Abstract
Background
Intravesical chemotherapy and bacillus Calmette-Guérin (BCG) reduce the recurrence rate in patients with stage Ta T1 urothelial bladder cancer; however, the benefit of BCG relative to chemotherapy for long-term end points is controversial, especially in intermediate-risk patients.
Objective
The aim of the study was to compare the long-term efficacy of BCG and epirubicin.
Design, setting, and participants
From January 1992 to February 1997, 957 patients with intermediate- or high-risk stage Ta T1 urothelial bladder cancer were randomized after transurethral resection to one of three treatment groups in the European Organization for Research and Treatment of Cancer Genito-Urinary Group phase 3 trial 30911.
Intervention
Patients received six weekly instillations of epirubicin, BCG, or BCG plus isoniazid (INH) followed by three weekly maintenance instillations at months 3, 6, 12, 18, 24, 30, and 36.
Measurements
End points were time to recurrence, progression, distant metastases, overall survival, and disease-specific survival.
Results and limitations
With 837 eligible patients and a median follow-up of 9.2 yr, time to first recurrence (p < 0.001), distant metastases (p = 0.046), overall survival (p = 0.023), and disease-specific survival (p = 0.026) were significantly longer in the two BCG arms combined as compared with epirubicin; however, there was no difference for progression. Three hundred twenty-three patients with stage T1 or grade 3 tumors were high risk, and the remaining 497 patients were intermediate risk. The observed treatment benefit was at least as large, if not larger, in the intermediate-risk patients compared with the high-risk patients.
Conclusions
In patients with intermediate- and high-risk stage Ta and T1 urothelial bladder cancer, intravesical BCG with or without INH is superior to intravesical epirubicin not only for time to first recurrence but also for time to distant metastases, overall survival, and disease-specific survival. The benefit of BCG is not limited to just high-risk patients; intermediate-risk patients also benefit from BCG.
Keywords: Bacillus Calmette-Guérin, Epirubicin, Instillation therapy, Isoniazid, Non–muscle-invasive bladder cancer, Randomized clinical trial, Urothelial carcinoma of the bladder
1. Introduction
The European Association of Urology (EAU) guidelines on non–muscle-invasive urothelial carcinoma of the bladder provide recommendations for the adjuvant treatment of patients after transurethral resection (TUR) based on their risk of recurrence and progression to muscle-invasive disease [1,2]. In patients at low risk of recurrence and progression, one immediate instillation of chemotherapy is recommended as the complete adjuvant treatment. In patients at high risk of progression, at least 1 yr of intravesical bacillus Calmette-Guérin (BCG) is recommended after an immediate instillation of chemotherapy. In the remaining intermediate-risk patients, the guidelines recommend that either further instillations of chemotherapy or a minimum of 1 yr of BCG be given after an immediate instillation of chemotherapy [1].
Meta-analyses have shown that adjuvant BCG with a maintenance schedule is superior to both TUR alone and TUR plus adjuvant chemotherapy in preventing recurrence [3–5]. Although two literature-based meta-analyses comparing BCG with mitomycin C (MMC) or other therapies concluded that BCG with maintenance prevents or at least delays progression to muscle-invasive disease [6,7], a recent individual patient data meta-analysis comparing BCG with MMC could not confirm these results [5].
Intravesical chemotherapy and intravesical BCG both reduce the recurrence rate in patients with stage Ta T1 urothelial bladder cancer; however, the benefit of BCG relative to chemotherapy for long-term end points in the intermediate-risk patients, approximately 50% of whom recur but only about 10% progress to muscle-invasive disease, remains controversial because any advantage in efficacy with BCG must be weighed against its increased risk of toxicity [8].
As an alternative to MMC, epirubicin has been commonly used in the intravesical treatment of non-muscle-invasive bladder cancer after TUR in both Europe and in Japan [9]. In 1992, the European Organization for Research and Treatment of Cancer (EORTC) launched a trial to compare, after TUR, the efficacy of six weekly intravesical instillations of epirubicin, BCG, and BCG plus isoniazid (INH) followed by three weekly maintenance instillations at months 3, 6, 12, 18, 24, 30, and 36 in patients with intermediate- and high-risk stage Ta T1 urothelial bladder cancer without carcinoma in situ (CIS). The first results, dealing mainly with recurrence and side-effects, were published in 2001 [10]. The present paper presents the final long-term efficacy results, taking into account patient risk groups.
2. Material and methods
EORTC phase 3 trial 30911 included patients with biopsy-proven, completely resected, single or multiple, primary or recurrent, stage Ta T1, grades 1–3 urothelial carcinoma of the bladder.
Exclusion criteria were a solitary primary tumor; CIS; stage T2 or higher tumors; age >85 yr; World Health Organization (WHO) performance status 3 or 4; previous treatment with doxorubicin, epirubicin, or BCG; and intravesical treatment during the previous 3 mo. Patient informed consent was obtained in accordance with the Declaration of Helsinki and/or existing national and local regulations.
Within 24 h after TUR and prior to receipt of the histology report, patients were randomized to one of three treatment groups: (1) epirubicin, 50 mg in 50 ml of saline weekly for 6 consecutive weeks starting within 24 h after TUR; (2) BCG (Tice), 5 × 108 colony-forming units weekly for 6 consecutive weeks starting 7–15 d after transurethral resection; or (3) BCG (Tice) as above plus INH, 300 mg orally, the day before, the same day as, and the day after instillation. In all three treatment groups, this initial treatment was followed by three weekly instillations at months 3, 6, 12, 18, 24, 30, and 36 for a total of 27 instillations for 3 yr.
Cystoscopy and urine cytology were repeated every 3 mo during the first 3 yr and every 6 mo thereafter. All visible lesions had to be resected with recurrence established only by histologic confirmation. Patients stopped protocol treatment at the second recurrence after randomization, progression to muscle-invasive disease, appearance of CIS, carcinoma in the upper urinary tract or prostatic urethra, or distant metastases. Further treatment was then at the discretion of the local investigator. Further details are available in the original publication [10].
The treatment groups were compared on a primary end point of time to first bladder recurrence (any stage or grade) and on secondary end points of time to progression to muscle-invasive disease (T2 or higher), time to distant metastases, overall duration of survival, and time to death due to bladder cancer.
The starting point was the date of randomization. Overall survival was estimated using the Kaplan-Meier technique and compared using the two-sided log-rank test. To take into account patients who died of unrelated causes (competing risks) prior to observing the event of interest, times to the other events listed above were estimated by cumulative incidence functions and compared using the two-sided Gray’s test. Patients without an event were censored at the date of the last follow-up unless they died from other causes (competing risk).
Because the treatment of intermediate-risk patients is controversial, patients were divided into two risk groups according to their risk of progression and death due to bladder cancer: intermediate-risk group (neither T1 nor grade 3 tumors) and high-risk group (stage T1 and/or grade 3 tumors). Separate exploratory analyses were carried out in these two subgroups to check for consistency of results.
3. Results
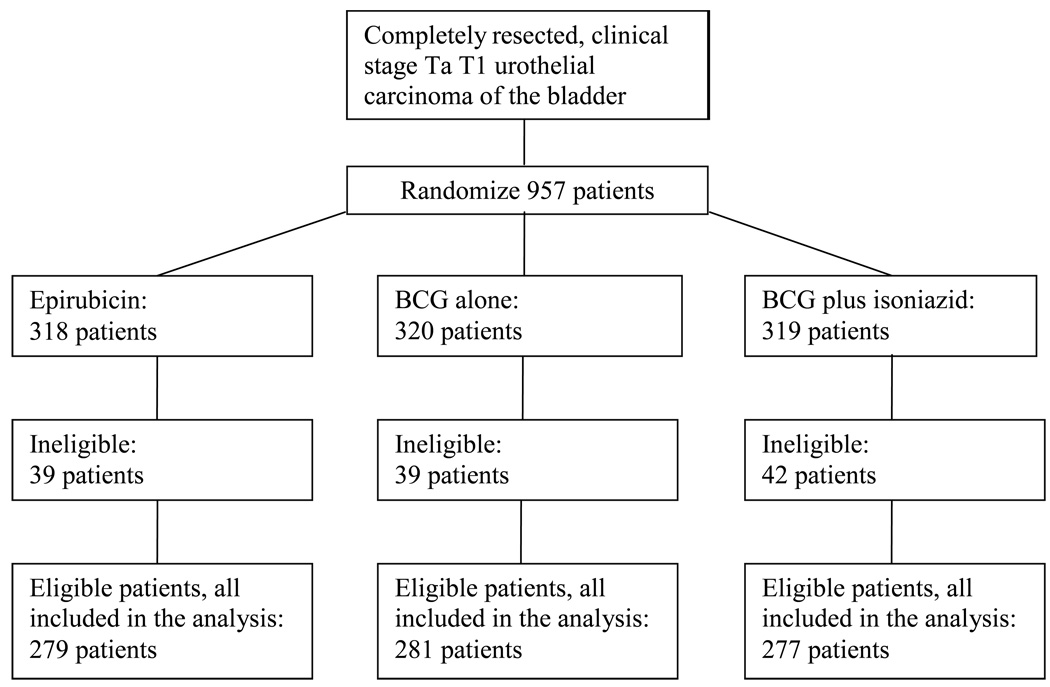
Nine hundred and fifty-seven patients were centrally randomized by 44 institutions at the EORTC headquarters between January 1992 and February 1997, with stratification by primary/recurrent tumors, solitary/multiple tumors, and institution using the minimization technique. One hundred and twenty ineligible patients were excluded from the comparisons of treatment efficacy (Fig. 1). Reasons for ineligibility were mainly related to an inappropriate tumor stage because histology was available only after randomization [10].
Fig. 1.
CONSORT flow diagram. BCG = bacillus Calmette-Guérin.
Table 1 summarizes patient characteristics for the 837 eligible patients by treatment group. The median age was 67 yr, 45% had primary tumors, 85% had multiple tumors, the median tumor size was 1 cm, 64% had Ta tumors, and 13% had grade 3 tumors. The median EORTC recurrence and progression scores were both 5, which correspond to intermediate risks of recurrence and progression [1,2]. Finally, 61% were intermediate risk, and 39% were high risk. Patient characteristics were well balanced in the three treatment groups.
Table 1.
Patient characteristics
| Epirubicin (n =279) |
BCG (n =281) |
BCG + INH (n = 277) |
Total (n = 837) |
|
|---|---|---|---|---|
| Age, yr, n (%) | ||||
| ≤60 | 74 (26.8) | 72 (26.2) | 74 (27.3) | 220 (26.8) |
| 61–70 | 100 (36.2) | 110 (40.0) | 104 (38.4) | 314 (38.2) |
| 71–80 | 90 (32.6) | 84 (30.5) | 82 (30.3) | 256 (31.1) |
| >80 | 12 (4.3) | 9 (3.3) | 11 (4.1) | 32 (3.9) |
| Median | 67 | 66 | 66 | 67 |
| IQR | 60–73 | 60–72 | 59–72 | 60–72 |
| Unknown | 3 | 6 | 6 | 15 |
| Prior recurrence rate, n (%) |
||||
| Primary | 127 (46.5) | 120 (44.6) | 118 (44.9) | 365 (45.3) |
| Recurrent ≤1/yr | 61 (22.3) | 76 (28.3) | 69 (26.2) | 206 (25.6) |
| Recurrent >1/yr | 85 (31.1) | 73 (27.1) | 76 (28.9) | 234 (29.1) |
| Unknown | 6 | 12 | 14 | 32 |
| Tumors, n (%) | ||||
| Single | 35 (13.0) | 45 (16.5) | 41 (15.3) | 121 (15.0) |
| Multiple | 234 (87.0) | 227 (83.5) | 227 (84.7) | 688 (85.0) |
| Unknown | 10 | 9 | 9 | 28 |
| Tumor size, n (%) | ||||
| ≤1 cm | 132 (50.6) | 138 (52.1) | 127 (49.0) | 397 (50.6) |
| >1 cm and ≤3 cm | 110 (42.1) | 100 (37.7) | 110 (42.5) | 320 (40.8) |
| >3 cm | 19 (7.3) | 27 (10.2) | 22 (8.5) | 68 (8.7) |
| Median | 1.0 | 1.0 | 1.5 | 1.0 |
| IQR | 0.7–2.0 | 0.7–2.0 | 0.8–2.0 | 0.7–2.0 |
| Unknown | 18 | 16 | 18 | 52 |
| T category, n (%) | ||||
| Ta | 179 (65.3) | 169 (61.7) | 173 (63.8) | 521 (63.6) |
| T1 | 95 (34.7) | 105 (38.3) | 98 (36.2) | 298 (36.4) |
| Unknown | 5 | 7 | 6 | 18 |
| WHO 1973 grade, n (%) |
||||
| G1 | 108 (39.7) | 103 (37.7) | 101 (37.4) | 312 (38.3) |
| G2 | 133 (48.9) | 133 (48.7) | 135 (50.0) | 401 (49.2) |
| G3 | 31 (11.4) | 37 (13.6) | 34 (12.6) | 102 (12.5) |
| Unknown | 7 | 8 | 7 | 22 |
| EORTC recurrence score |
||||
| Median | 5 | 6 | 5 | 5 |
| IQR | 4–7 | 4–8 | 4–7 | 4–7 |
| Unknown | 27 | 27 | 30 | 84 |
| EORTC progression score |
||||
| Median | 5 | 5 | 5 | 5 |
| IQR | 3–7 | 3–9 | 3–8 | 3–7 |
| Unknown | 27 | 27 | 30 | 84 |
| Risk group, n (%) | ||||
| Intermediate | 170 (62.0) | 161 (58.5) | 166 (61.3) | 497 (60.6) |
| High | 104 (38.0) | 114 (41.5) | 105 (38.7) | 323 (39.4) |
| Unknown | 5 | 6 | 6 | 17 |
BCG = bacillus Calmette-Guérin; EORTC = European Organization for Research and Treatment of Cancer; INH = isoniazid; IQR = interquartile range; WHO = World Health Organization.
A total of 267 patients on epirubicin, 266 on BCG, and 256 on BCG plus INH started treatment. The median duration of treatment was 12, 18, and 12 mo with 70 patients (25%), 81 patients (29%), and 68 patients (25%), respectively, completing all 36 mo of treatment. The main reasons for prematurely stopping treatment was treatment inefficacy, including second recurrence or progression, in 166 patients and toxicity in 115 patients: 16 (6%) on epirubicin and 99 (19%) on the two BCG arms. Table 2 describes the further treatment reported after stopping protocol treatment.
Table 2.
Further treatment received
| Epirubicin (n =279) |
BCG (n =281) |
BCG + INH (n =277) |
Total (n =837) |
|
|---|---|---|---|---|
| Intravesical BCG, n (%) |
46 (16.5) | 28 (10.0) | 31 (11.2) | 105 (12.5) |
| Intravesical chemotherapy, n (%) |
39 (14.0) | 28 (10.0) | 29 (10.5) | 96 (11.5) |
| Cystectomy, n (%) | 10 (3.6) | 12 (4.3) | 13 (4.7) | 35 (4.2) |
| Without progression | 4 | 3 | 2 | 9 |
| After progression | 6 | 9 | 11 | 26 |
BCG = bacillus Calmette-Guérin; INH = isoniazid.
Based on a median follow-up of 9.2 yr and a maximum follow-up of 14.3 yr, Table 3 presents the treatment outcome by treatment group. Globally, 43% recurred, 8% progressed to muscle-invasive disease, 6% developed distant metastases, 12% developed a second primary, 33% died, and 4.5% died due to bladder cancer. Because there was no beneficial or adverse effect of the addition of INH to BCG [10,11] and the main interest centers on the comparison of epirubicin to BCG (with or without INH), the two BCG treatment groups were pooled together for comparison purposes. Compared to epirubicin, and as summarized in Table 4, BCG reduced the risk of recurrence (hazard ratio [HR]: 0.62; p < 0.001; Fig. 2), the development of distant metastases (HR: 0.55; p = 0.046; Fig. 4), the risk of death (HR: 0.76; p = 0.023; Fig. 5), and the risk of death due to bladder cancer (HR: 0.47; p = 0.026; Fig. 6). There was no significant difference in the time to progression (HR: 0.84; p = 0.55; Fig. 3) or in non–bladder cancer mortality (p = 0.21).
Table 3.
Treatment outcome
| Epirubicin (n = 279) |
BCG (n = 281) |
BCG + INH (n = 277) |
Total (n = 837) |
|
|---|---|---|---|---|
| Recurrence, n (%) | ||||
| No | 132 (47.3) | 178 (63.3) | 167 (60.3) | 477 (57.0) |
| Yes | 147 (52.7) | 103 (36.7) | 110 (39.7) | 360 (43.0) |
| Progression, n (%) | ||||
| No | 255 (91.4) | 262 (93.2) | 254 (91.7) | 771 (92.1) |
| Yes | 24 (8.6) | 19 (6.8) | 23 (8.3) | 66 (7.9) |
| Distant metastases, n (%) |
||||
| No | 255 (91.4) | 265 (94.3) | 265 (95.7) | 785 (93.8) |
| Yes | 24 (8.6) | 16 (5.7) | 12 (4.3) | 52 (6.2) |
| Progression or distant metastases, n (%) |
||||
| No | 240 (86.0) | 257 (91.5) | 250 (90.3) | 747 (89.2) |
| Yes | 39 (14.0) | 24 (8.5) | 27 (9.7) | 90 (10.8) |
| Progression only | 15 | 8 | 15 | 38 |
| Distant metastases only |
15 | 5 | 4 | 24 |
| Both | 9 | 11 | 8 | 28 |
| Second primary, n (%) |
||||
| No | 244 (87.5) | 249 (88.6) | 247 (89.2) | 740 (88.4) |
| Yes | 35 (12.5) | 32 (11.4) | 30 (10.8) | 97 (11.6) |
| Survival, n (%) | ||||
| Alive | 173 (62.0) | 197 (70.1) | 189 (68.2) | 559 (66.8) |
| Dead | 106 (38.0) | 84 (29.9) | 88 (31.8) | 278 (33.2) |
| Bladder cancer | 19 (6.8) | 9 (3.2) | 10 (3.6) | 38 (4.5) |
| Cardiovascular | 40 (14.3) | 30 (10.7) | 36 (13.0) | 106 (12.7) |
| Other | 33 (11.8) | 28 (10.0) | 31 (11.2) | 92 (11.0) |
| Cause unknown | 14 (5.0) | 17 (6.1) | 11 (4.0) | 42 (5.0) |
BCG = bacillus Calmette-Guérin; INH = isoniazid.
Table 4.
Comparison of epirubicin and Bacillus Calmette-Guérin
| Intermediate risk | High risk | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Events/patients (%) |
HR (95% CI) | p value | Events/patients (%) |
HR (95% CI) |
p value |
Events/patients (%) |
HR (95% CI) | p value | |
|
Recurrence Epirubicin BCG |
100/170 (58.8) 131/327 (40.1) |
1 0.59 (0.45–0.76) |
– <0.001 |
46/104 (44.2) 82/219 (37.4) |
1 0.69 (0.48–1.05) |
– 0.09 |
147/279 (52.7) 213/558 (38.2) |
1 0.62 (0.50–0.76) |
– <0.001 |
|
Progression Epirubicin BCG |
12/170 (7.1) 13/327 (4.0) |
1 0.56 (0.26–1.23) |
– 0.14 |
12/104 (11.5) 29/219 (13.2) |
1 0.92 (0.47–1.82) |
– 0.93 |
24/279 (8.6) 42/558 (7.5) |
1 0.84 (0.51–1.39) |
– 0.55 |
|
Distant metastases Epirubicin BCG |
15/170 (8.8) 12/327 (3.7) |
1 0.42 (0.20–0.90) |
– 0.027 |
9/104 (8.7) 16/219 (7.3) |
1 0.66 (0.29–1.52) |
– 0.42 |
24/279 (8.6) 28/558 (5.0) |
1 0.55 (0.32–0.94) |
– 0.046 |
|
Progression or distant metastases Epirubicin BCG |
23/170 (13.5) 17/327 (5.2) |
1 0.39 (0.21–0.73) |
– 0.002 |
16/104 (15.4) 34/219 (15.5) |
1 0.80 (0.44–1.45) |
– 0.70 |
39/279 (14.0) 51/558 (9.1) |
1 0.63 (0.41–0.95) |
– 0.031 |
|
Death: all causes Epirubicin BCG |
66/170 (38.8) 99/327 (30.3) |
1 0.79 (0.58–1.09) |
– 0.14 |
40/104 (38.5) 73/219 (33.3) |
1 0.68 (0.46–1.00) |
– 0.051 |
106/279 (38.0) 172/558 (30.8) |
1 0.76 (0.59–0.96) |
– 0.023 |
|
Death: bladder cancer Epirubicin BCG |
12/170 (7.1) 8/327 (2.4) |
1 0.35 (0.14–0.86) |
– 0.020 |
7/104 (6.7) 11/219 (5.0) |
1 0.60 (0.23–1.56) |
– 0.39 |
19/279 (6.8) 19/558 (3.4) |
1 0.47 (0.25–0.89) |
– 0.026 |
BCG = bacillus Calmette-Guérin; CI = confidence interval; HR = hazard ratio.
Fig. 2.
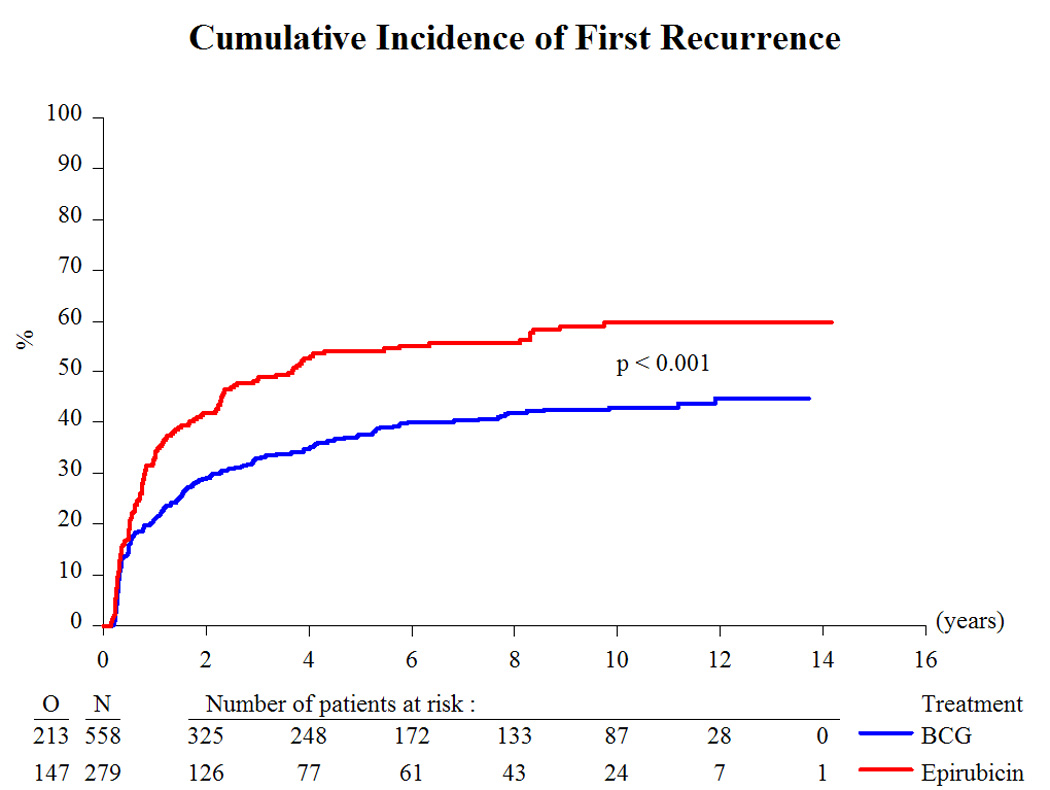
Cumulative incidence of first recurrence. BCG = bacillus Calmette-Guérin.
Fig. 4.
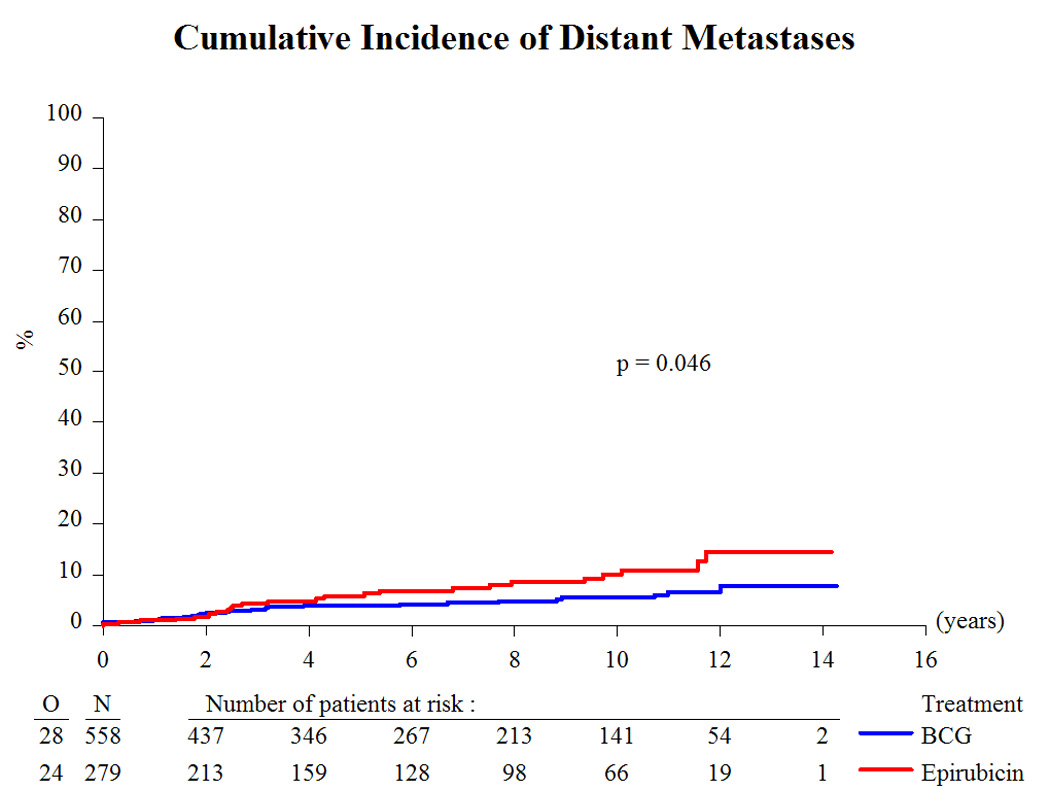
Cumulative incidence of distant metastases. BCG = bacillus Calmette-Guérin.
Fig. 5.
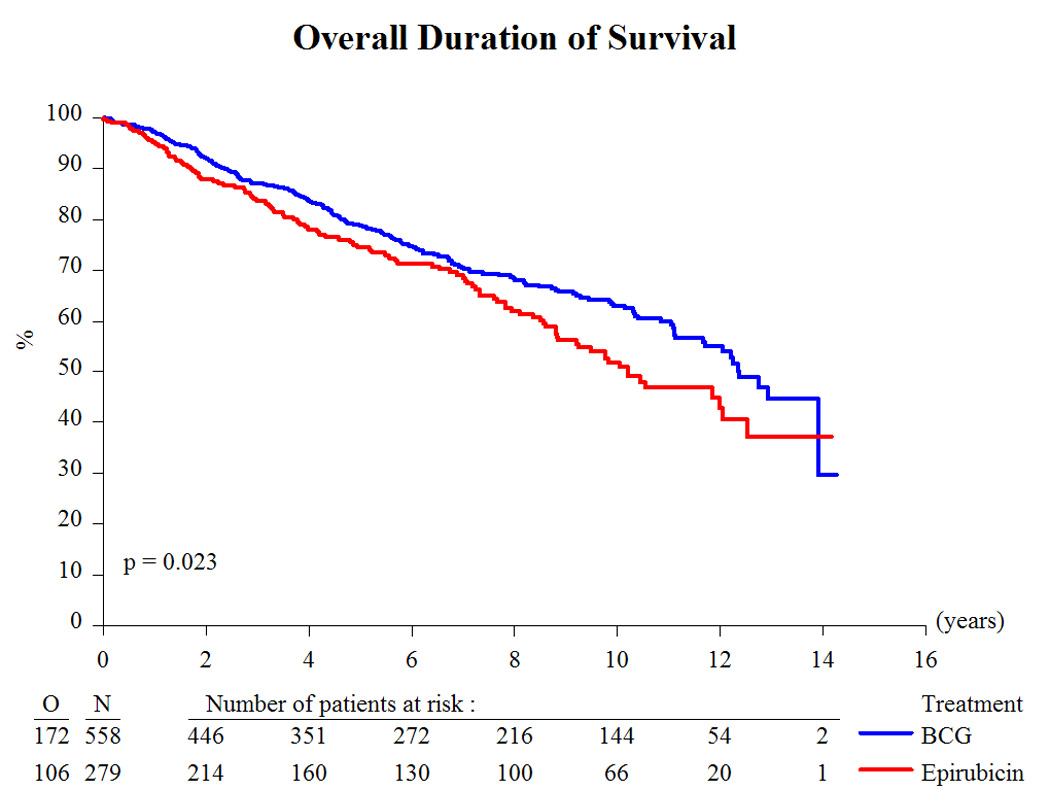
Overall duration of survival. BCG = bacillus Calmette-Guérin.
Fig. 6.
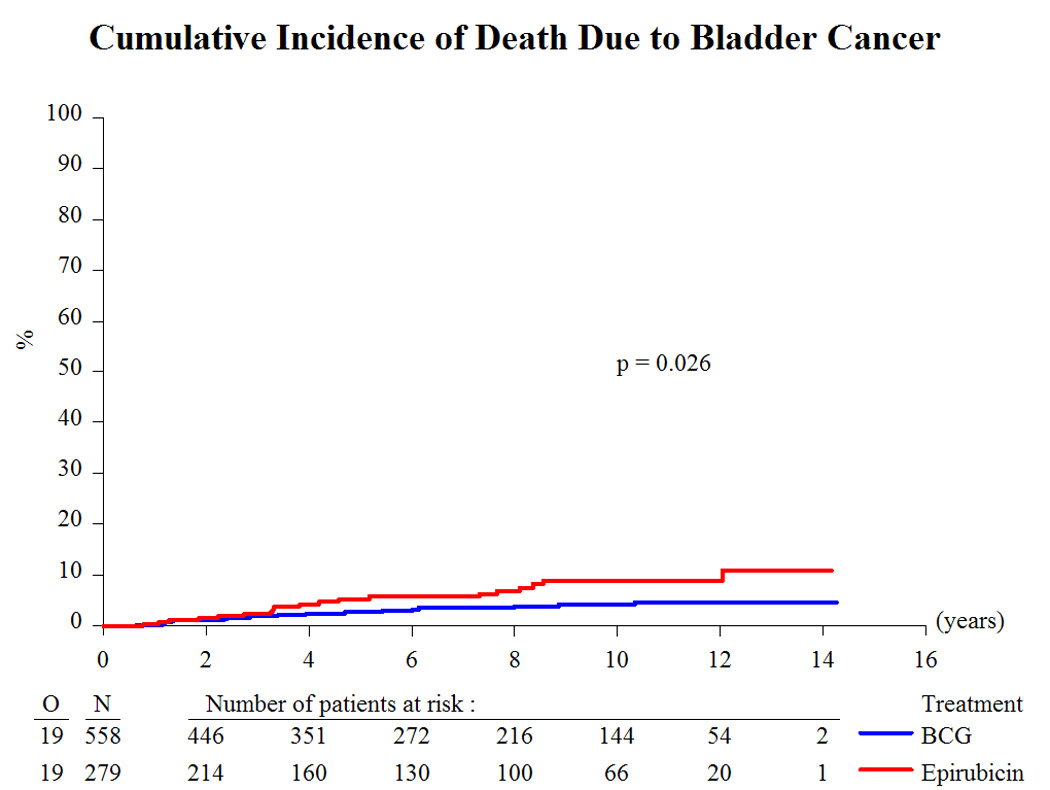
Cumulative incidence of death due to bladder cancer. BCG = bacillus Calmette-Guérin.
Fig. 3.
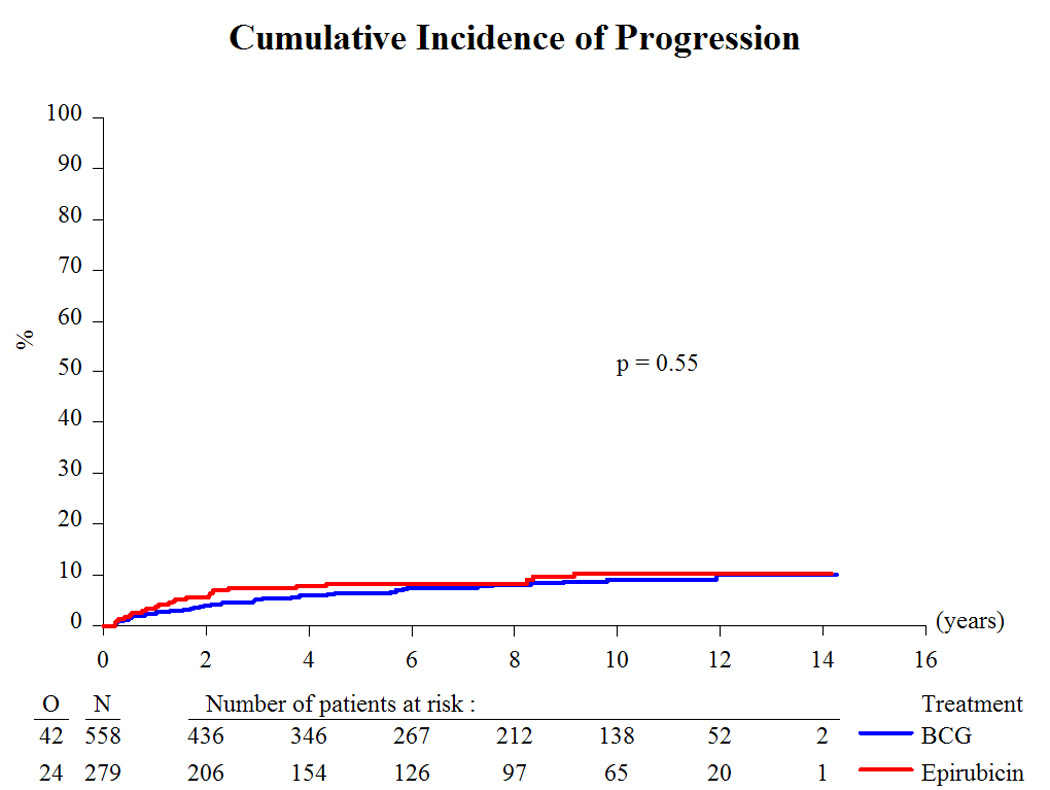
Cumulative incidence of progression. BCG = bacillus Calmette-Guérin.
Table 4 subdivides the comparisons according to whether the patients were at an intermediate or high risk of progression and/or death due to bladder cancer. Although the analyses are exploratory and in some cases are based on very small numbers of events, the analyses suggest that the differences in favor of BCG are at least as large, if not larger, in the intermediate-risk group patients as compared with the high-risk patients. Except for death due to any cause, the treatment-comparison HRs were more extreme in the intermediate-risk group patients, and none of the comparisons in the high-risk group was statistically significant.
4. Discussion
The long-term results of this study comparing epirubicin with BCG have confirmed BCG’s superiority for the time to first recurrence (Fig. 2). In addition, BCG favorably influenced the long-term end points of time to distant metastases (Fig. 4), overall duration of survival (Fig. 5), and time to death due to bladder cancer (Fig. 6). It was unexpected, however, that the treatment effect appeared to be larger in the intermediate-risk patients than in the high-risk patients. These results raise several questions.
First, why is there an apparently larger effect of BCG in the intermediate-risk patients than in the high-risk (T1 and/or grade 3) patients? Some grade 2 intermediate-risk patients might have been reclassified as high risk if the 2004 WHO grading system had been used, possibly decreasing the observed disparity in results. However, BCG, a nonspecific immunotherapy, may be more effective in patients with a lower tumor burden. Because a second-look transurethral resection of bladder tumor (TURBT) was not standard practice in the 1990s, some high-risk patients might have had residual T2 disease for which intravesical therapy is not effective. Although BCG has been shown to be superior to chemotherapy in patients with CIS [12], there are no convincing data to prove the superiority of BCG to chemotherapy in high-risk patients without CIS.
Second, why is there an effect on distant metastases but not on progression? There may be an effect of BCG on micrometastases via a systemic immune response. In the intermediate-risk group, BCG does appear to reduce the risk of progression (HR: 0.56), but there are too few progressions (25) to make meaningful comparisons. There is no suggestion of a benefit for progression in the high-risk group, but some patients may have had residual T2 disease because a second-look TURBT was not routinely done.
Third, why is there a significant effect of BCG on long-term end points in this study but not in the recent meta-analysis comparing BCG with MMC [5]? Is MMC, an alkylating agent, more effective than epirubicin, an anthracycline? MMC has a lower molecular weight than epirubicin, 334 kDa versus 579 kDa, and hence, at least theoretically, may be more readily absorbed into the layers of the bladder wall. There have thus been attempts to optimize the delivery of MMC [13,14] and to increase the penetration of MMC in the bladder wall either by combining MMC with hyperthermia or by electromotive drug administration [15]. But there are no randomized trials directly comparing MMC with epirubicin. Two small phase 2 chemoresection studies showed similar response rates on epirubicin and MMC [16]. There was no suggestion that epirubicin might be less effective than MMC when given as a single immediate postoperative instillation [17]. However, a meta-analysis of randomized studies suggested that MMC may be more effective than Adriamycin or epirubicin in the treatment of patients with CIS [12]. Thus although some evidence indicates that MMC may be more effective than epirubicin in high-risk patients, randomized studies are lacking.
The results on epirubicin in this study are disappointing, especially considering that the schedule and dose of epirubicin were adequate. An immediate instillation was given within 24 h, and instillations were continued for 3 yr. The dose and concentration of epirubicin employed, 50 mg in 50 ml of saline, is a classical one [9]. The epirubicin treatment schedule was feasible because just 16 patients (6%) stopped epirubicin due to toxicity [10]. Nevertheless, epirubicin at the schedule and dose used in this study cannot be recommended in clinical practice. More recently, in a study of 250 patients with 2 yr of maintenance, BCG was found to be more effective than epirubicin (50 mg) plus interferon in preventing recurrence [18].
In high-risk patients, the EAU guidelines recommend the use of maintenance BCG; however, further comparative data from randomized trials in patients with high-risk urothelial tumors without CIS, such as the patients included in this study, are needed. In the intermediate-risk patients, the relative advantages of intravesical BCG and chemotherapy need to be considered on a case-by-case basis, taking into account the patient’s previous history, overall condition, tumor characteristics, and the increased risk of toxicity with BCG [8].
5. Conclusions
In patients with intermediate- and high-risk stage Ta and T1 bladder cancer, this trial confirms the superiority of intravesical BCG with or without INH as compared with intravesical epirubicin not only for the time to first recurrence but also for the long-term end points of time to distant metastases, overall survival, and disease-specific survival. This study, however, shows that the benefit of BCG is not limited just to high-risk patients; intermediate-risk patients also benefit from BCG.
Acknowledgments
Funding/Support and role of the sponsor: This publication was supported by the EORTC Charitable Trust and by grant numbers 5U10 CA011488-37 through 5U10 CA011488-39 from the National Cancer Institute (Bethesda, MD, USA). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute. BCG Tice and epirubicin were supplied by Organon Teknika and Farmitalia Carlo Erba, respectively, in countries where these drugs were not registered during the time period that patients were receiving protocol treatment. EORTC participated in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; and the preparation, review, and approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial registration: This study was registered with the US National Cancer Institute clinical trials database [protocol ID: EORTC-30911].
Author contributions: Richard J. Sylvester had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sylvester, Brausi.
Acquisition of data: Brausi, Kirkels, Hoeltl, Da Silva, Powell, Prescott, Kirkali, van de Beek, de Reijke.
Analysis and interpretation of data: Sylvester.
Drafting of the manuscript: Sylvester.
Critical revision of the manuscript for important intellectual content: Sylvester, Brausi, Kirkels, Hoeltl, Da Silva, Powell, Prescott, Kirkali, van de Beek, Gorlia, de Reijke.
Statistical analysis: Sylvester, Gorlia.
Obtaining funding: Sylvester.
Administrative, technical, or material support: Sylvester.
Supervision: Sylvester.
Other (specify): None.
Financial disclosures: I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/ affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
References
- 1.Babjuk M, Oosterlinck W, Sylvester R, Kasinen E, Bohle A, Palou-Redorta J. EAU guidelines on non–muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2008;54:303–314. doi: 10.1016/j.eururo.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 2.Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–477. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 3.Shelley MD, Kynaston H, Court J, et al. A systematic review of intravesical bacillus Calmette-Guérin plus transurethral resection vs transurethral resection alone in Ta and T1 bladder cancer. BJU Int. 2001;88:209–216. doi: 10.1046/j.1464-410x.2001.02306.x. [DOI] [PubMed] [Google Scholar]
- 4.Bohle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guérin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol. 2003;169:90–95. doi: 10.1016/S0022-5347(05)64043-8. [DOI] [PubMed] [Google Scholar]
- 5.Malmstrom PU, Sylvester RJ, Crawford DE, et al. An individual patient data meta-analysis of the long-term outcome of randomized studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non–muscle-invasive bladder cancer. Eur Urol. 2009;56:247–256. doi: 10.1016/j.eururo.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 6.Bohle A, Bock PR. Intravesical bacilli Calmette-Guérin versus mitomycin C in superficial bladder cancer: formal meta-analysis of comparative studies on tumor progression. Urology. 2004;63:682–686. doi: 10.1016/j.urology.2003.11.049. [DOI] [PubMed] [Google Scholar]
- 7.Sylvester RJ, van der Meijden AP, Lamm DL. Intravesical bacillus Calmette-Guérin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized trials. J Urol. 2002;168:1964–1970. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 8.Sylvester RJ. Bacillus Calmette-Guérin versus mitomycin C for the treatment of intermediate risk non–muscle-invasive bladder cancer: the debate continues. Eur Urol. 2009;56:266–268. doi: 10.1016/j.eururo.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 9.Sylvester RJ, Oosterlinck, Witjes JA. The schedule and duration of intravesical chemotherapy in patients with non–muscle-invasive bladder cancer: a systematic review of the published results of randomized clinical trials. Eur Urol. 2008;53:709–719. doi: 10.1016/j.eururo.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van der Meijden APM, Brausi M, Zambon V, Kirkels W, de Balincourt C, Sylvester R. Intravesical instillation of epirubicin, bacillus Calmette-Guérin and bacillus Calmette-Guérin plus isoniazid for intermediate and high risk Ta, T1 urothelial carcinoma of the bladder: a European Organization for Research and Treatment of Cancer Genito-Urinary Group randomized phase III trial. J Urol. 2001;166:476–481. doi: 10.1097/00005392-200108000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Vegt PDJ, van der Meijden APM, Sylvester R, Brausi M, Hoeltl W, de Balincourt C. Does isoniazid reduce side effects of intravesical bacillus Calmette-Guérin therapy in superficial bladder cancer? Interim results of European Organization for Research and Treatment of Cancer protocol 30911. J Urol. 1997;157:1246–1249. [PubMed] [Google Scholar]
- 12.Sylvester RJ, van der Meijden APM, Witjes JA, Kurth KH. Bacillus Calmette-Guérin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. J Urol. 2005;174:86–92. doi: 10.1097/01.ju.0000162059.64886.1c. [DOI] [PubMed] [Google Scholar]
- 13.Au JL, Badalament RA, Wientjes MG, Young DC, Warner JA. Methods to improve the efficacy of intravesical mitomycin C: results of a randomized phase III trial. J Natl Cancer Inst. 2001;93:597–604. doi: 10.1093/jnci/93.8.597. [DOI] [PubMed] [Google Scholar]
- 14.Au JLS, Wientjes MG. Intravesical chemotherapy of superficial bladder cancer: optimization and novel agents. In: Lerner SP, Schoenberg MP, Sternberg CN, editors. Treatment and management of bladder cancer. London, UK: Informa Healthcare; 2008. pp. 23–33. [Google Scholar]
- 15.Malkowicz SB. The role of intravesical chemotherapy in the treatment of bladder cancer. In: Lerner SP, Schoenberg MP, Sternberg CN, editors. Treatment and management of bladder cancer. London, UK: Informa Healthcare; 2008. pp. 17–22. [Google Scholar]
- 16.Bono AV, Hall RR, Denis L, Lovisolo JA, Sylvester R. Chemoresection in Ta-T1 bladder cancer. Eur Urol. 1996;9:385–390. doi: 10.1159/000473784. [DOI] [PubMed] [Google Scholar]
- 17.Sylvester RJ, Oosterlinck W, van der Meijden APM. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol. 2004;171:2186–2190. doi: 10.1097/01.ju.0000125486.92260.b2. [DOI] [PubMed] [Google Scholar]
- 18.Duchek M, Johansson R, Jahnson S, et al. Bacillus Calmette-Guerin is superior to a combination of epirubicin and interferon-α2b in the intravesical treatment of patients with stage T1 urinary bladder cancer. A prospective, randomized, Nordic study. Eur Urol. 2010;57:25–31. doi: 10.1016/j.eururo.2009.09.038. [DOI] [PubMed] [Google Scholar]