Summary
Intravenous enzyme replacement therapy with recombinant human α-l-iduronidase (rhIDU) is used weekly to treat mucopolysaccharidosis (MPS) I. We tested continuous administration of rhIDU at two dosing levels (0.58 mg/kg/week and 2 mg/kg/week) in MPS I dogs, and compared the efficacy of continuous to the clinically-used 0.58 mg/kg weekly three-hour infusion. Peak plasma concentrations of rhIDU were much higher in weekly-treated dogs (mean 256 units/ml) than steady-state concentrations in dogs treated with continuous infusion (mean 1.97 units/ml at 0.58 mg/kg/week; 10.1 units/ml at 2 mg/kg/week). Dogs receiving continuous IV rhIDU, even at a higher (2 mg/kg/week) dose, had consistently lower iduronidase levels in tissues than dogs receiving a weekly (0.58 mg/kg/week) dose. GAG storage was also less improved by continuous intravenous infusion. Adverse events were similar in all dosing groups. We found that continuous administration of 2 mg/kg/week rhIDU to MPS I dogs was insufficient to achieve GAG storage reduction comparable to 0.58 mg/kg weekly dosing.
Synopsis.
Continuous intravenous enzyme replacement therapy was less successful than weekly three-hour infusions in the treatment of lysosomal storage due to canine mucopolysaccharidosis I.
Introduction
Enzyme replacement therapy (ERT) is used weekly or every other week to treat patients with the lysosomal storage disesases Gaucher syndrome, Fabry disease, Pompe disease, and mucopolysaccharidoses (MPS) I, II, and VI (Barton et al 1991; Kakkis et al 2001; Eng et al 2001; Harmatz et al 2005; Kishnani et al 2006; Muenzer J et al 2006). In MPS I patients, weekly infusions of 0.58 mg/kg intravenous recombinant human α-l-iduronidase (laronidase, rhIDU, EC 3.1.2.76) improve hepatosplenomegaly, pulmonary function, ambulation, joint mobility, and cardiac functional class (Kakkis et al 2001). The administration of weekly rhIDU leaves patients without a constant source of enzyme, such as occurs physiologically, and the intermittent dosing may be inconvenient.
Patients with some disorders, notably diabetes mellitus, benefit from continuous infusions of needed protein via an implanted catheter and pump. The pump is small, portable, and convenient to many of its users. We used MPS I dogs to investigate continuous administration of rhIDU using an infusion pump at two dosing levels, and compared the efficacy of continuous to the clinically-used 0.58 mg/kg weekly dose.
Materials and Methods
MPS I dogs (beagle/Plott hound mix) were bred and maintained at the Los Angeles Biomedical Research Institute at Harbor-UCLA, an AAALAC accredited facility.
Recombinant human iduronidase was donated by BioMarin Pharmaceutical (Novato, California) from preclinical lots not for human use. Enzyme activity was 117,000 to 350,000 units/ml in formulation buffer (150 mM NaCl; 100 mM sodium phosphate, pH 5.5-5.8). Some lots also contained 0.001% polysorbate 80. For weekly administration, rhIDU was diluted to 50 ml in 0.9% saline and 1 mg/ml canine albumin and infused into a cephalic vein over 3 h once per week using a Razel syringe pump connected to a 0.22 micron inline filter. For continuous administration, rhIDU was diluted as above and was delivered using a Broviac pediatric catheter (single lumen, with Surecuff Tissue Ingrowth cuff) implanted into the external jugular or femoral vein so that its tip lay within the superior vena cava above the right atrium. The catheter was routed subcutaneously to a skin exit site near a mesh vest containing a Dakmed ambulatory infusion pump connected to a sterile infusion bag. Bags were changed every 2 to 7 days, and enzyme stability verified by activity assay of rhIDU remaining in the reservoir.
Clinical assessments
Weekly complete blood cell counts, leukocyte differentials, blood chemistry assessments and total immunoglobulin assays were obtained on all animals throughout the study period. Urinalysis and urine creatinine testing was performed on a weekly basis for the first month, and every 3-4 weeks thereafter. Physical examinations noting weight, temperature, heart rate, posture, activity, mobility, demeanor and coat appearance were performed weekly. Behavior and skin condition were assessed daily.
Plasma rhIDU levels
Plasma rhIDU levels were determined for weekly-treated dogs on samples taken each hour during the infusion of enzyme, and then 2, 5, 10, 15, 30, 45, 60, 90, 120, and 240 minutes after the end of the infusions given in weeks 24 and 26. For continuously-treated dogs, samples were taken at weekly intervals.
Biochemical Analyses
Animals were euthanized by barbiturate injection forty-eight hours after the last intravenous infusion. Tissues were harvested immediately, frozen on dry ice and stored at −80°C until assayed for iduronidase activity and glycosaminoglycan (GAG) content. Frozen tissue samples (100-500 mg) were thawed and homogenized in three volumes PAD buffer (10 mM sodium phosphate, pH 5.8, 0.002% sodium azide, 0.1 mM dithiothreitol). Iduronidase activity was assessed using 250 μM 4-methylumbelliferyl α-l-iduronide substrate (4-MUI, Calbiochem, San Diego, CA) as previously described (Kakkis et al 1996), except that the incubation temperature was 37°C and the incubation time was 1 h. One unit is defined as the activity catalyzing the hydrolysis of 1 nmol 4-MUI substrate in 1 h. Urine and tissue GAG was measured using an Alcian blue dye binding method (Kakkis et al 1996). Urinary GAG was collected at weekly intervals. Urinary creatinine was quantified using the Roche Reagent for creatinine and measured at 500 nm on the COBAS MIRA® chemistry analyzer.
Results
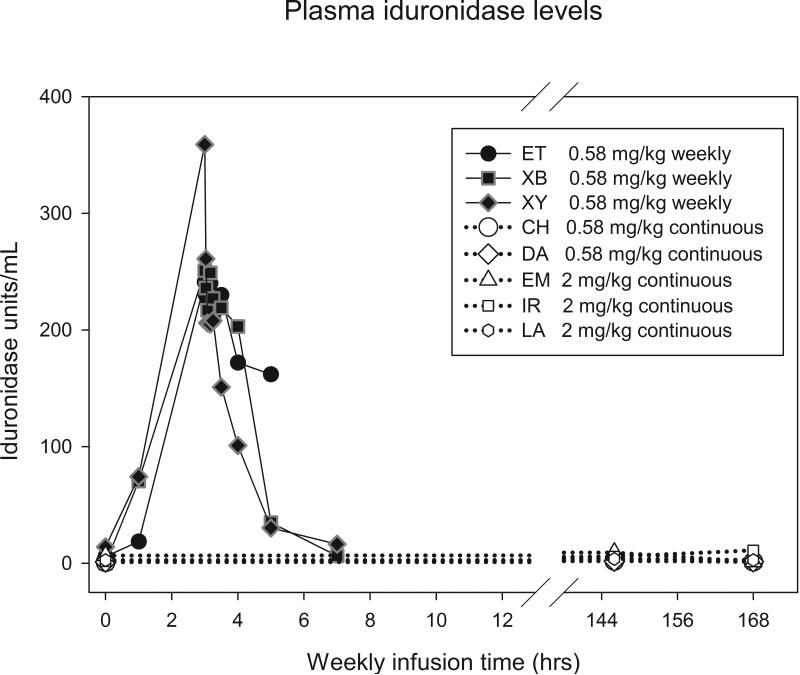
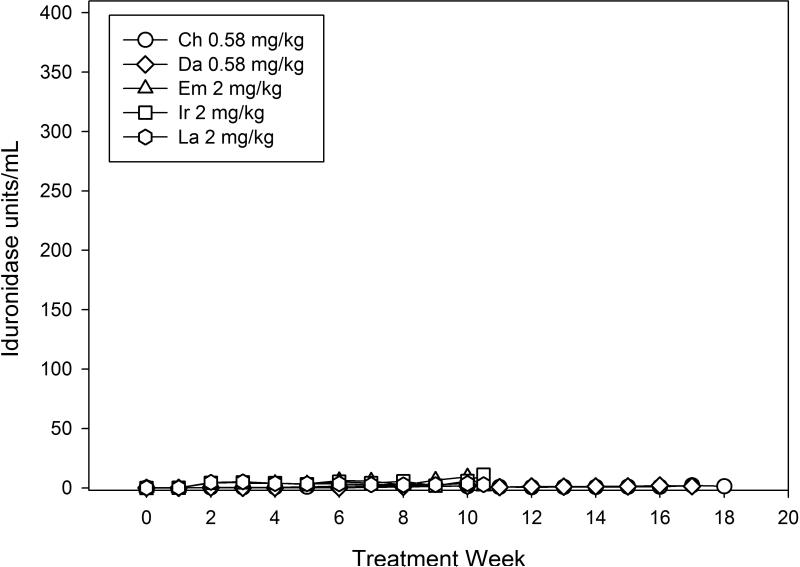
Two MPS I dogs received 744 units/kg body weight per hour (equivalent to 0.58 mg/kg/week of rhIDU in its current, clinically used formulation) IV rhIDU continuously (Table 1). Three MPS I dogs received 2976 units/kg per hour continuously (equivalent to 2 mg/kg/week). These were compared with nine MPS I dogs published elsewhere that received 0.58 mg/kg once per week (Dickson et al 2008). Plasma iduronidase levels were measured in four weekly-treated animals during treatments at week 24 or 26. Peak iduronidase in weekly-treated animals occurred at 180 minutes and reached a mean peak value of 284 units/ml (Fig. 1). Mean plasma iduronidase in animals receiving 0.58 mg/kg/week continuously was 1.97 units/ml during weeks 16-17, and was 8.44 units/ml in animals receiving 2 mg/kg/week continuously at weeks 9-10.
Table 1.
Experimental Canine Subjects
| Canine ID | Age at study end (months) | Dose | IV rhIDU duration (weeks) | Posttreatment Antibody Titer (OD units/μl) | Peak plasma rhIDU units/mL |
|---|---|---|---|---|---|
| Continuous rhIDU infusion | |||||
| Ch | 32 | 0.58 mg/kg | 16 | 57.3 | 2.02 |
| Da | 29 | 0.58 mg/kg | 16 | 47.1 | 1.91 |
| Em | 31 | 2.0 mg/kg | 10 | 175 | 19.4 |
| Ir | 17 | 2.0 mg/kg | 10 | 33.2 | 11.0 |
| La | 14 | 2.0 mg/kg | 10 | 84.2 | 5.09 |
|
Weekly rhIDU infusion | |||||
| Xb* | 31 | 0.58 mg/kg | 27 | 28.4 | 251 |
| Xy* | 31 | 0.58 mg/kg | 27 | 38.9 | 359 |
| Ub* | 35 | 0.58 mg/kg | 26 | 61.4 | nd |
| Et* | 13 | 0.58 mg/kg | 26 | 70.4 | 241 |
| Ni* | 40 | 0.58 mg/kg | 25 | 83.8 | nd |
| Ul* | 35 | 0.58 mg/kg | 26 | 113 | nd |
| Ye* | 19 | 0.58 mg/kg | 9 | 377 | nd |
| Um* | 33 | 0.58 mg/kg | 14 | 678 | 176 |
| Ru | 19 | 0.58 mg/kg | 14 | 2296 | nd |
Also received IT rhIDU.
Fig. 1.
a) Plasma iduronidase levels during and following a weekly rhIDU infusion. Plasma iduronidase levels were taken at hourly intervals during the infusion of enzyme, and then 2, 5, 10, 15, 30, 45, 60, 90, 120, and 240 minutes after the end of the infusion. Weeks 24 (ET) and 26 (XB, XY) are shown. Iduronidase levels tested once per week in continuous dogs (CH, DA, EM, IR, LA) are shown for comparison. Weeks 24-26 are shown. b) Iduronidase levels at all treatment weeks in continuous dogs.
Tissue iduronidase levels were also lower in dogs receiving continuous iduronidase, even at the higher dose (Fig. 2). Iduronidase levels in tissues of dogs treated with continuous infusion of 0.58 or 2 mg/kg/week ranged from 1.9 to 81% of those achieved using the clinically-used weekly dose. Continuous infusion was also inferior to weekly dosing in reducing GAG levels at the 0.58 mg/kg dosing level in most tissues (Table 2). Mean tissue GAG levels in dogs receiving 2 mg/kg continuous infusion were lower in most tissues when compared to the 0.58 mg/kg weekly group, although ranges overlap. Urinary GAG was similar in both weekly and continuously treated dogs (data not shown).
Fig. 2.
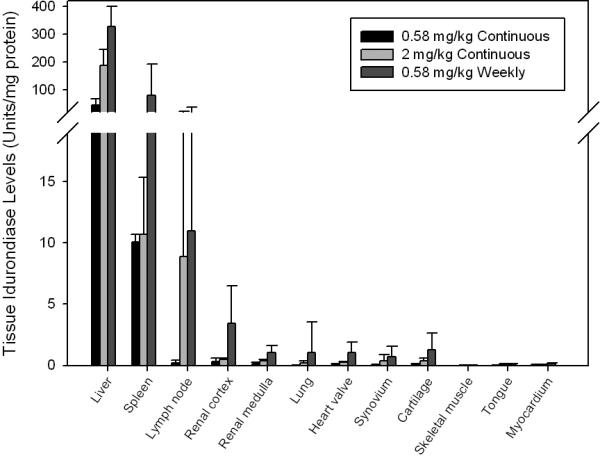
Tissue iduronidase levels in dogs treated weekly and continuously with IV rhIDU. Means of the means are shown. Error bars represent standard deviation.
Table 2.
Tissue GAG levels in MPS I dogs receiving IV rhIDU
| 0.58 mg/kg Continuous N=2 | 2 mg/kg Continuous N=3 | 0.58 mg/kg Weekly N=9 | |
|---|---|---|---|
| Liver | 8.34 [5.88, 10.8] | 4.03 [2.98 – 5.05] | 4.61 [2.61 – 7.84] |
| Spleen | 6.36 [4.77, 7.94] | 5.25 [3.51 – 7.84] | 3.90 [2.63 – 7.19] |
| Lymph node | 15.8 [13.6, 18.0] | 7.42 [3.54 – 11.7] | 13.4 [5.15 – 22.4] |
| Renal cortex | 38.1 [31.7, 44.5] | 16.0 [11.6 – 24.2] | 18.5 [10.2 – 32.0] |
| Renal medulla | 14.7 [13.9, 15.5] | 13.5 [12.5 – 15.1] | 23.8 [11.5 – 36.4] |
| Lung | 16.5 [14.4, 18.5] | 7.04 [6.75 – 7.20] | 9.39 [5.54 – 13.5] |
| Heart valve | 73.6 [66.0, 81.1] | 64.8 [49.3 – 82.8] | 77.5 [61.6 – 95.7] |
| Synovium | 2.75 [2.11, 3.38] | 4.86 [3.26 – 6.37] | 6.73 [1.82 – 12.6] |
| Skeletal Muscle | 0.535 [0.00, 1.07] | 0.586 [0.00 – 1.09] | 1.03 [0.00 – 2.18] |
| Tongue | 3.05 [2.32, 3.78] | 1.34 [0.00 – 2.08] | 3.33 [1.58 – 7.83] |
| Myocardium | 2.67 [1.94, 3.39] | 0.973 [0.00 – 1.47] | 1.53 [0.968 – 2.44] |
Mean [range] shown.
Three dogs receiving continuous IV rhIDU developed catheter-related infections, associated with an elevation in leukocyte count and ill appearance. Bacteria were isolated from the catheter in 3 of the 4 events. All animals recovered with antibiotics. The implanted catheter required replacement in two animals, resulting in interruptions in therapy of 6-11 days. Catheter obstruction occasionally required tissue plasminogen activator and resulted in interruptions of therapy (1-8 d). Dogs receiving continuous IV rhIDU required oral prednisone daily or every other day to prevent infusion reactions. All animals developed specific IgG antibodies against rhIDU (post-treatment titers shown in Table 1). Median time to positive antibody titer (> 20 OD units/μl) was 4 weeks in continuous dogs and 5.5 weeks in weekly-treated dogs (excluding Ni, Ul, Ub, Um, and Ru, which were previously exposed to rhIDU). Mean peak titer reached was 218 OD units/μl in continuous and 158 OD units/μl in weekly dogs, excluding those previously treated with rhIDU.
Discussion
MPS I patients receive intravenous enzyme replacement therapy with rhIDU on a weekly basis. In MPS I dogs we tested two doses of rhIDU administered continuously using a pump. Dogs receiving continuous IV rhIDU, even at a higher (2 mg/kg/week) dose, had consistently lower iduronidase levels in tissues than dogs receiving a weekly (0.58 mg/kg/week) dose. GAG storage was also less improved by continuous intravenous infusion. Adverse events were more frequent in dogs receiving continuous IV rhIDU and included catheter-related complications and the need for immune suppression to prevent infusion reactions.
Weekly dosing results in high peak plasma concentrations of rhIDU, ranging from 241 to 337 units/ml in dogs receiving 0.58 mg/kg/week. With a continuous infusion, plasma concentration at steady-state ranged from 0.6 to 2.0 units/ml. This plasma concentration is approximately 1/10 of the half-maximal uptake constant of iduronidase (1 nM). Thus the plasma concentration achieved in continuous dogs may not be large enough to drive diffusion into tissue of a concentration sufficient for uptake. Another possibility is that the concentration achieved with continuous dosing is below the threshold required to overcome clearance of the enzyme, leading to reduced therapeutic effect. The clearance may reflect uptake and metabolism by the liver. All of the treated dogs developed antibodies to rhIDU, which may prolong the clearance of rhIDU from plasma. In addition, the concentration of antibodies may be sufficient to bind up all of the enzyme when provided slowly during a continuous infusion. Higher concentrations achieved during 3-4 hour weekly infusions may allow more enzyme to escape binding from antibody late in the infusions as the titer falls (data not shown). The precise length of infusions on a weekly basis were not studied, as accelerating infusions did result in adverse events in the dogs as it can in humans. We did not test a shortened infusion duration in the weekly dogs, which may lead to safety issues if attempted in MPS I patients.
The study is limited by the small number of animals in continuous dosing groups. Statistical analysis was not performed. Treatment duration also differed among animals, though a subgroup analysis showed no difference in tissue GAG in the three weekly-treated dogs (Um, Ye, Ru) that received IV rhIDU for a shorter length of time than other animals in that group (data not shown). Most dogs in the weekly group received IT rhIDU in addition to IV rhIDU. While we have not examined whether enzyme administered intrathecally enters the bloodstream, the IT dose and frequency were low (0.46 to 1.38 mg at monthly or three-month intervals) relative to the IV rhIDU dose (0.58 mg per kg body weight, weekly). Tissue GAG varied among animals, and ranges of continuous and weekly-treated dogs overlapped for most tissues. Heart valve and synovium GAG responded poorly to therapy in all dosing groups in these nontolerant animals, as previously observed (Dickson et al 2008). The 48-hour timepoint was selected for analysis in dogs receiving weekly IV rhIDU, as it represents a maximum tissue enzyme distribution and avoids measuring extracellular enzyme. The enzyme has a long half-life intracellularly of five days, so that tissue levels are not likely to vary greatly between infusions (Kakkis et al 1994). However, tissue enzyme and GAG levels at other timepoints following weekly infusion were not studied.
We found that continuous administration of 2 mg/kg/week rhIDU to MPS I dogs was insufficient to achieve GAG storage reduction comparable to 0.58 mg/kg weekly dosing. This contrasts with data from patients with diabetes, who do not require increased insulin doses for continuous administration (Jakisch et al 2008). Elevated doses of 1.0 and 1.2 mg/kg weekly, and 1.2 and 1.8 mg/kg biweekly, have been studied in MPS I patients and were tolerated well (Giugliani et al 2008, Wraith et al 2007). However, the higher doses would increase the cost of ERT for patients up to four-fold and did not improve the effectiveness of treatment. Recently, we showed improved efficacy of intravenous rhIDU in dogs made tolerant to rhIDU and treated with 0.58 mg/kg/wk, and that a 2 mg/kg weekly dose had an even greater effect in tolerant dogs (Dickson et al 2008). Achieving efficacy with continuous infusion of rhIDU that is comparable to weekly treatment would require still higher doses (greater than 2 mg/kg/week), making continuous infusion impractical. However, in the absence of antibodies, the outcome may be different. Regardless, it is clear that the optimal duration of infusion to treat distal tissues is not well established and could benefit from more research.
Acknowledgements
Funding provided by the Ryan Foundation for MPS Children and BioMarin Pharmaceutical Inc. Thanks to Michael McEntee, Rita Esquivel, and Dan Garner for their technical assistance. Thanks to Paul Fu, Sr., for measurement of urinary creatinine.
The study was funded by BioMarin Pharmaceutical Inc., and the Ryan Foundation for MPS Children. The sponsors did not influence the content of the work.
Abbreviations
- MPS
mucopolysaccharidosis
- GAG
glycosaminoglycan
- rhIDU
recombinant human α-l-iduronidase
- ERT
enzyme replacement therapy
Footnotes
Competing interests: Dr. Kakkis and Mr. Lester are employees of BioMarin Pharmaceutical Inc., and have financial interest in laronidase (rhIDU).
Ethics approval: Animal studies were reviewed and approved by the Animal Care and Use Committee at the Los Angeles Biomedical Research Institute at Harbor-UCLA (an AALAC-accredited facility).
References
- Barton NW, Brady RO, Dambrosia JM, et al. Replacement therapy for inherited enzyme deficiency: macrophage-targeted glucocerebrosidase for Gaucher's disease. N Engl J Med. 1991;324:1464–1470. doi: 10.1056/NEJM199105233242104. [DOI] [PubMed] [Google Scholar]
- Dickson P, Peinovich M, McEntee M, et al. Immune tolerance improves the efficacy of enzyme replacement therapy in the canine model of mucopolysaccharidosis I. J Clin Invest. 2008;118:2868–2876. doi: 10.1172/JCI34676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng CM, Guffon N, Wilcox WR, et al. Safety and efficacy of recombinant human a-galactosidase A replacement therapy in Fabry's disease. N Engl J Med. 2001;345:9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- Giugliani R, Rojas VM, Martins AM, et al. A dose-optimization trial of laronidase (Aldurazyme) in patients with mucopolysaccharidosis I. Mol Genet Metab. 2009;96:13–19. doi: 10.1016/j.ymgme.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Harmatz P, Kramer WG, Hopwood JJ, et al. Pharmacokinetic profile of recombinant human N-acetylgalactosamine 4-sulphatase enzyme replacement therapy in patients with mucopolysaccharidosis VI (Maroteaux-Lamy syndrome): a phase I/II study. Acta Pædiatr Suppl. 2005;94:61–68. doi: 10.1111/j.1651-2227.2005.tb02115.x. [DOI] [PubMed] [Google Scholar]
- Jakisch BI, Wagner VM, Heidtmann B, et al. Comparison of continuous subcutaneous insulin infusion (CSII) and multiple daily infusions (MDI) in paediatric Type 1 diabetes: a multicentre matched-pair cohort analysis over 3 years. Diabetic Med. 2008;25:80–85. doi: 10.1111/j.1464-5491.2007.02311.x. [DOI] [PubMed] [Google Scholar]
- Kakkis ED, Muenzer J, Tiller GE, et al. Enzyme-replacement therapy in mucopolysaccharisosis I. N Engl J Med. 2001;344:182–188. doi: 10.1056/NEJM200101183440304. [DOI] [PubMed] [Google Scholar]
- Kakkis ED, McEntee MF, Schmidtchen A, et al. Long-Term and High-Dose Trials of Enzyme Replacement Therapy in the Canine Model of Mucopolysaccharidosis I. Biochem Molec Med. 1996;58:156–167. doi: 10.1006/bmme.1996.0044. [DOI] [PubMed] [Google Scholar]
- Kakkis ED, Matynia A, Jonas AJ, et al. Overexpression of the human lysosomal enzyme α-l-iduronidase in Chinese hamster ovary cells. Prot Expr Purif. 1994;5:225–232. doi: 10.1006/prep.1994.1035. [DOI] [PubMed] [Google Scholar]
- Kishnani PS, Nicolino M, Voit T, et al. Chinese hamster ovary cell-derived recombinant human acid [alpha]-glucosidase in infantile-onset Pompe disease. J Pediatr. 2006;149:89–97. doi: 10.1016/j.jpeds.2006.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenzer J, Wraith JE, Beck M, et al. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome). Genet Med. 2006;8:465–473. doi: 10.1097/01.gim.0000232477.37660.fb. [DOI] [PubMed] [Google Scholar]
- Wraith JE, Beck M, Lane R, et al. Enzyme replacement therapy in patients who have mucopolysaccharidosis I and are younger than 5 years: results of a multinational study of recombinant human {alpha}-l-iduronidase (laronidase). Pediatr. 2007;120:e37–e46. doi: 10.1542/peds.2006-2156. [DOI] [PubMed] [Google Scholar]