal gyrus, right thalamus, right and left DLPFC (BA9/46), left vlPFC (BA47), when subjects were medicated relative to not medicated. To neutral pictures, decreased activity was found in the left ACG (BA24)(Table 2b, Figure 1); however an increase in activity was found in the vmPFC (BA11) when subjects were medicated relative to not medicated.
Finally, during emotion induction in the Anger paradigm, paired t-tests comparisons showed reduced activity in the right amygdala. Exploratory analyses revealed significant activity reduction when subjects were medicated relative to not medicated in the left and right insula, right and left thalamus, left and right caudate, right and left ACG (BA24/BA32), right and left vlPFC (BA47), right and left DLPFC (BA09/46), left hippocampus, right and left parahippocampal gyrus, right and left putamen (Table 2c, Figure 1). To neutral pictures, BOLD signal was reduced in the bilateral insula in the medicated relative to the unmedicated state (Table 2c).
DISCUSSION
This fMRI study evaluated the neural responses to visually presented emotional stimuli in healthy subjects during 4-week of repeated use of low doses of clomipramine (mean 37.5 Mg/day) and after a 4-week washout period. Healthy subjects had no personal or family history of psychiatric disorders, and showed no detectable subjective changes in mood or emotional reactivity to everyday stimuli under repeated use of clomipramine. We were able to replicate previous findings of reduction of amygdala activity after antidepressant use in response to negative stimuli. However, our analysis did not find change in amygdala activity in response to positive stimuli. Although there were some degree of variability in the specific locations of emotion-related neural activity changes across the three emotion-provoking paradigms employed (happiness, fear and anger), we identified a consistent pattern of regionally decreased activity after repeated clomipramine use. The decreased activity occurred in neural regions implicated in the processing of emotional stimuli in healthy individuals, such as the parahippocampal gyrus, anterior cingulate gyrus, insula, subcortical nuclei (striatum and thalamus), and prefrontal cortex (Phillips et al. 2003; Phillips et al. 2008). The healthy subjects in the study showed no detectable subjective changes in mood or emotional reactivity to everyday stimuli under repeated use of clomipramine. Thus, the reduced BOLD signal during the medicated state may reflect intrinsic pharmacological effects of clomipramine upon neural activity, rather than improvements in sub-clinical psychopathology or differences in emotional engagement of the subjects during the processing of visual emotion stimuli between two consecutive scanning sessions.
Our findings of decreased activity in amygdala after repeated clomipramine use during the Fear and Anger paradigm parallels previous studies in healthy volunteers. Studies of acute antidepressant use of citalopram revealed decrease amygdala response to aversive faces (Del-Ben et al. 2005). Moreover, decreased amygdala response was also demonstrated after acute fluvoxamine use in response to aversive faces (Takahashi et al. 2005). Finally, a reduction of amygdala activity after seven days use of citalopram and reboxetine was demonstrated after fearful faces (Harmer et al. 2006; Norbury et al. 2007). The consistent observation of reduction in the amygdala activity in acute, subacute and repeated antidepressant use is consistent with the idea of antidepressant effects in emotion appraisal rather than regulatory emotion processing mechanisms (Norbury et al. 2007). However, one study found increased activation in the amygdala after acute use of citalopram (Bigos et al. 2008). The contrasting findings are interesting and need replication. Moreover, studies in unipolar depression groups using sophisticated functional connectivity analysis methods, reported increased connectivity between amygdala and subgenual ACC, a regulatory area (Anand et al. 2007).
Interestingly, we did not find any significant change in the amygdala activity in response to positive stimuli. We might speculate a specific effect of antidepressant in the neural response only after negative stimuli. However, more studies using positive stimuli are necessary to replicate this finding.
Across the three emotion-provoking paradigms, the brain regions with the most significant activity differences between the medicated and the unmedicated state were the anterior cingulate gyrus, putamen and insula. These three regions have been implicated in the brain network critical for the mediation of emotional responses to emotional stimuli (Phillips et al. 2008; Critchley 2005). In particular, the anterior cingulate gyrus and insula areas are thought to be relevant to the cortical mapping of information pertaining to bodily responses that accompany emotional reactions (Drevets et al. 1997; Craig 2002; Critchley 2005), while the putamen is involved with reward behavior (Forbes et al. 2008). Furthermore, the anterior cingulate gyrus has been implicated in the generation of changes in autonomic arousal, and the insula is thought to play a key role in the central representation of internal visceral responses during emotional processing (Bechara et al. 1997). In addition, the putamen has been implicated in emotional appraisal and identification (Phillips et al. 2008). Contemporary theories propose that the central mapping of such emotion-based somatic markers is crucial to the guiding of decisions and behaviors in complex situations, consciously or unconsciously (Del-Ben et al. 2005; Harmer et al. 2006; Norbury et al. 2007).
Curiously, we found reductions in brain activity in response to negative stimuli (Fear and Anger paradigm) but not to positive stimuli in dorso- and ventro-lateral prefrontal cortical regions. These regions are known to be involved with regulation of behavior and emotion. The use of sophisticated analysis determining the function integration between these regions might help to elucidate the effect of antidepressants in the regulatory process and coupling between the subcortical regions and the prefrontal cortex.
To our knowledge, this is the first study reporting significant activity decrements in the insula and anterior cingulate gyrus in healthy subjects after repeated clomipramine use (4 weeks). Previous fMRI studies on healthy subjects during the presentation of emotion-inducing stimuli have examined the changes in neural activity following only acute (single dose) or subacute (seven days) administration of 5-HT/NA re-uptake inhibiting drugs (Del-Ben et al. 2005; Harmer et al. 2006; Norbury et al. 2007; Bigos et al. 2008; Takahashi et al. 2005). Thus, our findings suggest that a sustained 5-HT/NA re-uptake inhibition (4 weeks) may induce changes in regions involved in the central representation of emotion-related autonomic and visceral responses. Since the subjects in the study were selected with exclusion criteria of vulnerability to depressive, anxiety or other mental disorders (they had neither personal nor familiar history of any psychiatric disorders), our findings suggest an intrinsic capacity of clomipramine to modify brain activity patterns involved in the normal processing of emotional responses, irrespectively of pathological mood features.
Although the duration of clomipramine treatment in this study is close to the latency for antidepressants effects of this drug, the low doses and the absence of anxiety or depressive psychopathology limit the extrapolation of the current findings to clinical states. Nevertheless, one could tentatively take our findings as suggestive that treatment-induced modifications in the processing of emotion-related bodily information are relevant to the mediation of the clinical efficacy of antidepressant drugs. This is consistent with the proposed role of the anterior cingulate gyrus as a surrogate biomarker of antidepressant treatment response, as indicated in previous neuroimaging studies of major depressed patients evaluated before and after antidepressant treatment (Fraguas et al. 2007). Moreover, there is evidence that the intensity of autonomic responses during the processing of emotion-provoking stimuli in unmedicated major depressed patients predicts depressive symptom improvement after chronic antidepressant treatment (Fu et al. 2004).
It is noteworthy that as other tricyclic antidepressants with SNRI properties, clomipramine displays high affinity to histaminergic H1, α1-adrenergic, 5-HT2A and muscarinic postsynaptic receptors (Marcourakis et al. 1993). Therefore, the differences observed in emotion-related neural activity between medicated and non-medicated states may be due to the effect of clomipramine on one or more of those postsynaptic receptor sites.
The choice of clomipramine for this experiment was based on our group’s previous experiments showing that it is clinically effective in doses lower than those usually recommended for major depression, and therefore is likely to decrease unwanted side-effects that could mask the more subtle effects on normal mood regulation (Lotufo-Neto et al. 2001). Indeed, clomipramine is highly efficacious in relatively low doses in panic disorder (Wijkstra et al. 2006) Also, its antidepressant efficacy may be higher than that of non-tricyclic antidepressants in severe depression (Gillman 2007). Moreover, low doses of clomipramine, such as 10Mg, has been demonstrated to occupy 80% of 5-HT transporter, similar with the occupation pattern of more selective inhibitors in clinical doses, (20–40Mg of fluoxetine occupy 80% of the 5-HT transporter)(Wong et al. 2008). Finally, pharmacological studies using animal tissue or human cloned receptors have shown that clomipramine displays strong, dual action both as 5-HT and NA reuptake inhibitor, fulfilling the criteria of a serotonin/noradreline reuptake inhibiting (SNRI) antidepressant even in greater conformity than the more recently marketed agents venlafaxine and duloxetine (Gillman 2007).
As we emphasized the detection of any patterns of changes in subjective mood under clomipramine use (and the exclusion from the present report of responders to this drug), we chose to use a fixed scanning order with the first session under the medicated state. Therefore, it is possible that the findings were influenced by non-specific practice effects from the first, chronically medicated, to the second, not medicated, fMRI session. However, such practice effects are likely to have been minimized since all subjects underwent a simulated scan session one week prior to the medicated assessment. Moreover, previous studies have shown decreased activity in neural regions involved in emotional processing after repeated exposure to emotion-provoking stimuli, rather than the increase in neural activity seen in the post-treatment scan in our subjects. Therefore, the differences in activity in regions implicated in the representation of autonomic states may be related to the direct action of clomipramine, decreasing activity in those neural regions, rather than resulting from practice effects.
Our sample was not balanced in gender distribution and we did not control for possible influence of menstrual cycle phase in our female subsample. Other studies should replicate our finding assessing the menstrual cycle and/or study a larger sample with only females.
In conclusion, the findings of this study indicate that clomipramine, possibly by sustained 5-HT/NA re-uptake inhibition, reduces the central representation of somatic states in response to positive and negative emotional stimuli in healthy subjects. Our finding will inform future research on patients with major depression or panic/agoraphobia in order to clarify the therapeutic effect of antidepressants on neural activity changes in regions involved in the processing of the somatic markers that accompany the generation of emotional states.
Acknowledgments
We thank Antonio Cesário Cruz for technical assistance on the preparation of the devices for stimuli presentation and scale response recording, as well as for his continuing support during neuroimaging data acquisition.
Disclosure/Conflict of Interest
This study was supported by a grant from the ‘Fundação de Amparo à Pesquisa do Estado de São Paulo’ (FAPESP-Brazil; 01/00189-9). JRCA was supported by “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior”(CAPES-Brazil; 190105-2)
References
- Aizenstein HJ, Butters MA, Wu M, Mazurkewicz LM, Stenger VA, Gianaros PJ, Becker JT, Reynolds CF, 3rd, Carter CS. Altered Functioning of the Executive Control Circuit in Late-Life Depression: Episodic and Persistent Phenomena. Am J Geriatr Psychiatry. 2008 doi: 10.1097/JGP.0b013e31817b60af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Gardner K, Lowe MJ. Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating circuit: an FMRI study. J Neuropsychiatry Clin Neurosci. 2007;19(3):274–82. doi: 10.1176/appi.neuropsych.19.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275(5304):1293–5. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR. Acute 5-HT Reuptake Blockade Potentiates Human Amygdala Reactivity. Neuropsychopharmacology. 2008;33(13):3221. doi: 10.1038/npp.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Cooney RE, Goldin P, Shah M, Sivers H, Thomason ME, Whitfield-Gabrieli S, Gabrieli JD, Gotlib IH. Amygdala reactivity to emotional faces predicts improvement in major depression. Neuroreport. 2005;16(12):1267–70. doi: 10.1097/01.wnr.0000174407.09515.cc. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493(1):154–66. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Del-Ben CM, Deakin JF, McKie S, Delvai NA, Williams SR, Elliott R, Dolan M, Anderson IM. The effect of citalopram pretreatment on neuronal responses to neuropsychological tasks in normal volunteers: an FMRI study. Neuropsychopharmacology. 2005;30(9):1724–34. doi: 10.1038/sj.npp.1300728. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Willians JBW. Structured Clinical Interview for DSM-IV Axis I Disorders _ (SCID-, version 2.0) New York: Biometric research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Brown SM, Ryan ND, Birmaher B, Axelson DA, Dahl RE. Altered Striatal Activation Predicting Real-World Positive Affect in Adolescent Major Depressive Disorder. Am J Psychiatry. 2008 doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33(5):636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fraguas R, Jr, Marci C, Fava M, Iosifescu DV, Bankier B, Loh R, Dougherty DD. Autonomic reactivity to induced emotion as potential predictor of response to antidepressant treatment. Psychiatry Res. 2007;151(1–2):169–72. doi: 10.1016/j.psychres.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Statistical parametric mapping: the analysis of funtional brain images. Amsterdam; Boston: Elsevier/Academic Press; 2007. [Google Scholar]
- Fu CHY, Williams SCR, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore ET. Attenuation of the Neural Response to Sad Faces in Major Depression by Antidepressant Treatment: A Prospective, Event-Related Functional Magnetic Resonance Imaging Study. Arch Gen Psychiatry. 2004;61(9):877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Gentil V, Zilberman ML, Lobo D, Henna E, Moreno RA, Gorenstein C. Clomipramine-induced mood and perceived performance changes in selected healthy individuals. J Clin Psychopharmacol. 2007;27(3):314–5. doi: 10.1097/01.jcp.0000270084.15253.5c. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J Neurosci. 2008;28(4):990–9. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman PK. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol. 2007;151(6):737–48. doi: 10.1038/sj.bjp.0707253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, V, Mattay S, Tessitore A, Fera F, Smith WG, Weinberger DR. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology. 2002;27(6):1036–40. doi: 10.1016/S0893-133X(02)00373-1. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Bhagwagar Z, Perrett DI, Vollm BA, Cowen PJ, Goodwin GM. Acute SSRI Administration Affects the Processing of Social Cues in Healthy Volunteers. Neuropsychopharmacology. 2002;28(1):148. doi: 10.1038/sj.npp.1300004. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. Am J Psychiatry. 2004;161(7):1256–63. doi: 10.1176/appi.ajp.161.7.1256. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry. 2006;59(9):816–20. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biol Psychiatry. 2005;58(6):495–503. doi: 10.1016/j.biopsych.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58(11):843–53. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Gray MA, Silberstein RB, Armstrong SM, Nathan PJ. Augmentation of serotonin enhances pleasant and suppresses unpleasant cortical electrophysiological responses to visual emotional stimuli in humans. Neuroimage. 2004;22(3):1084–1096. doi: 10.1016/j.neuroimage.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Kruger S, Mayberg HS, Meyer JH, McCann S, Arifuzzman AI, Houle S, Vaccarino FJ. Changes in Regional Brain Glucose Metabolism Measured With Positron Emission Tomography After Paroxetine Treatment of Major Depression. Am J Psychiatry. 2001;158(6):899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS):Technical Manual and Affective Ratings. Gainesville, FL: University of Florida, Center for Research in Psychophysiology; 1997. [Google Scholar]
- Lotufo-Neto F, Bernik MA, Ramos RT, Andrade L, Gorenstein C, Cordas T, Melo M, Gentil V. A dose-finding and discontinuation study of clomipramine in panic disorder. J Psychopharmacol. 2001;15(1):13–17. doi: 10.1177/026988110101500103. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Marcourakis T, Gorenstein C, Gentil V. Clomipramine, a better reference drug for panic/agoraphobia. II. Psychomotor and cognitive effects. J Psychopharmacol. 1993;7(4):325–330. doi: 10.1177/026988119300700403. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48(8):830–43. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- McKie S, Del-Ben C, Elliott R, Williams S, del Vai N, Anderson I, Deakin JF. Neuronal effects of acute citalopram detected by pharmacoMRI. Psychopharmacology (Berl) 2005;180(4):680–6. doi: 10.1007/s00213-005-2270-y. [DOI] [PubMed] [Google Scholar]
- Norbury R, Mackay CE, Cowen PJ, Goodwin GM, Harmer CJ. Short-term antidepressant treatment and facial processing. Functional magnetic resonance imaging study. Br J Psychiatry. 2007;190:531–2. doi: 10.1192/bjp.bp.106.031393. [DOI] [PubMed] [Google Scholar]
- Norbury R, Mackay CE, Cowen PJ, Goodwin GM, Harmer CJ. The effects of reboxetine on emotional processing in healthy volunteers: an fMRI study. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002091. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54(5):504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. The Neural Basis of Voluntary and Automatic Emotion Regulation: Implications for Understanding the Neurodevelopment of Bipolar Disorder. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.65. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer HS, Putnam KM, Benca RM, Davidson RJ. Event-related functional magnetic resonance imaging measures of neural activity to positive social stimuli in pre- and post-treatment depression. Biol Psychiatry. 2006;60(9):974–86. doi: 10.1016/j.biopsych.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50(9):651–8. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Siegle GJ. A neural network model of attention biases in depression. Prog Brain Res. 1999;121:407–32. doi: 10.1016/s0079-6123(08)63085-x. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am J Psychiatry. 2006;163(4):735–8. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SC, Phillips ML. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57(3):201–9. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yahata N, Koeda M, Takano A, Asai K, Suhara T, Okubo Y. Effects of dopaminergic and serotonergic manipulation on emotional processing: a pharmacological fMRI study. Neuroimage. 2005;27(4):991–1001. doi: 10.1016/j.neuroimage.2005.05.039. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H, Olfson M. Brief screening for family psychiatric history: the family history screen. Arch Gen Psychiatry. 2000;57(7):675–82. doi: 10.1001/archpsyc.57.7.675. [DOI] [PubMed] [Google Scholar]
- Wijkstra J, Lijmer J, Balk FJ, Geddes JR, Nolen WA. Pharmacological treatment for unipolar psychotic depression: Systematic review and meta-analysis. Br J Psychiatry. 2006;188:410–5. doi: 10.1192/bjp.bp.105.010470. [DOI] [PubMed] [Google Scholar]
- Wong DF, Tauscher J, Grunder G. The Role of Imaging in Proof of Concept for CNS Drug Discovery and Development. Neuropsychopharmacology. 2008;34(1):187. doi: 10.1038/npp.2008.166. [DOI] [PubMed] [Google Scholar]


