Abstract
N-methyl-D-aspartate (NMDA) receptors play crucial roles in excitatory synaptic transmission as well as in excitotoxicity. A growing body of evidence suggests that the regulation of both subunit composition and the number of NMDA receptors reaching the surface membrane are tightly regulated. Recently, we have shown that the third membrane domains (M3) of both NR1 and NR2B subunits contain endoplasmic reticulum (ER) retention signals that prevent the unassembled subunits from leaving the ER. Furthermore, these membrane domains together with NR1 M4 are necessary for negating the ER retention signals found in M3 of NR1 and NR2B. In this addendum, we present new electrophysiological data showing that mutation of the HLFY motif, located immediately after M4 of the NR2B subunit, abolishes the surface trafficking of full-length NR1/NR2B complexes (supporting previous immunofluorescent experiments from our lab); however, the deletion of the NR2B C-terminus including the HLFY motif did not affect the formation of functional receptors when two pieces of the NR2B subunit, NR2B truncated before M4 and NR2B M4, were co-expressed together with the NR1 subunit. These observations will help to uncover the processes involved in the assembly of NR1 and NR2 subunits into functional NMDA receptors.
Keywords: ER retention, glutamate receptor, ion channel, masking, patch-clamp, recombinant, subunit assembly
N-methyl-D-aspartate (NMDA) receptors are glutamate-gated ionotropic receptors mediating excitatory neurotransmission in the central nervous system.1 It is believed that these receptors are heterotetramers composed of two NR1 and two NR2 and/or NR3 subunits.2 All NMDA receptor subtypes share the same structural topology with three membrane domains (M1, M3 and M4), a re-entrant pore-forming domain (M2), and extracellular (N-terminus and loop between M3 and M4) and intracellular (C-terminus) regions. The most abundant NR1 splice variant (NR1-1) and all NR2 subunits are retained in the ER when expressed by themselves in various heterologous cells and neurons; however, co-expression of NR1-1 and NR2 leads to surface expression of assembled complexes.3–5 Thus, these data suggest the presence of a mechanism(s) regulating ER retention of unassembled subunits, correct assembly of the subunits into functional receptors, as well as release of the functional receptors from the ER.
There have been a number of reports studying the role of ER retention in the assembly of NMDA receptors. First, the localization of ER retention signals was studied using chimeric proteins composed of tac (an interleukin-2 receptor subunit) and NMDA receptor C-termini.6–9 Since both NR1-1 and NR2B C-termini cause the chimeric protein to be retained in the ER, an extensive mutagenesis study was performed. Hence, an RXR ER retention motif in the NR1-1 C-terminus was identified. Efforts to localize a particular motif responsible for the ER retention of tac-NR2B chimera have been unsuccessful. Second, using truncated and chimeric NMDA receptor subunits, we showed recently that M3 of both NR1 and NR2B possess ER retention signals and that these domains as well as M4 of NR1 are responsible for masking the ER retention signals in the M3 domains.10 Third, NR1/NR2 complexes can reach the cell surface membrane even when either one or both C-terminal regions are deleted five or six amino acid residues after M4.10–12 However, full-length NR2B constructs lacking the HLFY motif, located one amino acid residue after the M4 domain, are not delivered to the surface membrane.6 Fourth, a later study showed that replacement of the HLFY motif with alanines in the NR2B subunit, truncated immediately after the mutated HLFY motif, does not affect the formation of functional channels.13 Since this study also showed that at least three amino acid residues after NR2B M4 are necessary for surface trafficking of the receptors, this indicates that the NR2B M4 domain and surrounding segments are critical for correct assembly and formation of functional receptors. Finally, co-expression of the NR2 subunit truncated before M4 (NR2 preM4) with the full-length NR1 subunit fails to produce functional receptors. However, the formation of functional receptors can be rescued when the peptide containing NR2 M4 and C-terminus is present.10,14 Interestingly, each of these separate pieces of NR2 subunits contains at least one ER retention signal, which has to be masked during assembly of the functional receptor in order for the assembled receptor to exit the ER.
To further explore the roles of NR2 domains in the assembly and masking of the ER retention signals, we performed whole-cell patch-clamp recordings from human embryonic kidney 293 cell (HEK293) cells expressing various NMDA receptor subunit combinations. As expected, co-expression of full-length NR1-1a and NR2B subunits produced functional receptors, but co-expression of the NR1-1a subunit with either the NR2B subunit truncated immediately after M4 (NR2B-0) or the full-length NR2B subunit without the HLFY motif (NR2BHLFY/AAAA) produced no current (Fig. 1). This suggests that the release of receptors from the ER is tightly regulated.
Figure 1. Role of the HLFY motif in the formation of functional NMDA receptors.
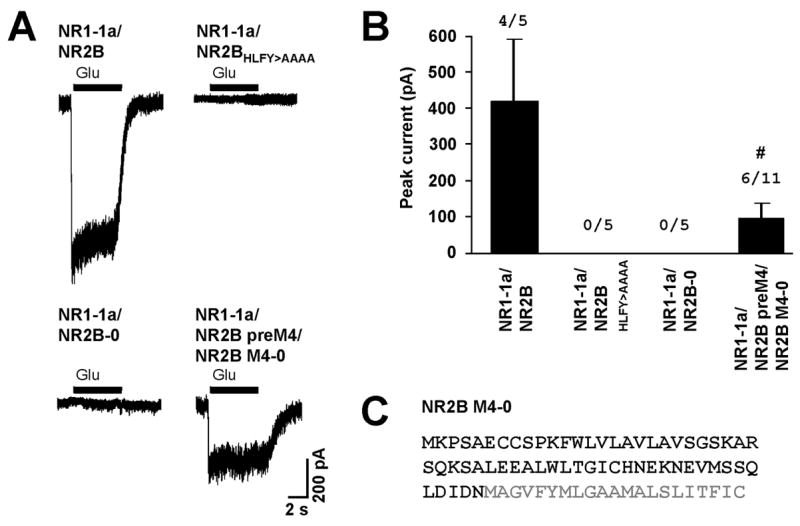
(A) Currents elicited with 1 mM glutamate and 1 mM glycine during whole-cell patch-clamp recordings from HEK293 cells expressing indicated combinations of NMDA receptor subtypes are shown (holding potential at −60 mV). (B) Bar graph representing the mean peak current amplitudes ± SEM of evoked whole-cell currents. The numbers of cells responding to agonists out of the total number of recorded cells are shown above the columns. Data from cells expressing NR1-1a/NR2B preM4/NR2B M4-0 were pooled together from two separate experiments (#). (C) The amino acid sequence of NR2B M4-0 is depicted. Grey color indicates the M4 domain.
Next, we asked whether the HLFY motif is necessary for formation of functional receptors from truncated NR2B subunits. Surprisingly, co-expression of NR1-1a, NR2B preM4, and the peptide containing only NR2B M4 domain (NR2B M4-0) led to the formation of functional receptors (Fig. 1). Since we showed recently that the NR1/NR2B preM4 complex can reach the surface membrane,10 our data show that the HLFY motif is not necessary for formation of functional channels when the receptor has already exited the ER. Our results also suggest that NR2B M4 associates with the remaining segments of the NR2B and/or NR1 subunits and thus helps to govern the formation of the functional receptors.
Acknowledgments
This work was supported by the NIDCD Intramural Program.
Abbreviations
- ER
endoplasmic reticulum
- HEK293
human embryonic kidney 293 cell
- M
membrane domain
- NMDA
N-methyl-D-aspartate
- SEM
standard error of the mean
- tac
interleukin-2 receptor subunit
Footnotes
Addendum to: Masking of the endoplasmic reticulum retention signals during assembly of the NMDA receptor, Martin Horak, Kai Chang, Robert J. Wenthold, The Journal of Neuroscience 2008; 28(13):3500-3509
References
- 1.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–26. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- 2.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–35. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 3.Fukaya M, Kato A, Lovett C, Tonegawa S, Watanabe M. Retention of NMDA receptor NR2 subunits in the lumen of endoplasmic reticulum in targeted NR1 knockout mice. Proc Natl Acad Sci U S A. 2003;100:4855–60. doi: 10.1073/pnas.0830996100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McIlhinney RA, Le Bourdelles B, Molnar E, Tricaud N, Streit P, Whiting PJ. Assembly intracellular targeting and cell surface expression of the human N-methyl-D-aspartate receptor subunits NR1a and NR2A in transfected cells. Neuropharmacology. 1998;37:1355–67. doi: 10.1016/s0028-3908(98)00121-x. [DOI] [PubMed] [Google Scholar]
- 5.Okabe S, Miwa A, Okado H. Alternative splicing of the C-terminal domain regulates cell surface expression of the NMDA receptor NR1 subunit. J Neurosci. 1999;19:7781–92. doi: 10.1523/JNEUROSCI.19-18-07781.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawkins LM, Prybylowski K, Chang K, Moussan C, Stephenson FA, Wenthold RJ. Export from the endoplasmic reticulum of assembled N-methyl-d-aspartic acid receptors is controlled by a motif in the c terminus of the NR2 subunit. J Biol Chem. 2004;279:28903–10. doi: 10.1074/jbc.M402599200. [DOI] [PubMed] [Google Scholar]
- 7.Scott DB, Blanpied TA, Swanson GT, Zhang C, Ehlers MD. An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J Neurosci. 2001;21:3063–72. doi: 10.1523/JNEUROSCI.21-09-03063.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Standley S, Roche KW, McCallum J, Sans N, Wenthold RJ. PDZ domain suppression of an ER retention signal in NMDA receptor NR1 splice variants. Neuron. 2000;28:887–98. doi: 10.1016/s0896-6273(00)00161-6. [DOI] [PubMed] [Google Scholar]
- 9.Xia H, Hornby ZD, Malenka RC. An ER retention signal explains differences in surface expression of NMDA and AMPA receptor subunits. Neuropharmacology. 2001;41:714–23. doi: 10.1016/s0028-3908(01)00103-4. [DOI] [PubMed] [Google Scholar]
- 10.Horak M, Chang K, Wenthold RJ. Masking of the endoplasmic reticulum retention signals during assembly of the NMDA receptor. J Neurosci. 2008;28:3500–9. doi: 10.1523/JNEUROSCI.5239-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vissel B, Krupp JJ, Heinemann SF, Westbrook GL. A use-dependent tyrosine dephosphorylation of NMDA receptors is independent of ion flux. Nat Neurosci. 2001;4:587–96. doi: 10.1038/88404. [DOI] [PubMed] [Google Scholar]
- 12.Zheng X, Zhang L, Wang AP, Bennett MV, Zukin RS. Protein kinase C potentiation of N-methyl-D-aspartate receptor activity is not mediated by phosphorylation of N-methyl-D-aspartate receptor subunits. Proc Natl Acad Sci U S A. 1999;96:15262–7. doi: 10.1073/pnas.96.26.15262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang W, Zheng C, Song Q, Yang X, Qiu S, Liu C, Chen Z, Duan S, Luo J. A three amino acid tail following the TM4 region of the N-methyl-D-aspartate receptor (NR) 2 subunits is sufficient to overcome endoplasmic reticulum retention of NR1-1a subunit. J Biol Chem. 2007;282:9269–78. doi: 10.1074/jbc.M700050200. [DOI] [PubMed] [Google Scholar]
- 14.Schorge S, Colquhoun D. Studies of NMDA receptor function and stoichiometry with truncated and tandem subunits. J Neurosci. 2003;23:1151–8. doi: 10.1523/JNEUROSCI.23-04-01151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


