Abstract
Reduced cardiac output is associated with increased white matter hyperintensities (WMH) and executive dysfunction in older adults, which may be secondary to relations between systemic and cerebral perfusion. This study preliminarily describes the regional distribution of cerebral WMH in the context of a normal cerebral perfusion atlas and aims to determine if these variables are associated with reduced cardiac output. Thirty-two participants (72 ± 8 years old, 38% female) with cardiovascular risk factors or disease underwent structural MRI acquisition at 1.5 T using a standard imaging protocol that included FLAIR sequences. WMH distribution was examined in common anatomical space using voxel-based morphometry and as a function of normal cerebral perfusion patterns by overlaying a single photon emission computed tomography (SPECT) atlas. Doppler echocardiogram data was used to dichotomize the participants on the basis of low (n = 9) and normal (n = 23) cardiac output. Global WMH count and volume did not differ between the low and normal cardiac output groups; however, atlas-derived SPECT perfusion values in regions of hyperintensities were reduced in the low versus normal cardiac output group (p < 0.001). Our preliminary data suggest that participants with low cardiac output have WMH in regions of relatively reduced perfusion, while normal cardiac output participants have WMH in regions with relatively higher regional perfusion. This spatial perfusion distribution difference for areas of WMH may occur in the context of reduced systemic perfusion, which subsequently impacts cerebral perfusion and contributes to subclinical or clinical microvascular damage.
Keywords: Cardiovascular disease, SPECT, MRI, Perfusion, Cardiac output, White matter hyperintensities
Many vascular risk factors, such as hypertension, diabetes mellitus, and atherosclerosis, are associated with central nervous system (CNS) injury, including abnormal age-associated neuroanatomic (Carmelli et al., 1999; DeCarli et al., 1999) and cognitive changes (Muller et al., 2007) as well as clinical dementia (Borenstein et al., 2005; Hofman et al., 1997; Kivipelto et al., 2005). A related but poorly understood aspect of vascular aging is the association between cardiac function and CNS injury. Though cardiac dysfunction has been related to neuropsychological impairments among patients with severe cardiomyopathies (Zuccala et al., 1997), less is known about how subtle cardiac dysfunction impacts brain aging through reductions in systemic blood flow in the absence of end-stage heart failure.
Recent studies from our laboratory suggest that subtle reductions in cardiac output are related to abnormal brain aging in the absence of end-stage heart failure. In particular, reduced cardiac output is associated with specific elements of executive dysfunction (Jefferson et al., 2007a,b) and increased white matter hyperintensities (WMH) (Jefferson et al., 2007a,b) adjacent to the subcortical nuclei in older patients with cardiovascular disease. The mechanism accounting for these associations remains unknown, but reduced systemic perfusion may impact cerebral perfusion homeostasis. The cerebral vasculature plays an essential role in maintaining cerebral perfusion. Microvasculature and perfusion disruptions can cause CNS damage and neuronal death, contributing to clinical or subclinical brain injury through the development and progression of microvascular changes. For instance, mouse models suggest that chronic cerebral hypoperfusion induces the development and progression of WMH (Shibata et al., 2004). In humans, reduced cerebral blood flow is associated with hyperintensities but not areas of normal appearing white matter as evidenced by structural MRI (Hatazawa et al., 1997) and perfusion weighted MRI (Marstrand et al., 2002).
Convention suggests that auto-regulatory mechanisms augment blood flow to the brain and other critical organs during periods of acute reduced systemic perfusion (Saxena and Schoemaker, 1993); however, these mechanisms are less effective with subtle or chronic reductions in systemic blood flow. For example, research in macaque monkeys has demonstrated that lowering systemic blood flow, specifically cardiac output, directly reduces cerebral blood flow (Tranmer et al., 1992). In humans, cerebral blood flow is significantly reduced among end-stage heart failure patients, but values are restored to healthy levels following heart transplantation (Gruhn et al., 2001). These studies suggest that auto-regulatory mechanisms for maintaining cerebral perfusion may be disrupted during conditions of reduced cardiac function, such that blood flow from the heart to the brain is not consistently maintained. Such disruptions in systemic blood flow (which impact cerebral blood flow homeostasis) may contribute to clinical or subclinical brain injury. Thus, reduced cardiac output may exacerbate the vulnerability of neuroanatomical regions that are uniquely susceptible to changes in perfusion and thereby contribute to white matter damage and related cognitive impairment (Gunning-Dixon and Raz, 2000).
The purpose of the present study is to preliminarily explore the cross-sectional associations between normal patterns of cerebral perfusion as defined by an existing single photon emission computed tomography (SPECT) atlas (Holland et al., 2008) in relation to WMH spatial distribution in the context of reduced cardiac output. Among a referral sample of patients with prevalent but stable cardiovascular disease, we hypothesize that WMH will be present among areas of reduced cerebral perfusion, as defined by the perfusion atlas, for patients with low cardiac output as compared to patients with normal cardiac output values.
1. Methods
1.1. Participants
Participants were 32 community-dwelling individuals recruited from local hospitals, rehabilitation programs, private practices, and general advertisements. Inclusion criteria required a documented history of cardiovascular disease (i.e., prior myocardial infarction, heart failure, coronary artery disease, cardiac surgery) or risk factor (i.e., hypertension). Exclusion criteria included end-stage heart failure; history of traumatic brain injury with loss of consciousness greater than 10 min, neurological disease (e.g., Parkinson’s disease, multiple sclerosis, dementia), major psychiatric illness (e.g., schizophrenia); current depressed mood as assessed by the Beck Depression Inventory (Beck et al., 1961); previous drug or alcohol abuse requiring hospitalization; or MRI contraindication, including ferrous metal implants or pacemakers. Participants consisted of 20 males and 12 females, ranging 57–85 years of age with 10–18 years of education. The sample’s mean global cognitive functioning was in the normal range based on the Mini-Mental State Examination (Folstein et al., 1975) and Dementia Rating Scale (Mattis, 1973). Each participant provided written informed consent prior to testing, and the study was approved by the local IRB.
1.2. Echocardiogram
A complete, transthoracic echocardiogram was obtained from each participant according to standards put forth by the American Society of Echocardiography. From these data, cardiac output was derived. Cardiac output is the amount of blood in liters per minute (L/min) that is pumped from the heart into systemic circulation and is a function of stroke volume and heart rate. Forward stroke volume can be calculated as the flow of blood leaving the left ventricle recorded with Doppler echocardiography multiplied by the area of left ventricular outflow tract measured from the 2D echo image (Jefferson et al., 2007a,b). This noninvasive method is strongly correlated with invasive measures of cardiac output (Moulinier et al., 1991).
1.3. Brain MRI acquisition and WMH quantification
Our neuroimaging protocol has been described in detail elsewhere (Gunstad et al., 2005; Jefferson et al., 2007b). Briefly, brain MRI was acquired using a Siemens Symphony 1.5 T magnet and a standard imaging protocol was obtained consisting of axial T1-, T2-, and fluid attenuated inversion recovery (FLAIR; TR/TE = 6000/105, slice thickness = 5 mm/2 mm gap, inplane resolution = 1 mm × 1 mm) images. For this particular study, we utilized the FLAIR sequence because its suppression of CSF signal increases the sensitivity to detecting WMH. Post-processing was conducted using the commercially available Mayo Clinic software program ANALYZE® (Biomedical Imaging Resource, Mayo Foundation, Rochester, MN). The skull, brain stem, and cerebellum were removed manually to leave only the cerebral brain parenchyma. A semi-automated thresholding method was applied to FLAIR images to segment and quantify WMH. Intra- and inter-rater reliability for WMH quantification were consistently >0.90 (Jefferson et al., 2007b).
Participant MR images and WMH segmentations were registered into the ICBM452 coordinate space for analysis with the FMRIB linear image registration tool (FLIRT, Analysis Group, FMRIB Oxford, UK) (Jenkinson and Smith, 2001), using 12 degrees of freedom (affine) transformation. Quantitative WMH counts and volume were calculated for each participant in standard (atlas) space to control for inter-subject variation in head size and total brain volume.
WMH distribution was then examined in the context of normal cerebral perfusion patterns by superimposing WMH segmentations with an atlas of normal cerebral perfusion derived from whole brain SPECT images of 47 healthy individuals aged 22–49 (34.3 ± 7.6 years). These images are publicly available for research via an archive maintained by The Society of Nuclear Medicine Brain Imaging Council (http://brainscans.indd.org/brncncl4.htm). Briefly, the SPECT scans were acquired using the tracer Tc-99m ethylene cysteinate dimer (Neurolite, DuPont Pharma, Billerica, MA), 1000 MBq of which was injected intravenously. Images were acquired with a triple-headed Picker camera (Prism 3000, Picker International, Cleveland, OH) with a 2.2 mm3 × 2.2 mm3 × 3.6 mm3 nominal voxel size. The images were reconstructed using a standardized low pass filter (order = 4.0/cutoff = 0.26) and spatially normalized to Talairach space.
We generated a SPECT atlas based on these healthy adult images, which we restricted to archive participants under age 50 years. Contemporaneous MR images are not available for comparison with the normal SPECT data for each healthy subject, so the condition of the white matter of these healthy individuals is unknown. Therefore, the age restriction (i.e., adults under age 50 years) was determined a priori to minimize the likelihood of incidental WMH in the archive SPECT sample, because the incidence of subclinical WMH is known to increase substantially in individuals age 60 years and older (Meyer et al., 1992). Our goal for this study was to generate a normative SPECT perfusion atlas based on data from healthy adults that would provide an estimate of normal perfusion patterns as a context for analyzing the distribution of white matter damage in patients with cardiovascular risk factors and disease. Because perfusion is dynamic and fluctuates in response to many factors, including regional brain activity, an atlas based on the average of many healthy adults removes some of the temporal and functional variability present in individual scans. The atlas then yields mean perfusion values for all brain regions in arbitrary units with a range of 1–1000.
We related the SPECT atlas perfusion values to each voxel of WMH for our cardiovascular patients in native participant space. Our utilization of SPECT allowed for the comparison of relative but not absolute differences in perfusion within the white matter (De Cristofaro et al., 1990; Lycke et al., 1993).
1.4. Statistical Analysis
Group dichotomization was based on cardiac output status: low (<4.0 L/min) and normal (≥4.0 L/min). The rationale for this dichotomy was based on two factors. First, we did not anticipate any meaningful variations in normal cardiac output that would correspond to white matter changes. Second, and most clinically relevant, the dichotomization of cardiac output was based on widely accepted cut-offs utilized by cardiologists to identify impaired vs. normal cardiac function (Grossman and Baim, 1995). This dichotomization allows our data to be applied in a clinical context.
Descriptive statistics and between-group comparisons were conducted to summarize the sample’s clinical characteristics (age, education, sex, Mini-Mental State Examination, Dementia Rating Scale, vascular risk factors, and prevalent cardiovascular disease). Between-group comparisons for quantitative WMH counts, WMH volume, and perfusion values co-localized with WMH were calculated using the Student’s t-test. For all analyses, statistical significance was defined a priori as p < 0.05.
2. Results
2.1. Clinical characteristics
There were no between-group differences for the low (n = 9) and normal (n = 23) cardiac output patients for age (t(31) = 0.15, p = 0.88), education level (t(30) = −0.41, p = 0.69), sex (x2 = 1.74, p = 0.19), global cognitive status as assessed by the Mini-Mental State Exam (Folstein et al., 1975) (t(31) = 1.01, p = 0.32) or Dementia Rating Scale (Mattis, 1973) (t(31) = −0.28, p = 0.78). Chi-square analyses yielded no significant between-group differences for vascular risk factors or prevalent cardiovascular disease (all p-values > 0.39). Descriptive statistics for demographic, cognitive, and cardiovascular variables are displayed in Table 1.
Table 1.
Clinical characteristics.
| Normal cardiac output | Low cardiac output | |
|---|---|---|
| Age, years | 72.4 ± 6.8 | 72.8 ± 7.9 |
| Education level, years | 13.9 ± 2.1 | 13.4 ± 2.2 |
| Sex, % female | 30 | 46 |
| Mini-mental state exam | 28.8 ± 1.1 | 29.2 ± 1.3 |
| Dementia rating scale | 138.6 ± 4.5 | 137.6 ± 4.4 |
| Cardiac output, L/min* | 5.2 ± 1.5 | 3.4 ± 0.5 |
| Family history of heart disease, % | 64 | 50 |
| Hypertension, % | 83 | 78 |
| Hypercholesterolemia, % | 50 | 63 |
| Diabetes mellitus, % | 18 | 0 |
| Atrial fibrillation, % | 17 | 11 |
| Prior coronary artery bypass, % | 48 | 33 |
| Heart failure, % | 11 | 11 |
| Prior myocardial infarction, % | 48 | 22 |
Note: Data presented as mean ± standard deviation or as percentage; vascular risk factors and prevalent vascular disease data are based on self report and confirmed via medical records when possible;
Significant between-group difference, p < 0.05.
2.2. Quantitative comparison of WMH & perfusion correlates
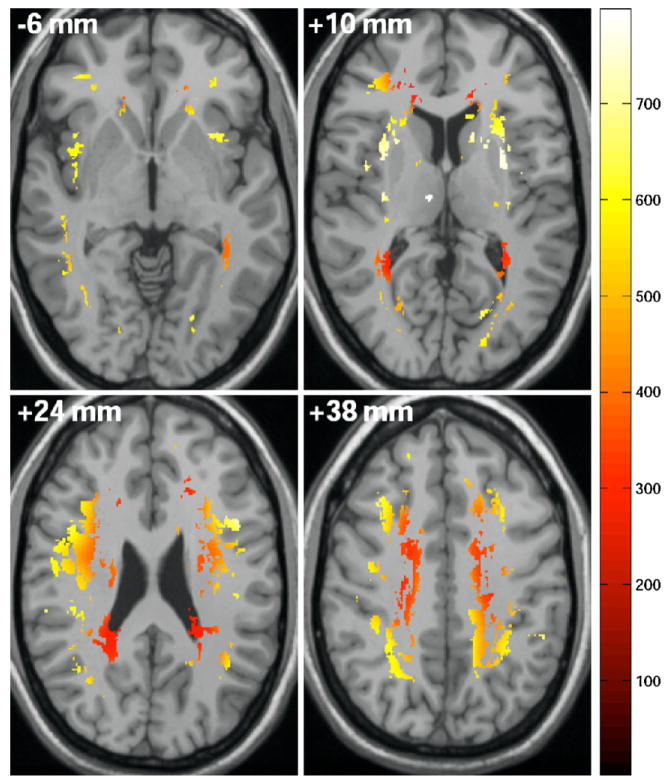
A total of 975 unique hyperintensities were identified for the sample. In Table 2, we present the quantitative WMH statistics and WMH perfusion values for the entire cohort and for each cardiac output group. There were no between-group differences for the average number of hyperintensities (t(30) = −0.08, p = 0.93) or mean volume of WMH per participant (t(30) = −0.97, p = 0.34); however, the distribution of hyperintensities across levels of cerebral perfusion differed between the two groups. Specifically, WMH were present in regions of significantly lower cerebral perfusion in the low, as compared to the normal, cardiac output group according to the normative perfusion atlas (t(30) = −30.1, p < 0.0001). Qualitative illustration of the WMHs unique to the low cardiac output group and the corresponding arbitrary perfusion values is provided in Fig. 1.
Table 2.
Quantitative WMH and atlas-derived perfusion data.
| Normal cardiac output | Low cardiac output | t-test (normal vs. low output) | |
|---|---|---|---|
| WMH count mean (range) | 30.2 (2–75) | 31.1 (4–124) | p = 0.93 |
| WMH volume (mL) per participant median (25th, 75th) | 4.6 (2.9, 7.7) | 4.2 (2.3, 16.4) | p = 0.34 |
| WMH perfusion median (25th, 75th) | 542 (457, 635) | 507 (422, 593) | p < 0.0001 |
Fig. 1.
Illustration of perfusion values in regions of WMH unique to the low cardiac output subgroup.
2.3. Qualitative illustration of WMH & perfusion correlates
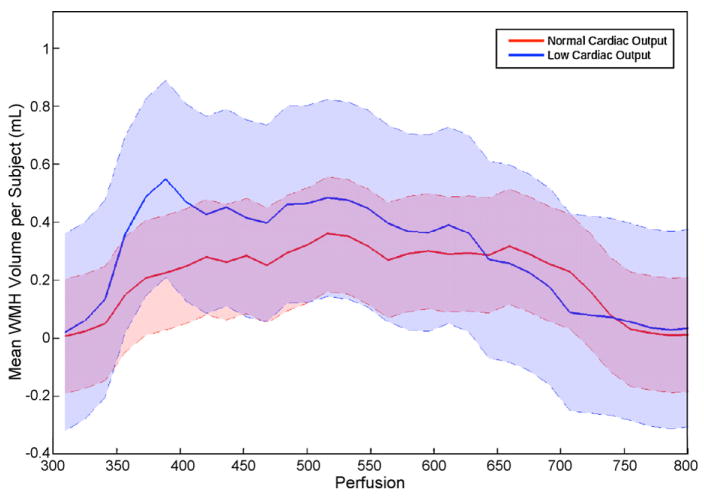
Examining WMH distribution in relation to normal perfusion patterns, as defined by the healthy adult SPECT perfusion atlas, yielded the histogram of WMH volume per participant (mL/participant) presented in Fig. 2. Shaded regions correspond to 95% confidence intervals. The illustration suggests increased WMH prevalence in regions of lower relative normal perfusion, which is supported by the quantitative findings above. A peak is present at approximately 500–550 (arbitrary perfusion units) for both groups, representing a similar distribution that may reflect a common mechanism of white matter damage in regions of white matter in this perfusion range. However, the strong peak in regions of white matter with the relative perfusion (i.e., 350–400 arbitrary perfusion units) qualitatively appears to be unique to the low cardiac output group.
Fig. 2.
WMH frequency histogram in relation to normal atlas-derived perfusion values for normal cardiac output and low cardiac output subgroups. Data reflects mean WMH volume per subject measured in mL in relation to arbitrary perfusion units. Shaded error regions indicate 95% confidence intervals for each group, with the purple area illustrating subgroup overlap.
3. Discussion
Our preliminary cross-sectional findings suggest that, despite having comparable total WMH burden (i.e., WMH count and volume), the cardiac output groups differed with respect to normative cerebral perfusion patterns in areas of hyperintensities based on an atlas generated from healthy adults. Among participants with low cardiac output, hyper-intensities were present in regions with lower normative perfusion values. Though there are auto-regulatory mechanisms that augment cerebral blood flow at the expense of other organs during critical and sudden reductions in systemic blood flow (Saxena and Schoemaker, 1993), these mechanisms are less effective when systemic blood flow reductions are chronic or subtle and result in reduced cerebral perfusion (Gruhn et al., 2001; Tranmer et al., 1992). Therefore, chronic reductions in cardiac output may impact cerebral perfusion and exacerbate the susceptibility of these regions to hypoperfusion or ischemia, thereby leading to white matter damage (Shibata et al., 2004; Tranmer et al., 1992).
The between-group differential distribution of perfusion values in regions of hyperintensities is qualitatively supported by the histogram curves observed for each group (Fig. 2). The cross-sectional nature of the present study precludes inferences about causality; however, systemic perfusion may be the distinguishing feature for the unique peak observed at lower perfusion levels for the low cardiac output group. Reduced cardiac output may act as a unique mechanism of injury, or it may lead to white matter damage in normally spared regions (Jefferson et al., 2007b). These interpretations are purely speculative, and additional investigation comparing vascular risk factors and systolic function among larger samples will extend these preliminary data and provide insight regarding perfusion differences relative to WMH lesion burden.
It is worthwhile to note that the subgroups share a peak around 500–550 arbitrary perfusion units in Fig. 2 and have comparable mean WMH burden, which is likely due to a common or shared mechanism of white matter injury for the two groups. One probable shared mechanism is advanced age, which is a well-known risk factor for hyperintensities (Meyer et al., 1992). Alternatively, the comparable vascular risk factor burden and prevalent cardiovascular disease seen in the low and normal cardiac output groups may be a common mechanism of injury. Specifically, microvascular disease can occur in the absence of reduced cardiac output, and vascular risk factors known to be associated with white matter damage, including blood pressure (Murray et al., 2005) and diabetes (Carmelli et al., 1999), are prevalent in our sample regardless of subgroup status. Future studies with larger samples are needed to determine the distribution and cerebral perfusion correlates of hyperintensities and relations between white matter injury and systemic blood flow to confirm whether subtle systolic dysfunction is a risk factor for abnormal brain aging.
There are limitations to the current study that restrict the generalizability of our preliminary findings. First, the demographics and referral source of our sample may limit generalizability, because the sample was predominantly college educated, White of European descent, with a history of cardiovascular disease or vascular risk factor. As acknowledged above, our sample size was small, which precludes more rigorous statistical comparisons due to insufficient power. Low spatial resolution is a well-known limitation of perfusion imaging techniques, including SPECT, and can lead to partial voluming effects. Finally, the SPECT atlas was based on an archival dataset of healthy adults, so detailed clinical characteristics are unavailable for this normative dataset. As a solution, we restricted inclusion of SPECT scans to those adults under age 50 years to minimize confounding clinical characteristics (Holland et al., 2008). However, limiting the sample to a younger cohort (age 22–49 years) may have introduced another limitation of excessive homogeneity in perfusion characteristics versus the heterogeneity that might be seen in an older cohort.
With these limitations in mind, the present preliminary findings support previous research that links reductions in cerebral perfusion to the presence and progression of WMH in animal (Shibata et al., 2004) and human models (Marstrand et al., 2002). The present results also extend our prior work, which found that reduced cardiac output is related to very specific elements of executive dysfunction, including sequencing and planning difficulties (Jefferson et al., 2007a), and regional WMH adjacent to the subcortical nuclei (Jefferson et al., 2007b). In light of the association between cardiac output and abnormal brain aging, chronic alterations in systemic perfusion may have important implications for the development and progression of cognitive impairment secondary to cerebral perfusion changes and microvascular disease. Additional investigation is warranted to further assess the mechanism(s) accounting for cardiac function and brain aging relations, to assess the longitudinal implications of reduced cardiac output for cognitive aging and white matter integrity, and to better understand relations between cerebral perfusion and white matter disease with age.
Acknowledgments
Funding sources: This research was supported in part by K23-AG030962 (Paul B. Beeson Career Development Award in Aging; ALJ), IIRG-08-88733 (Alzheimer’s Association Investigator Initiated Award; ALJ), F30-NS049808 (CMH), K23-MH073416 (DFT), R01-AG017975 (RAC), and P30-AG013846 (Boston University Alzheimer’s Disease Core Center).
Footnotes
Disclosure: There are no actual or potential conflicts of interest for this work. The Lifespan Institutional Review Board approved this study, and informed consent was obtained from all subjects prior to participation.
References
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Borenstein AR, Wu Y, Mortimer JA, Schellenberg GD, McCormick WC, Bowen JD, McCurry S, Larson EB. Developmental and vascular risk factors for Alzheimer’s disease. Neurobiology of Aging. 2005;26:325–334. doi: 10.1016/j.neurobiolaging.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Carmelli D, Swan GE, Reed T, Wolf PA, Miller BL, DeCarli C. Midlife cardiovascular risk factors and brain morphology in identical older male twins. Neurology. 1999;52:1119–1124. doi: 10.1212/wnl.52.6.1119. [DOI] [PubMed] [Google Scholar]
- De Cristofaro MT, Mascalchi M, Pupi A, Nencini P, Formiconi AR, Inzitari D, Dal Pozzo G, Meldolesi U. Subcortical arteriosclerotic encephalopathy: single photon emission computed tomography-magnetic resonance imaging correlation. American Journal of Physiologic Imaging. 1990;5:68–74. [PubMed] [Google Scholar]
- DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Garner J, Jack L, Carmelli D. Predictors of brain morphology for the men of the NHLBI twin study. Stroke. 1999;30:529–536. doi: 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Grossman W, Baim D. Cardiac Catheterization, Angiography and Intervention. Lea & Febiger; Philadelphia: 1995. Blood flow measurement: the cardiac output; pp. 109–124. [Google Scholar]
- Gruhn N, Larsen FS, Boesgaard S, Knudsen GM, Mortensen SA, Thomsen G, Aldershvile J. Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke. 2001;32:2530–2533. doi: 10.1161/hs1101.098360. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14:224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Cohen RA, Tate DF, Paul RH, Poppas A, Hoth K, Macgregor KL, Jefferson AL. Blood pressure variability and white matter hyperintensities in older adults with cardiovascular disease. Blood Pressure. 2005;14:353–358. doi: 10.1080/08037050500364117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatazawa J, Shimosegawa E, Satoh T, Toyoshima H, Okudera T. Subcortical hypoperfusion associated with asymptomatic white matter lesions on magnetic resonance imaging. Stroke. 1997;28:1944–1947. doi: 10.1161/01.str.28.10.1944. [DOI] [PubMed] [Google Scholar]
- Hofman A, Ott A, Breteler MM, Bots ML, Slooter AJ, van Harskamp F, van Duijn CN, Van Broeckhoven C, Grobbee DE. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet. 1997;349:151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- Holland CM, Smith EE, Csapo I, Gurol ME, Brylka DA, Killiany RJ, Blacker D, Albert MS, Guttmann CR, Greenberg SM. Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke. 2008;39:1127–1133. doi: 10.1161/STROKEAHA.107.497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson AL, Poppas A, Paul RH, Cohen RA. Systemic hypoperfusion is associated with executive dysfunction in geriatric cardiac patients. Neurobiology of Aging. 2007a;28:477–483. doi: 10.1016/j.neurobiolaging.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson AL, Tate DF, Poppas A, Brickman AM, Paul RH, Gunstad J, Cohen RA. Lower cardiac output is associated with greater white matter hyperintensities in older adults with cardiovascular disease. Journal of the American Geriatrics Society. 2007b;55:1044–1048. doi: 10.1111/j.1532-5415.2007.01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, Helkala EL, Tuomilehto J, Soininen H, Nissinen A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Archives of Neurology. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- Lycke J, Wikkelso C, Bergh AC, Jacobsson L, Andersen O. Regional cerebral blood flow in multiple sclerosis measured by single photon emission tomography with technetium-99m hexamethylpropyleneamine oxime. European Neurology. 1993;33:163–167. doi: 10.1159/000116926. [DOI] [PubMed] [Google Scholar]
- Marstrand JR, Garde E, Rostrup E, Ring P, Rosenbaum S, Mortensen EL, Larsson HB. Cerebral perfusion and cerebrovascular reactivity are reduced in white matter hyperintensities. Stroke. 2002;33:972–976. doi: 10.1161/01.str.0000012808.81667.4b. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale, Professional Manual. Psychological Assessment Resources; Odessa, FL: 1973. [Google Scholar]
- Meyer JS, Kawamura J, Terayama Y. White matter lesions in the elderly. Journal of the Neurological Sciences. 1992;110:1–7. doi: 10.1016/0022-510x(92)90002-3. [DOI] [PubMed] [Google Scholar]
- Moulinier L, Venet T, Schiller NB, Kurtz TW, Morris RC, Jr, Sebastian A. Measurement of aortic blood flow by Doppler echocardiography: day to day variability in normal subjects and applicability in clinical research. Journal of the American College of Cardiology. 1991;17:1326–1333. doi: 10.1016/s0735-1097(10)80143-3. [DOI] [PubMed] [Google Scholar]
- Muller M, Grobbee DE, Aleman A, Bots M, van der Schouw YT. Cardiovascular disease and cognitive performance in middle-aged and elderly men. Atherosclerosis. 2007;190:143–149. doi: 10.1016/j.atherosclerosis.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Murray AD, Staff RT, Shenkin SD, Deary IJ, Starr JM, Whalley LJ. Brain white matter hyperintensities: relative importance of vascular risk factors in nondemented elderly people. Radiology. 2005;237:251–257. doi: 10.1148/radiol.2371041496. [DOI] [PubMed] [Google Scholar]
- Saxena PR, Schoemaker RG. Organ blood flow protection in hypertension and congestive heart failure. The American Journal of Medicine. 1993;94:4S–12S. [PubMed] [Google Scholar]
- Shibata M, Ohtani R, Ihara M, Tomimoto H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 2004;35:2598–2603. doi: 10.1161/01.STR.0000143725.19053.60. [DOI] [PubMed] [Google Scholar]
- Tranmer BI, Keller TS, Kindt GW, Archer D. Loss of cerebral regulation during cardiac output variations in focal cerebral ischemia. Journal of Neurosurgery. 1992;77:253–259. doi: 10.3171/jns.1992.77.2.0253. [DOI] [PubMed] [Google Scholar]
- Zuccala G, Cattel C, Manes-Gravina E, Di Niro MG, Cocchi A, Bernabei R. Left ventricular dysfunction: a clue to cognitive impairment in older patients with heart failure. Journal of Neurology, Neurosurgery, and Psychiatry. 1997;63:509–512. doi: 10.1136/jnnp.63.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]