Abstract
OBJECTIVES
To examine the frequency of MCI in African American older adults. The study also plans to explore the specific cognitive domains of impairment as well as whether there are differences in demographics, health, and cognitive performance between MCI and normal participants.
DESIGN
Cross-sectional.
SETTING
Independent-living sample of urban dwelling elders in Baltimore, Maryland.
PARTICIPANTS
The sample consisted of 554 subjects ranging in age from 50 to 95 (mean = 68.79 ± 9.60).
MEASUREMENTS
Socio-demographics and health were assessed. Several cognitive measures were administered to assess inductive reasoning, declarative memory, perceptual speed, working memory, executive functioning, language, global cognitive functioning.
RESULTS
Approximately 22% of participants were considered MCI (i.e. 18% non-amnestic vs. 4% amnestic). A majority of the non-amnestic MCI participants had impairment in one cognitive domain, particularly language and executive function. Individuals classified as non-amnestic MCI were significantly older and had more years of education than normal individuals. The MCI groups were not significantly different than cognitively normal individuals on health factors. Individuals classified as MCI performed significantly worse on global cognitive measures as well as across specific cognitive domains than cognitively normal individuals.
CONCLUSION
This study demonstrates that impairment in a non-memory domain may be an early indicator of cognitive impairment, particularly among African Americans.
Keywords: mild cognitive impairment, cognitive domains of impairment, African Americans
Researchers have focused on identifying the transitional period of normal cognition and dementia. Several terms have been used to describe this period, but the term Mild Cognitive Impairment (MCI) has often be used in the literature (Albert, 2008). Depending on the diagnostic guidelines used to measure MCI, the prevalence rates of MCI range from 3% to 53.8% (Panza, D’Introno, Colacicco, Capurso, Del Parigi, Caselli et al., 2005). Although previous research of racially mixed samples has suggested that African Americans are at a greater risk for the development of MCI than Caucasians (Manly, Tang, Schupf, Stern, Vonsattel, and Mayeux, 2008), there is still limited research exploring MCI among older African Americans. The purpose of this investigation was to examine the frequency of MCI among a sample of older African Americans and the specific cognitive domains of impairment for individuals meeting the criteria for MCI.
The prevalence of dementia in older African Americans has shown to be significantly higher than in older Caucasians at baseline testing (33.7% vs. 20%) and at a one year follow-up (32.1% vs. 18.9%; Gurland et al., 1999). The incidence of dementia, specifically Alzheimer’s disease, is almost double among African Americans compared to Caucasians (Tang, Cross, Andrews, Jacobs, Small, Bell et al., 2001). Even though African Americans are disproportionately affected by dementia, this has not led to many studies on MCI in this understudied population. This is surprising, considering racial differences have been observed in clinical and genetic etiologies of dementia (Farrer, Cupples, Haines, Hyman, Kukull, Mayeux et al., 1997; Fillenbaum, Prosnitz, Raiford, Burchett, and Clark, 1991; Froehlich, Bogardus, and Inouye, 2001;). In fact, certain clinical types of dementia, such as vascular or multi-infarct dementia, are more common in African Americans than Caucasians (de la Monte, Hutchins, and Moore, 1989; Heyman et al., 1991). In contrast, the presence of apolipoprotein E(APOE)-e 4 allele is less of genetic risk for Alzheimer’s disease in African Americans than Caucasians (Farrer et al., 1997; Tang, Maestre, Tsai, Liu, Feng, Chung et al., 1996). Thus, specific cognitive domains of initial impairment among this population may differ from what previous studies of predominately Caucasian samples have reported, and there might be a unique onset and course of MCI among African Americans. Culturally relevant factors such as disparities in health could explain cognitive functioning within African Americans (Whitfield, Allaire, Belue, and Edwards, 2008). For example, the prevalence of cardiovascular disease is highly prevalent among African Americans (Cooper, Cutler, Desvigne-Nickens, Fortmann, Friedman, Havlik et al., 2000; Whitfield, Weidner, Clark, and Anderson, 2002), and cardiovascular disease or increased cardiovascular risk factors are associated with impairments in cognitive domains other than memory (Lezak, Howieson, and Loring, 2004), such as executive function and speed (Knopman, Boland, Mosely, Howard, Liao, Szklo et al., 2001). Research indicates that cognitively normal individuals with cardiovascular disease are at an increased risk for developing MCI (Tervo, Kivipelto, Hanninen, Vanhanen, Hallikainen, Mannermaa et al., 2004)and, subsequently, vascular dementia, which is highly prevalent among African Americans (Froehlich et al., 2001). Given this, early cognitive impairments may be observed in non-memory tasks and several health as well as demographic factors may explain these impairments, particularly among African Americans.
The purpose of this investigation was to examine the frequency of MCI among African Americans and the specific cognitive domains of impairment for individuals meeting the criteria for MCI. The current study was guided by three specific aims. The first aim was to investigate the frequency of MCI in our sample. The second aim was to examine within the MCI group the number and types of cognitive domains in which individuals showed impairment. Finally, the third aim was to examine whether there are significant differences between MCI and cognitively normal individuals in demographic characteristics, health factors, and cognitive performance.
Methods
Participants
The study sample consisted of 554 urban dwelling, independently living African American older adults drawn from the first wave of the Baltimore Study of Black Aging: Patterns of Cognitive Aging (BSBA: PCA). The overarching goal of the BSBA is to examine cognition, health, and other critical factors in older African Americans. Although the BSBA: PCA has a total sample of 602, 48 were not included in the analysis due to meeting criteria for dementia or having insufficient cognitive data. A description of the excluded participants is described in a previously published article examining the BSBA: PCA sample (Allaire, Gamaldo, Ayotte, Sims, and Whitfield, 2009). The 554 participants (24% males) included in the analysis ranged in age from 50 to 95 years (mean = 68.79 ± 9.60). Participants were recruited from 29 senior housing facilities that consisted primarily (>75%) of African Americans living in the West Baltimore area. Participants’ average monthly income was $1000 (SD = $600; range = < $100 – >$2300) and the average years of education was 11.80 ± 2.82 years (range = 3–20).
Cognitive Battery
A large cognitive battery was administered to assess inductive reasoning, declarative memory, perceptual speed, working memory, executive functioning, and language. Specific information regarding the battery’s measures is described in a previously published article examining the BSBS: PCA sample (Allaire et al., 2009).
Overall cognitive function was assessed using the Mini-Mental State Examination (MMSE; Folstein, Folstein, and McHugh, 1975) and the Short Portable Mental Status Questionnaire (SPMSQ; Pfeiffer, 1975). The MMSE is commonly used to measure orientation, memory, attention, calculation, language, and visuospatial skills. The SPMSQ is a brief measure of cognitive function, which primarily assesses short-term memory, long-term memory, orientation, and serial operation. Unlike the MMSE, the SPMSQ has shown to be less culturally biased than the MMSE because it is less reliant on items that may be influenced by education (Teresi, Holmes, Ramírez, Gurland, and Lantigua, 2002).
Mental and Physical Health
The Center for Epidemiological Studies-Depression (CES-D) scale was used to measure depressive symptoms (Radloff, 1977). The CES-D is commonly used in detecting depressive symptoms in older adults across diverse populations (Foley, Reed, Mutran, and DeVellis, 2002). A vascular risk composite score was created by summing participants’ responses as to whether a physician had informed them that they had any of the following conditions: heart condition, circulation problems, high blood pressure, diabetes, and stroke. Three assessments of blood pressure (BP) were taken using an oscillometric automated device (A & D model UA-767; Milpitas California), while the participant was sitting and standing (Beevers, Lip, and O’Brien, 2001). For each individual, average systolic and diastolic values were calculated. The average of three pulmonary function readings was calculated using a mini-Wright peak flow meter (Cook, Evans, Scherr, Speizer, Taylor, and Hennekens, 1991). For each reading, participants were asked to blow as hard as possible into the end of a peak flow meter after taking a deep breath for 1 second.
Socio-Demographic
A self-reported questionnaire was used to measure demographics. The items on this questionnaire include age, sex, martial status, education, residency, family health, and income.
Procedure
Participants were individually tested in a vacant, public room of their apartment building. During the testing session, participants were administered an assessment battery that includes the paper and pencil measures described above. Each participant was compensated $30.
MCI Classification
Following the procedure outlined by Allaire and colleagues (Allaire et al., 2009), all of the cognitive tests were standardized to a mean of 50 and standard deviation of 10, and composite scores were created by taking the mean of all the tests assessing a specific cognitive domain. The influence of education was residualized from the composites which were standardized (M = 50, SD = 10) again. This process resulted in composite scores for six cognitive domains: Reasoning (i.e. Letter Series and Shipley), Memory (i.e. HVLT, AVLT, and Immediate Recall), Perceptual Speed (i.e. Number Comparison, Identical Pictures, and DSST), Executive Function (i.e. 3:25 CDT and 11:10 CDT), Working Memory (i.e. Alpha Span, OSPAN, and Backward Digit Span), and Language (i.e. Verbal Ability Test and Shipley Institute of Living Verbal Meaning Test).
For each computed cognitive composite, individuals were considered impaired on that ability if they were 1.5 SD (i.e. the bottom 6.68% of the sample distribution) below the sample mean. MCI status was determined used a modified version of the age-associated cognitive decline criterion (Levy, 1994; Ritchie, Artero, and Touchon, 2001), which classifies individuals if they indicated impairment on at least one cognitive domain (i.e. language, memory, reasoning, executive functioning, working memory, and perceptual speed). Unlike other existing criteria of MCI, this criterion does not require a subjective memory complaint and allows for some impairment in everyday functioning. This criterion is shown to have high predictive validity in detecting dementia years following a baseline assessment in a non-clinical sample (Busse, Bischkopf, Riedel-Heller, and Angermeyer, 2003a). Following this classification criterion, the MCI group was further divided into two groups: amnestic MCI and non-amnestic MCI (Petersen and O’Brien, 2006). Individuals were considered amnestic MCI if there was any impairment observed on the memory domain. Whereas, individuals were considered non-amnestic MCI if there was any impairment observed on the non-memory domains.
Descriptive statistics were conducted to estimate the frequency of MCI in the current sample as well as the frequency of specific cognitive domains of impairment. Three multivariate analyses of variances (MANOVA) were conducted to examine whether there were significant differences among the amnestic, and non-amnestic, and normal control groups on demographic characteristics, health variables, and cognitive performance. Although impairment in cognitive performance was used to define MCI status, the current study wanted to explore whether there might be subtle deficits across a board array of cognitive abilities, which might not have been captured by the current’s study impairment criterion. Thus, the current study explored whether there were differences among the groups on global scales of cognition, which are commonly used in clinical settings, as well as across the specific domains. Chi-square tests were performed to determine whether there were gender differences between the two groups.
Results
Frequency of MCI
Out of the 554 participants included in this study, 124 participants met criteria for MCI. That is, roughly 4% of our African American older adults were classified as amnestic MCI, while approximately 18% were classified as non-amnestic MCI.
Impaired Cognitive Domains within MCI groups
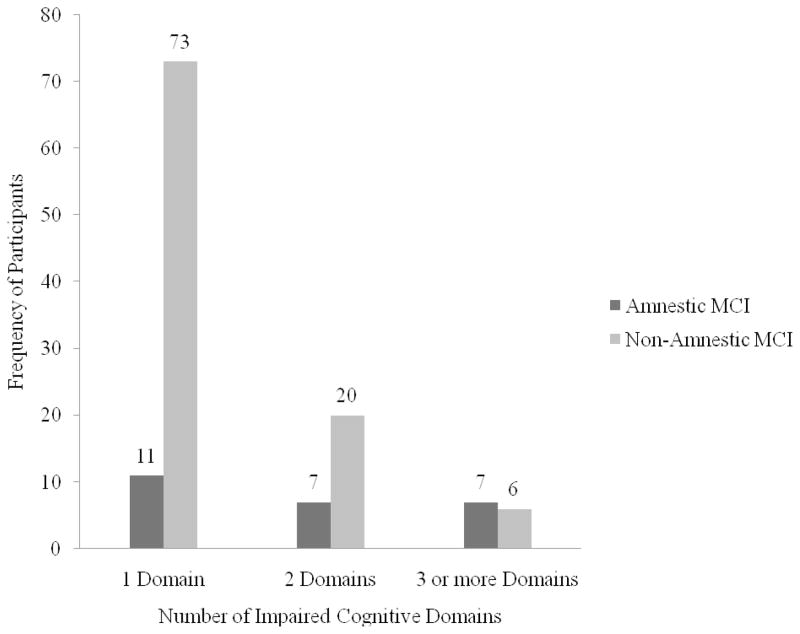
Additional descriptive analyses broke down the MCI groups based on whether participants were impaired in one, two, or more than three cognitive domains. As can be seen in Figure 1, a majority of participants were impaired in only a single cognitive domain. Among the non-amnestic group, however, there appears to be a relatively high number of participants with at least two impaired cognitive domains.
Figure 1.
Frequency of Amnestic and Non-Amnestic MCI Participants Impaired on 1, 2, and 3 or more Cognitive Domains
After further decomposing the MCI groups, non-amnestic participants with impairment in only one cognitive domain were impaired in language and executive functioning (see Table 1). Most non-amnestic participants with impairment in two or more cognitive domains were impaired in reasoning (see Table 1). Among the amnestic participants with impairments in three or more cognitive domains, impairment on working memory was almost as frequently observed as impairment in memory (Table 2).
Table 1.
Breakdown of specific impaired cognitive domains by the number of impaired domains (i.e. 1 domain, 2 domains, 3 or more domains impaired) for the non-amnestic MCI group
| Number of Impaired Domains |
|||
|---|---|---|---|
| Cognitive Domain | 1 | 2 | 3 or more |
| Memory | - | - | - |
| Reasoning | 11 | 14 | 6 |
| Language | 18 | 9 | 3 |
| Perceptual Speed | 14 | 6 | 4 |
| Executive Function | 17 | 4 | 4 |
| Working Memory | 13 | 7 | 3 |
Table 2.
Breakdown of specific impaired cognitive domains by the number of impaired domains (i.e. 1 domain, 2 domains, 3 or more domains impaired) for the amnestic MCI group
| Number of Impaired Domains |
|||
|---|---|---|---|
| Cognitive Domain | 1 | 2 | 3 or more |
| Memory | 11 | 7 | 7 |
| Reasoning | - | 2 | 4 |
| Language | - | 3 | 2 |
| Perceptual Speed | - | 1 | 3 |
| Executive Function | - | 1 | 3 |
| Working Memory | - | 0 | 6 |
Demographic and Health differences among the diagnostic groups
MANOVA results revealed significant differences among the groups for age and education (F (4, 1100) = 4.30, p < .05, η2 = .01). The non-amnestic MCI group was significantly older and had more years of education than the cognitively normal group (see Table 3). No significant differences on the health variables controlling for age, education, and gender were found (F (10, 1036) = 0.96, p > .05, η2 = .01; see Table 3).
Table 3.
Means for control participants and MCI participants across demographic & health variables and cognitive domains
| Demographic & Health Variables |
|||||||
|---|---|---|---|---|---|---|---|
| Age | Education | Vascular Risk Factors | CES-D | Peak Flow | Systolic BP | Diastolic BP | |
| Control | 68.24 (9.48) | 11.67 (2.75) | 1.83 (1.16) | 11.80 (6.07) | 286.33 (92.56) | 145.24 (23.11) | 86.81 (11.99) |
| Amnestic MCI | 69.40 (9.34) | 11.44 (3.00) | 1.45 (0.91) | 12.68 (6.83) | 280.21 (98.28) | 147.58 (26.05) | 86.49 (17.09) |
| Non-Amnestic MCI | 70.96 (10.00)a | 12.44 (2.97)a | 2.02 (1.17) | 11.62 (4.80) | 271.82 (84.06) | 148.91 (24.17) | 86.79 (13.69) |
| Cognitive Domains |
||||||||
|---|---|---|---|---|---|---|---|---|
| MMSE | SPMSQ | Reasoning | Language | Memory | Perceptual Speed | Executive Function | Working Memory | |
| Control | 9.42 (0.81) | 26.21 (2.99) | 52.11 (9.59) | 52.84 (8.78) | 52.57 (8.65) | 52.94 (8.70) | 52.39 (7.34) | 52.71 (9.15) |
| Amnestic MCI | 8.88 (1.24)a | 23.68 (3.38)a | 43.92 (9.20)a | 40.76 (6.39)a | 29.85 (4.84)a | 43.22 (6.93)a | 46.50 (11.12)a | 39.29 (4.79)a |
| Non-Amnestic MCI | 9.12 (0.96)a | 24.96 (2.91)a | 42.93 (7.69)a | 42.39 (9.94)a | 47.30 (7.46)a,b | 42.74 (9.40)a,b | 43.60 (12.99)a | 43.84 (9.10)a |
Note. Standard deviations are in parentheses. C, control; MCI, Mild Cognitive Impairment; CES-D, Center for Epidemiological Studies-Depression; BP, Blood Pressure.; MMSE, Mini-Mental State Examination.; SPMSQ, Short Portable Mental Status Questionnaire.
denotes normal group significantly different from MCI groups.
denotes Amnestic MCI group significantly different from Non-Amnestic MCI group.
A significant gender difference was observed (X2 (2, n = 554) = 6.68, p < .05), suggesting that more males (20% non-amnestic; 8% amnestic) than females (17% non-amnestic; 3% amnestic) were considered MCI.
Cognitive Performance differences among the diagnostic groups
A significant multivariate main effect for group was observed on the global cognitive assessments (i.e. MMSE, SPMSQ) and across specific cognitive domains (i.e., reasoning, language, memory, perceptual speed, executive function, and working memory; F (16, 1084) = 25.94, p < .05, η2 = .28) even after controlling for controlling for age, education, and gender. Follow-up univariate ANOVAs suggested that both MCI groups performed significantly worse on all of the cognitive measures than the normal controls (see Table 3). Although the amnestic group performed significantly worse on memory tasks than the non-amnestic group, the non-amnetic group performed significantly worse on perceptual speed tasks than the amnestic group.
Discussion
Previous literature using similar MCI criteria has estimated that 20.9% of a Caucasian sample appeared mildly impaired based on performing 1 SD below a sample mean (Ritchie et al., 2001). Given that the current study used a 1.5 SD impairment cutoff score, the frequency rate of 22% would increase within our African American sample with a less stringent impairment cutoff of 1 SD. In fact, when we adjusted the impairment cutoff score to 1 SD, the frequency of MCI became 40%. Differences in the cognitive measures used to assess MCI, as addressed further in the discussion, could explain why the current study’s frequency rate appear to be much higher than previous studies. However, given that MCI is considered to be the precursor to dementia, our findings appear to conform to the previous literature, which suggests that African Americans tend to have a higher rate of dementia than Caucasians (Demirovic, Prineas, Loewenstein, Bean, Duara, Sevush et al., 2003; Gurland, Wilder, Lantigua, Stern, Chen, Killeffer et al., 1999).
Further exploration of the frequency and type of impaired cognitive domains among both MCI groups revealed that a higher percentage of participants were impaired in non-memory domains than memory domains. Given that vascular dementia is highly prevalent in African Americans (Froehlich et al., 2001)and deficits in cognitive domains other than memory tend to be associated with vascular dementia, domains that show early impairments may differ between African Americans and Caucasians (Zanetti, Ballabio, Abbate, Cutaia, Vergani, and Bergamaschini, 2006). However, further research is needed to explore whether these domains are most often impaired in other African American samples.
A majority of the current study’s participants were impaired on one non-memory cognitive domain. These results are supported by previous studies, which have suggested that a higher percentage of individuals with MCI are impaired in a single non-memory domain than multiple domains or a single memory domain (Busse, Bischkopf, Riedel-Heller, and Angermeyer, 2003b). Unlike the current investigation, these studies do not specify the cognitive domain in which they are impaired. In the current study, MCI participants were often impaired on language, reasoning, and executive functioning. The specific domains impaired, however, varied depending on the number of impaired domains. Participants with one domain of impairment were often impaired on language and executive function, while those participants with two domains of impairment were often impaired on reasoning and language. Most participants with three or more domains of impairment were impaired on reasoning, perceptual speed, and executive functioning.
Participants with multiple domain deficits have shown to be further along in the progression of cognitive impairment (Bäckman, Jones, Berger, Laukka, and Small, 2005). More specifically, participants with more marked deficits on episodic memory (Albert, Blacker, Moss, Tanzi, and McArdle, 2007; Bäckman et al., 2005), executive functioning (Albert, Blacker, Moss, Tanzi, and McArdle, 2007; Bäckman et al., 2005), and perceptual speed (Bäckman et al., 2005) have shown to be more likely to convert to dementia than those participants with deficits on verbal ability and attention. Partially consistent with these previous results, a high frequency of amnestic MCI participants with multiple domains deficits (i.e. 3 or more impaired cognitive domains) had impairments on memory and working memory. In contrast, a high frequency of non-amnestic MCI participants with multiple domain deficits had impairments on reasoning, perceptual speed, and executive function. These results further support the idea that varying deficits across cognitive domains may be associated with a particular dementia subtype (Nordlund, Polstad, Klang, Lind, Hansen, and Wallin, 2007; Zanetti et al., 2006).
Several studies have reported a significant difference between individuals with MCI and cognitively normal individuals in demographic variables (Grundman, Petersen, Ferris, Thomas, Aisen, Bennett et al., 2004; Manly, Bell-McGinty, Tang, Schupf, Stern, and Mayeux, 2005). The current study only observed a significant difference between the normal group and non-amnestic MCI group in age and education. Partially consistent with previous literature (Luck, Riedel-Heller, Kaduszkiewicz, Bickel, Jenssen, Pentzek et al., 2007; Manly et al., 2005), the current study’s non-amnestic MCI group was older. In contrast to the previous literature (Grundman et al., 2004; Manly et al., 2005), however, the current studied observed that the non-amnestic MCI group had more years of education on average than the cognitively normal group. The role of stereotype threat on cognitive performance may be a possible explanation for this finding in that our participants with more years of education may be more concerned about disconfirming negative stereotypes regarding African Americans’ cognitive ability, which, as a result, may have decreased their attention and lack of confidence in successfully completing the study’s cognitive assessments (Blascovich, Spencer, Quinn, and Steele, 2001; Steele and Aronson, 1997). No significant differences between the groups were observed in health characteristics (i.e. vascular risk factors, depressive symptoms, peak flow, systolic blood pressure, and diastolic blood pressure). Considering previous research has suggested that health may explain African Americans’ cognitive functioning (Whitfield et al., 2008), these non-significant findings were somewhat surprising. Our inconsistent findings could be a result of different cognitive measures used to assess impairment as well as different criteria used to assess MCI status. The findings could also suggest that it is too early in the course of MCI to observe significant differences in health among our diagnostic groups and with further cognitive decline in the MCI groups, one may observe a clear distinction among the diagnostic groups. Finally, the results may suggest that the cognitive abilities do not necessarily converge with the health factors assessed in our cross-sectional study. Thus, a longitudinal study that examines the potential differences in health trajectories among the diagnostic groups may be insightful.
Individuals with MCI tended to indicate worse cognitive performance than cognitively normal individuals. These results are consistent with several studies that have indicated significant differences between the groups (Grundman et al., 2004; Ribeiro, Guerreiro, and De Mendonca, 2007). Although the criteria used to assess MCI as well as the specific measures included in the current study may differ from previous studies, we have been able to identify a group that tends to perform worse on specific cognitive domains as well as on global scales of cognition. Interestingly, we observed that performance on memory and perceptual speed tasks can potentially differentiate amnestic and non-amnestic MCI. Differences in memory performance between the MCI groups are not surprising, but it was interesting to observe that the non-amnestic group tended to have worse perceptual speed than the amnestic group. Further exploration is needed, however, to examine whether deficits in perceptual speed continue to differentiate the amnestic and non-amnestic MCI over time as well as whether deficits in perceptual speed differentiate dementia sub-types within African American samples.
Several limitations could possibly explain our findings. Cognitive impairment cutoff scores were established using the current study’s sample distribution, which may not be representative of other African American samples. Although MCI classification is typically based upon performance on neuropsychological measures and a clinical consensus of experts in neurology and neuropsychology, MCI classification in the current study was based primarily on performance on psychometric measures. Thus, the individuals classified as MCI using the current methodology may have led to some misclassification of participants. The current study’s frequency of MCI, however, is relatively consistent with the previous literature. Since the current study’s measures of reasoning and language are influenced by literacy and/or quality of education, deficits in these domains may represent poor quality of education rather than mild cognitive impairment and might not be observed in other samples. MCI classification was also solely based upon one testing occasion, which limits the study from exploring whether there is a decline in cognitive functioning. Likewise, individuals who have been classified as MCI at one occasion have shown to exhibit normal performance at subsequent testing (Larrieu, Letenneur, Orgogozo, Fabrigoule, Amieva, Carret et al., 2002; Ritchie et al., 2001), which suggests that there can be fluctuations in MCI status (Holtzer, Verghese, Wang, Hall, and Lipton, 2008; Schretlen, Munro, Anthony, and Pearlson, 2003). Thus, it may be difficult to accurately diagnosis MCI after a single assessment.
Conclusion
Almost a quarter of our African American sample was considered to be mildly impaired, particularly on non-memory cognitive domains. Given the current study used a cross-sectional approach, further research utilizing a longitudinal design is needed to determine which domains are likely to be initially impaired for those individuals who develop dementia. Future research should also explore whether early deficits in specific domains are associated with an underlying etiology of dementia. The BSBA is scheduled to complete follow-up data on the current study’s sample in 2010. At that time, analyses will be conducted to examine some of these aims.
Acknowledgments
The research described here was supported by grants from the National Institute of Aging (AG24108-01A1 and AG024108-02S1) to Keith E. Whitfield.
Sponsor’s Role: Sponsors did not participate in the design or data analysis of any aspect of the study or in manuscript preparation.
The data for this paper came from a project supported by the National Institute on Aging (NIA; R01 AG24108 and AG24108-S1) to K.E.W. Additional support was support was provided to R.S. from NIA (T32 AG00029).
Footnotes
Conflict of Interest: None
Author Contributions: Alyssa Gamaldo contributed to the conceptualization and design of the study, statistical analysis, and drafted the manuscript. Jason Allaire assisted in writing the manuscript, data analyses, and interpretation of data analyses. Regina Sims made substantial contributions to the revisions of the manuscript and assisted in the interpretation of the results. Keith Whitfield led the conceptualization, design, and implementation of the study as well as made important theoretical contributions and revisions to the paper.
Contributor Information
Alyssa A. Gamaldo, North Carolina State University.
Jason C. Allaire, North Carolina State University.
Regina C. Sims, Duke University.
Keith E. Whitfield, Duke University.
References
- Allaire JC, Gamaldo A, Ayotte BJ, Sims R, Whitfield K. Mild cognitive impairment and objective instrumental everyday functioning: The everyday cognition battery memory test. JAGS. 2009;57:120–125. doi: 10.1111/j.1532-5415.2008.02054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS. The neuropsychology of the development of Alzheimer’s disease. In: Craik FIM, Salthouse S, editors. The Handbook of Aging and Cognition. 3. Psychology Press; New York, NY: 2008. pp. 97–132. [Google Scholar]
- Albert M, Blacker D, Moss MB, Tanzi R, McArdle JJ. Longitudinal change in cognitive performance among individuals with mild cognitive impairment. Neuropsychology. 2007;21:158–169. doi: 10.1037/0894-4105.21.2.158. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Jones S, Berger A-K, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer’s disease: A meta-analysis. Neuropsychology. 2005;19:520–531. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- Beevers G, Lip GYH, O’Brien E. ABC of hypertension: Blood pressure measurement; Part I - Sphygmomanometry: factors common to all techniques. British Medical Journal. 2001;322:981–985. doi: 10.1136/bmj.322.7292.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blascovich JM, Spencer SJ, Quinn D, Steele C. African Americans and high blood pressure: The role of stereotype threat. Psychological Science. 2001;12:225–229. doi: 10.1111/1467-9280.00340. [DOI] [PubMed] [Google Scholar]
- Busse A, Bischkopf J, Riedel-Heller SG, Angermeyer MC. Mild cognitive impairment: Prevalence and incidence according to different diagnostic criteria. British Journal of Psychiatry. 2003a;182:449–454. [PubMed] [Google Scholar]
- Busse A, Bischkopf J, Riedel-Heller SG, Angermeyer MC. Subclassifications for mild cognitive impairment: Prevalence and predicitve validity. Psychological Medicine. 2003b;33:1029–1038. doi: 10.1017/s0033291703007839. [DOI] [PubMed] [Google Scholar]
- Cook NR, Evans DA, Scherr PA, Speizer FE, Taylor JO, Hennekens CH. Peak expiratory flow rate and 5-year mortality in an elderly population. American Journal of Epidemiology. 1991;133:784–794. doi: 10.1093/oxfordjournals.aje.a115957. [DOI] [PubMed] [Google Scholar]
- Cooper R, Cutler J, Desvigne-Nickens P, Fortmann SP, Friedman L, Havlik R, et al. Trends and disparities in coronary heart disease, stroke, and cardiovascular diseases in the United States: Findings of the national conference on cardiovascular disease prevention. Circulation. 2000;102:3137–3147. doi: 10.1161/01.cir.102.25.3137. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Hutchins GM, Moore GW. Racial differences in the etiology of dementia and frequency of Alzheimer lesions in the brain. Journal of the National Medical Association. 1989;81:644–652. [PMC free article] [PubMed] [Google Scholar]
- Demirovic J, Prineas R, Loewenstein D, Bean J, Duara R, Sevush S, et al. Prevalence of dementia in three ethnic groups: The south Florida program on aging and health. Annals of Epidemiology. 2003;13:472–478. doi: 10.1016/s1047-2797(02)00437-4. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples A, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Foley KL, Reed PS, Mutran EJ, DeVellis RF. Measurement adequacy of the CES-D among a sample of older African-Americans. Psychiatry Research. 2002;109:61–69. doi: 10.1016/s0165-1781(01)00360-2. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Froehlich TE, Bogardus ST, Inouye SK. Dementia and race: Are there differences between African Americans and Caucasians? Journal of American Geriatrics Society. 2001;49:477–484. doi: 10.1046/j.1532-5415.2001.49096.x. [DOI] [PubMed] [Google Scholar]
- Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, et al. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Archives of Neurology. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- Gurland BJ, Wilder DE, Lantigua R, Stern Y, Chen J, Killeffer EHP, et al. Rates of dementia in three ethnoracial groups. International Journal of Geriatric Psychiatry. 1999;14:481–493. [PubMed] [Google Scholar]
- Heyman A, Fillenbaum G, Prosnitz B, Raiford K, Burchett B, Clark C. Estimated prevalence of dementia among elderly black and white community residents. Archives of Neurology. 1991;48:594–598. doi: 10.1001/archneur.1991.00530180046016. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Verghese J, Wang C, Hall CB, Lipton RB. Within-person across-neuropsychological test variability and incident dementia. JAMA. 2008;300:823–830. doi: 10.1001/jama.300.7.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman D, Boland LL, Mosely T, Howard G, Liao D, Szklo M, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- Larrieu S, Letenneur L, Orgogozo JM, Fabrigoule C, Amieva H, Carret NLe, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59:1594–1599. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- Levy R. Report: Aging-associated cognitive decline. International Psychogeriatrics. 1994;6:63–68. [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4. Oxford University Press; New York: 2004. [Google Scholar]
- Luck T, Riedel-Heller SG, Kaduszkiewicz H, Bickel H, Jessen F, Pentzek M, et al. Mild cognitive impairment in general practice: Age-specific prevalence and correlate results from the German study on ageing, cognition and dementia in primary care patients. Dementia and Geriatric Cognitive Disorders. 2007;24:307–316. doi: 10.1159/000108099. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Tang M-X, Schupf N, Stern Y, Vonsattel J-PG, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Annuals of Neurology. 2008;63:494–506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly JJ, Bell-McGinty S, Tang M-X, Schupf N, Stern Y, Mayeux R. Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Archives of Neurology. 2005;62:1739–1746. doi: 10.1001/archneur.62.11.1739. [DOI] [PubMed] [Google Scholar]
- Nordlund A, Rolstad S, Klang O, Lind K, Hansen S, Wallin A. Cognitive profiles of mild cognitive impairment with and without vascular disease. Neuropsychology. 2007;21:706–712. doi: 10.1037/0894-4105.21.6.706. [DOI] [PubMed] [Google Scholar]
- Panza F, D’Introno A, Colacicco AM, Capurso C, Del Parigi A, Caselli RJ, et al. Current epidemiology of mild cognitive impairment and other predementia syndromes. American Journal of Geriatric Psychiatry. 2005;13:633–644. doi: 10.1176/appi.ajgp.13.8.633. [DOI] [PubMed] [Google Scholar]
- Petersen RC, O’Brien J. Mild cognitive impairment should be considered for DSM-V. Journal of Geriatric Psychiatry and Neurology. 2006;19:147–154. doi: 10.1177/0891988706291085. [DOI] [PubMed] [Google Scholar]
- Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. Journal of American Geriatrics Society. 1975;23:433–41. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;3:385–401. [Google Scholar]
- Ribeiro F, Guerreiro M, De Mendonca A. Verbal learning and memory deficits in mild cognitive impairment. Journal of Clinical and Experimental Neuropsychology. 2007;29:187–197. doi: 10.1080/13803390600629775. [DOI] [PubMed] [Google Scholar]
- Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment. Neurology. 2001;56:37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- Schretlen DJ, Munro CA, Anthony JC, Pearlson GD. Examining the range of normal intraindividual variability in neuropsychological test performance. Journal of the International Neuropsychological Society. 2003;9:864–870. doi: 10.1017/S1355617703960061. [DOI] [PubMed] [Google Scholar]
- Steele CM, Aronson J. Stereotype threat and test performance of academically successful African Americans. In: Jencks C, Phillips M, editors. The Black-White Test Score Gap. Brookings Institution Press; Washington, DC: 1998. pp. 401–427. [Google Scholar]
- Tang MK, Cross P, Andrews H, Jacobs DM, Small S, Bell K, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- Tang M-X, Maestre G, Tsai W-Y, Liu X-H, Feng L, Chung W-Y, et al. Relative risk of Alzheimer disease and age age-at-onset distributions, based on APOE genotypes among elderly African Americans, Caucasians, and Hispanics in New York City. American Journal of Human Genetics. 1996;58:574–584. [PMC free article] [PubMed] [Google Scholar]
- Teresi JA, Holmes D, Ramirez M, Gurland BJ, Lantigua R. Performance of cognitive tests among different racial/ethnic and education groups: Findings of differential item functioning and possible item bias. In: Skinner JH, Holmes D, editors. Multicultural Measurement in Older Populations. Springer; New York: 2002. pp. 85–96. [Google Scholar]
- Tervo S, Kivipelto M, Hanninen T, Vanhanen H, Hallikainen M, Mannermaa A, et al. Incidence and risk factors for mild cognitive impairment: A population-based three-year follow-up study of cognitively health elderly subjects. Dementia Geriatric Cognitive Disorders. 2004;17:196–203. doi: 10.1159/000076356. [DOI] [PubMed] [Google Scholar]
- Whitfield KE, Allaire JC, Belue R, Edwards CL. Are comparisons the answer to understanding behavioral aspects of aging in racial and ethnic groups? Journal of Gerontology: Psychological Sciences. 2008;63B:301–308. doi: 10.1093/geronb/63.5.P301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield KE, Weidner G, Clark R, Anderson NB. Sociodemographic diversity and behavioral medicine. Journal of Consulting and Clinical Psychology. 2002;70:463–481. doi: 10.1037//0022-006x.70.3.463. [DOI] [PubMed] [Google Scholar]
- Zanetti M, Ballabio C, Abbate C, Cutaia C, Vergani C, Bergamaschini L. Mild cognitive impairment subtypes and vascular dementia in community-dwelling elderly people: a 3-year follow-up study. Journal of American Geriatric Society. 2006;54:580–586. doi: 10.1111/j.1532-5415.2006.00658.x. [DOI] [PubMed] [Google Scholar]