Abstract
Objective
To examine the course of depression during the treatment of depressed adolescents who had recently attempted suicide.
Method
Adolescents (N=124), age 12–18 years, with a 90-day history of suicide attempt, a current diagnosis of depressive disorder (96.0% had major depressive disorder), and a Children's Depression Rating Scale-Revised (CDRS-R) score of 36 or greater, entered a 6-month treatment with antidepressant medication, cognitive-behavioral therapy focused on suicide prevention, or their combination (Comb), at five academic sites. Treatment assignment could be either random or chosen by study participants. Intent-to-treat, mixed effects regression models of depression and other relevant ratings were estimated. Improvement and remission rates were computed with the last observation carried forward.
Results
Most patients (N=104 or 84%) chose treatment assignment and, overall, three-fourths (N=93) received Comb. In Comb, CDRS-R declined from a baseline adjusted mean of 49.6 (SD 12.3) to 38.3 (8.0) at week 12, and to 27.0 (10.1) at week 24 (p<0.0001), with a Clinical Global Impression-defined improvement rate of 58.0% at week 12, and 72.2% at week 24; and a remission (CDRS-R ≤28) rate of 32.5% at week 12 and 50.0% at week 24. CDRS-R and the Scale for Suicidal Ideation (SSI) scores were correlated at baseline (r=0.43, p<0.0001), and declined in parallel.
Conclusions
When vigorously treated with a combination of medication and psychotherapy, depressed adolescents who have recently attempted suicide show rates of improvement and remission of depression that appear comparable to those observed in non-suicidal depressed adolescents.
Keywords: adolescents, suicide, depression, treatment
Suicidal ideation and behavior are among the possible manifestations of depression, and the risk for suicide is intrinsic in the depressive psychopathology.1,2 When depression is associated with prominent suicidal symptoms, such as a recent suicide attempt, the underlying psychopathology may be more complex and the treatment response different, with respect to both efficacy and safety, than observed in depression without significant suicidal symptoms.
The efficacy of selective serotonin reuptake inhibitors (SSRI), cognitive-behavioral therapy (CBT), and their combination for the treatment of adolescent depression is well documented.3–7 The evidence, however, is in large part derived from randomized controlled clinical trials that excluded or restricted the participation of youths who recently attempted suicide. Most depression treatment studies have excluded patients deemed at high risk for suicide due to ethical and practical reasons as these patients are considered to be in need of more comprehensive treatment and intensive monitoring than usually provided under a research protocol. Consequently, clinicians have been left with incomplete information about the therapeutic benefits of interventions for depressed suicidal adolescents. This gap is especially problematic since depression is a major but malleable risk factor for suicide, and its treatment constitutes the main approach to suicide prevention.8
The Treatment of Adolescent Suicide Attempters Study (TASA) was an uncontrolled, pilot investigation to assess the feasibility of systematically treating adolescents who were clinically depressed and had recently attempted suicide.9 Participants in TASA received a thorough evaluation at study entry and were then treated with antidepressant medication and/or manualized CBT, which was developed specifically to address the suicidal risk with the aim of preventing recurrence of suicidal behavior (Cognitive Behavioral Therapy for Suicide Prevention; CBT-SP).10 The primary analyses showed that 12% of the patients experienced another suicide attempt during the 24weeks of TASA.9
In this paper, we report on the change in the ratings of depressive symptoms, global scores of illness severity, and level of functioning observed over the 24 weeks of treatment in TASA. The purpose of these analyses is to describe trends in depressive symptom change and to estimate, within the limitations imposed by the non-experimental, uncontrolled nature of the study, whether treatment response appears to be consistent with that reported in non-suicidal depressed adolescents.
Method
Sample and Design
TASA was a multi-site study to evaluate the feasibility of identifying, enrolling, and treating a sample of depressed adolescents with a recent history of suicide attempt.9 Treatment consisted of antidepressant medication and cognitive-behavioral therapy with focus on suicide prevention (CBT-SP). Females and males youths, aged 12–18 years, were eligible for participation if they had made a suicide attempt in the last 90 days, met current criteria for major depressive disorder, dysthymic disorder, or depressive disorder not otherwise specified, on the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version,11 and had a score of 36 or greater on the Children's Depression Rating Scale- Revised (CDRS-R).12 Exclusion criteria included substance dependence, bipolar disorder, psychosis, and pervasive developmental disorders. Patients were enrolled at five academic sites (University of Texas-Southwestern Medical Center, Dallas, TX; Western Psychiatric Institute and Clinic,Pittsburgh, PA; Johns Hopkins University, Baltimore, MD; Duke University Medical Center, Durham, NC; and New York State Psychiatric Institute-Columbia University/New York University, New York, NY), between December 2003 and June 2006.
The study started as a randomized trial, but, after about nine months of low enrollment despite intensive recruitment efforts, the design was changed so that patients and their families could either accept randomization or choose which treatment to receive. The study was reviewed and approved by the local institutional review boards, and monitored by an independent data and safety monitoring board.
Treatment
The treatment lasted 6 months and consisted of manualized CBT-SP, antidepressant pharmacotherapy (Med), following an adaptation of the Texas Medication Algorithm for adolescent depression,13 or a combination of these two modalities (Comb). A detailed description of CBT-SP is separately provided.10 In brief, CBT-SP was specifically aimed at modifying known risk factors for suicide, such as depression, with the goal of preventing recurrence of suicidal behavior.10 CBT-SP consisted of up to 22 sessions, including both individual and parent-youth sessions, and incorporated five key elements: 1) chain analysis of the index suicide attempt; 2) safety planning to reduce current suicide risk; 3) case conceptualization (i.e., formulation of the patient's specific cognitive, behavioral, affective, and contextual problems); 4) skill acquisition and enhancement (e.g., behavioral activation, cognitive restructuring, and problem-solving); and 5) relapse prevention. Med followed an established treatment algorithm for pharmacological treatment of depression in youths, which started with a comprehensive evaluation for possible use of antidepressant and included, as a first step, monotherapy with a selective serotonin reuptake inhibitor (SSRI), followed, in case of non response, by a different SSRI as step 2, and an alternate class (venlafaxine, duloxetine, mirtazapine, or bupropion) as step 3, with the option of augmenting with lithium or other antidepressant in case of partial response. 13
Trained psychotherapists provided the CBT-SP under the supervision of senior experts, and across site weekly telephone conferences were held to review cases and discuss treatment strategies. Psychopharmacologists provided the medication treatment, and weekly telephone conferences were held to review cases and ensure consistency across sites.
Assessments
The primary outcome of TASA was recurrence of a suicide event. Suicidal events included suicide attempts, interrupted suicide attempts, as well as high levels of suicidal ideation that necessitated emergency evaluation or hospitalization. As separately reported, a suicide event occurred in 19.5% of the participants, with 12.0% making a suicide attempt (9). The present report focuses on depressive symptoms and other related outcomes.
The primary measure of depressive symptoms was the total score on the CDRS-R, which is a clinician-rated 17-item scale based on direct interviewing of patient and parent. The CDRS-R was administered by independent evaluators, who were experienced clinicians with a master degree education or higher. Independent evaluators were trained to ensure acceptable inter-rater reliability and remained blind to patient treatment assignment. Ratings were obtained at study entry and at 6, 12, 18, and 24 weeks afterwards.
In addition, the Montgomery-Asberg Depression Rating Scale (MADRS)14 was administered by the pharmacotherapists at each visit. The MADRS is a 10-item clinician rating scale that was originally developed for use in adults, but has been applied to the assessment of depression symptoms in children.15 Patients completed the Beck Depression Inventory16 and the Beck Scale for Suicidal Ideation (SSI)17 at baseline and weeks 6, 12, 18, and 24. Anxiety symptoms were measured on the Multidimensional Anxiety Scale for Children (MASC),a self-rated, 39-item questionnaire.18
Overall severity of psychopathology and improvement were rated on the Clinical Global Impression Severity (CGI-S) and Improvement (CGI-I) scales,19 and level of functioning on the Child Global Assessment Scale (CGAS)20 by the independent evaluator at baseline and weeks 6, 12, 18, and 24. At the same time points, the patient completed the Social Adjustment Scale (SAS).21
Data Analysis
Data management and quality control were centralized at KAI-Research, Inc., under an NIMH contract. The data were analyzed with intent-to treat approach. For the continuous dependent variables, mixed effects regression models were estimated in which the intercepts and the slopes for change in the outcome over the course of the study were allowed to vary randomly across individuals. Gender, race (white vs. non-white), comorbidity (presence of mental disorder other than depression), pre-treatment history of additional suicide attempts, time from most recent suicide attempt at entry into TASA, and impairment severity (CGI-S > 4) were assessed as potential time-invariant predictors of this variation and of end-of-treatment improvement (i.e., final CGI-1 below 3) or remission status (i.e., final CDRS-R below 29). Site was controlled as a fixed effect in the model.
Although regression curves on the CDRS-R were computed for each treatment group, variation in treatment effect across group is difficult to interpret because group assignment occurred largely through self-selection and the large majority of subjects ended up in the combined treatment group (Comb). For this reason, more detailed analyses of the outcome measures were conducted for Comb alone.
Results
Baseline
A total of 124 adolescents, age 12–18 (mean 15.7 ± SD 1.5), 77.4% females, 79.8% White, participated in the study (Table 1). The large majority (96.0%) met diagnostic criteria for major depressive disorder (10.5% had both major depressive disorder and dysthymic disorder). Most (N=104 or 83.9%) chose their treatment rather than being randomized, and 93 (75.0%) received Comb. Comb patients had more severe depression (mean CDRS-R=52.1 ± SD 12.0) than Med patients (43.4 ± 11.1, t=2.58, p=0.01) or CBT patients (46.9 ± 14.7, t=2.56, p=0.01). There was also a higher prevalence of comorbid anxiety and more functional impairment in Comb than in the other two treatment groups.
Table 1.
Sample Characteristics at Study Entrya
| All N=124 | CBT N=17 | Med N=14 | Comb N=93 | P | |
|---|---|---|---|---|---|
| Females (%) | 77.4 | 94.1 | 92.9 | 72.0 | 0.05 |
|
| |||||
| White (%) | 79.8 | 70.6 | 85.7 | 80.7 | 0.54 |
|
| |||||
| Age, mean (SD) | 15.7 (1.5) | 15.7 (1.5) | 15.6 (1.4) | 15.7 (1.6) | 0.98 |
|
| |||||
| Diagnosis (%) | |||||
| MDD only | 85.5 | 94.1 | 92.9 | 82.8 | 0.34 |
| DD only | 0.8 | 0 | 0 | 1.1 | 0.85 |
| Both | 10.5 | 0 | 7.1 | 12.9 | 0.11 |
| D-NOS | 3.2 | 5.9 | 0 | 3.2 | 0.66 |
|
| |||||
| Comorbidity (%) | |||||
| Anxiety | 54.0 | 23.5 | 28.6 | 63.4 | 0.001 |
| ADHD | 21.0 | 11.8 | 14.3 | 23.7 | 0.44 |
| ODD/CD | 15.3 | 0.0 | 35.7 | 15.1 | 0.02 |
|
| |||||
| History of Multiple Suicide attempts N(%) | 54(43.6) | 6(35.3) | 4(28.6) | 44(47.3) | 0.32 |
|
| |||||
| CDRS-R, mean (SD) | 50.4 (12.6) | 46.9 (14.7) | 43.4 (11.1) | 52.1 (12.0) | 0.01 b Comb>Med, CBT |
|
| |||||
| MADRS,mean(SD) | 24.8 (11.7) | - c | 22.4 (12.8) | 25.2(11.5) | 0.08 b |
|
| |||||
| BDI, mean (SD) | 22.8 (13.2) | 18.1 (13.7) | 20.2 (10.3) | 23.9 (13.4) | 0.40 b |
|
| |||||
| CGI-S, mean (SD) | 4.5 (0.9) | 4.0 (0.8) | 2.8 (1.4) | 4.6 (0.9) | 0.003 b Comb>Med, CBT |
|
| |||||
| C-GAS, mean (SD) | 48.4(11.1) | 54.9(15.1) | 57.6(8.1) | 45.9 (9.5) | 0.0003 b Comb<Med, CBT |
|
| |||||
| SAS, mean (SD) | 57.1 (14.4) | 50.3 (13.2) | 57.1 (9.3) | 58.0 (15.1) | 0.12 b |
|
| |||||
| SSI, mean (SD) | 6.3 (7.7) | 5.0 (6.0) | 3.9 (6.0) | 6.9 (8.2) | 0.14 b |
Abbreviations: CBT: cognitive behavioral therapy; Med: antidepressant pharmacotherapy; Comb: combination of both CBT and antidepressant pharmacotherapy; MDD: major depressive disorder; DD: dysthymic disorder; D-NOS: depressive disorder not otherwise specified; CDRS-R: Child Depression Rating Scale-Revised; Montgomery-Asberg Depression Rating Scake; BDI: Beck Depression Inventory; CGI-S: Clinical Global Impressions-Severity; C-GAS: Children Global Assessment Scale; SAS: Social Adjustment Scale (self-report); SSI: Scale for Suicidal Ideation
Raw (unadjusted) scores from rating scales
Adjusted for site
The MADRS was completed by the pharmacotherapist (in Med and Comb only).
Premature discontinuation
Overall, 39 patients (31%) dropped out prior of the end of the 24-week study (22 patients or 18% in the first 12 weeks). In the Comb group, 14 patients dropped out by week 12 (15%) and 27 (29%) by week 24. The reasons for discontinuation were: suicidal intent with inability to commit to safety plan (N=2), lack of adherence to treatment (N=5), need for different treatments and services (N=9), and withdrawal of consent or failure to return for visits for unspecified reasons (N=23). One patient, a 16-year old boy, died of suicide 20 days after he completed the 24-week study; he had chosen and received combined treatment, and had shown improvement in TASA, reaching remission from the depressive disorder by week 8. Prior to entering TASA, this patient had attempted suicide three times using methods such as gunshot attempt, hanging, and carbon monoxide poisoning.
Change during Treatment
In all three treatment groups there was a significant decline in depressive symptoms as measured by the CDRS-R (Fig.1). The slope of the CDRS-R decline was not influenced by gender (gender*time p=0.70), race (White vs. non-White*time p=0.31, or history of multiple suicide attempts (attempt*time p=0.76).
Figure 1. Child Depression Rating Scale-Revised Scores Over Timea.
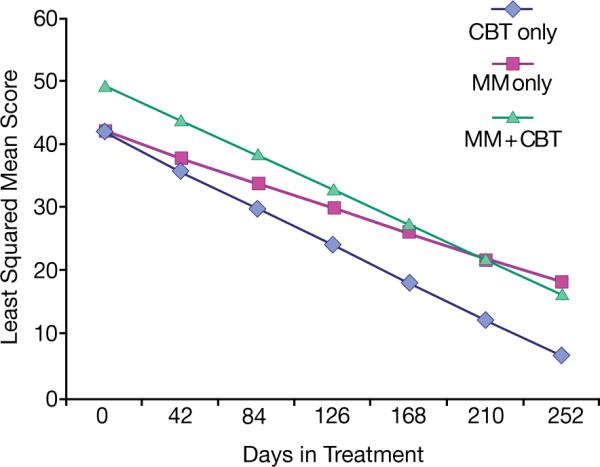
CBT: cognitive-behavioral therapy (N=17). Med: algorithmic SSRI medication treatment (N=14). Comb: combination of CBT and Med (N=93).
aAdjusted means based on mixed effects regression models of the independent evaluator ratings. Seventeen patients completed treatment after the end of the 6 months.
Mixed-model regression model: time: F=91.0, p<0.0001; treatment group differences at baseline: F=4.3, p=0.001 (COMB>CBT, Med); treatment*time: F=0.93, p=0.40.
In Comb, based on intent-to-treat mixed effect regression models, CDRS-R declined from a baseline adjusted mean of 49.6 ± SD 12.3 to 38.3 ± 8.0 at week 12, and to 27.0 ± 10.1 at week 24 (p<0.0001). The mean change from baseline was 14.6±15.1 at week 12, and 18.1±16.7 at week 24. The improvement rate, based on a CGI-I of 1 or 2, was 58.0% at week 12, and 72.2% at week 24. The remission rate, defined by a CDRS-R ≤28, was 32.5% at week 12, and 50.0% at week 24 (Table 2). The C-GAS scores improved from 49.3± 10.4 at baseline to 61.0±8.0 at week 12, and to 72.6 ± 11.4 at week 24. The SAS scores improved from 58.0±15.1 at baseline to 48.6±16.6 at week 12 and to 39.8±12.3 at week 24.
Table 2.
Depressive Symptoms Ratings during Combined CBT/Medication Treatment in TASA and Other Multisite Studies in Adolescent Depression
| Study | TASA | TADS5,6 | ADAPT22 | TORDIA7 |
|---|---|---|---|---|
| Design | patient choice | randomized | randomized | randomized |
| Na | 93b | 107 | 105 | 166 |
|
| ||||
| Females | 72% | 56% | 75% | 70% |
|
| ||||
| Age, yr, mean (SD) | 15.7 (1.6) | 14.6 (1.5) | 14.0 (1.5) | 16.0 (1.5) |
|
| ||||
| Diagnosis | Major depressive (96% of the sample) | Major depressive disorder | Major depressive disorder or 4 depressive symptoms | Major depressive disorder |
|
| ||||
| Suicidal history | Suicide attempt in the 90 days prior to study entry | Youths at high-risk for suicidality were excluded, but 29% of the participants had significant suicidal ideation | About half of the sample had history of suicidal behavior | 58% of the participants had clinically significant suicidal ideation |
|
| ||||
| Comorbidity | 68% | 52% | 88% | 52% |
|
| ||||
| Drop-out by Week 12 | 15% | 14% | 11% | 16% |
| Drop-out by Week 24a | 29% | 20% | 17% | -e |
|
| ||||
| CDRS-Rc | ||||
| Baseline, mean (SD) | 49.6 (12.3) | 60.8 (6.2) | 58.9 (10.5)e | 59.2 (11.0) |
| Week 12, mean (SD) | 38.3 (8.0) | 33.6 (8.6) | 42.5 (16.8)e | 36.9 (13.9) |
| Week 24,e mean (SD) | 27.0 (10.1) | 30.0 (8.1) | 36.4 (15.3)e | -e |
| CGI-I-Response ratef | ||||
| Week 12 | 58.0% | 71.0% | 41.6% | 59.0% |
| Week 24e | 72.2% | 77.6% | 53.1% | -h |
| Remission rateg | ||||
| Week 12 | 32.5% | 37% | -h | 31% |
| Week 24e | 50.0% | 56% | -h | -h |
|
| ||||
| C-GASc | ||||
| Baseline, mean (SD) | 49.3 (10.4) | 50.0 (7.5) | 41.6 (6.0) | 50.6 (7.7) |
| Week 12, mean (SD) | 61.0 (8.0) | 66.6 (11.9) | 52.1 (14.3) | 65.1 (11.8) |
| Week 24,e mean (SD) | 72.6 (11.4) | -h | 57.2 (16.4) | -h |
|
| ||||
| Incidence of suicidal attemptsi | ||||
| By Week 12 | 12.9% | 2.8% | 6.9% | 6.0% |
| By Week 24e | 15.1% | 3.7% | 7.1% | -h |
Abbreviations: TASA: Treatment of Adolescent Suicide Attempters; TADS: Treatment for Adolescents with Depression Study; ADAPT: Antidepressant and Psychotherapy Trial; TORDIA: Treatment of Resistant Depression in Adolescents; CDRS-R: Child Depression Rating Scale-Revised; CGI-I: Clinical Global Impressions-Improvement; C-GAS: Children Global Assessment Scale
Combined treatment group only
86 chose treatment and 7 were randomized
Adjusted means based on intent-to-treat regression analyses, except for ADAPT (see foot-note d)
dActual scores. N=104 at baseline, N=100 at week 12 and N=98 at week 28.
Week 28 for ADAPT
Response: score of 1 (very much improved) or 2 (much improved) on the CGI-I, with last observation carried forward
Remission: CDRS-R ≤28, with last observation carried forward.
Data not available
Actual suicide attempt (i.e., excluding aborted or interrupted attempts)
Still in Comb, statistically significant changes from baseline were detected on the other measures. The CGI-S declined from an adjusted mean of 4.3± SD 1.0 at entry to 3.2 ± 0.8 at week 12 and 2.0 ± 1.0 at week 24; the BDI from 21.9 ±13.5 at entry to 13.8±9.0 at week 12 and 5.7 ±9.3 at week 24; and the MADRS from 22.9±10.5 at entry to 13.8±6.9 at week 12 and 4.7±7.7 at week 24. The patient-rated MASC declined from 46.0±18.1 at entry to 40.0±14.9 at week 12 and 33.6±16.4 at week 24, while the parent-rated MASC went from 49.1±15.4 at entry to 44.9±12.0 at week 12, and 40.6±12.5 at week 24.
No predictors of improvement and remission status at week 24 were identified among the examined baseline variables, which included patient's gender, race, comorbidity, history of more than one suicide attempt, time from most recent suicide attempt, severity of illness, and study site.
The CDRS-R and the Scale for Suicidal Ideation (SSI) scores were correlated at baseline (r=0.43, p<0.001), and both declined significantly during treatment (time effect p<0.001). When the CDRS-R was entered as a covariate in the SSI regression, the time effect of SSI became non significant. Of the 23 suicidal events that occurred in TASA (involving a total of 15 patients), 35% occurred during the first 4 weeks, and 83% during the first 12 weeks.
Discussion
In this study, depressed adolescents who had recently attempted suicide were systematically diagnosed and treated in a 6-month feasibility study. As the study allowed for self-selection of treatment by the participants, no causal inferences are possible. The three treatment groups were diverse as to size, severity of depression and level of dysfunction, as more severe patients preferred combined treatment over monotherapy (Table 1). However, the data offer an opportunity to examine the changes in depressive symptoms and related indexes of psychopathology and functioning that occurred over a period of 6 months during which patients were intensively treated and closely monitored.
The large majority of the participants received the combination of psychotherapy (CBT-SP) and SSRI antidepressant medication (Comb). Thus, the results mainly apply to this treatment group. In Comb, the rate of improvement (58% at week 12 and 72% at week 24) was consistent with that observed in non-suicidal depressed adolescents treated with a combination of CBT and SSRI medication in recently completed randomized clinical trials (Table 2). The CDRS-R at study entry was, on average, lower in TASA than in the randomized trials, probably reflecting the entry criteria used for this study. While caution must be exercised when comparing results between studies, especially between randomized trials and non-experimental studies, the remission rate in TASA was 32% at week 12 and 50% at week 24, which is comparable to that in the Treatment for Adolescents with Depression Study (TADS), which was 37% at week 12 and 56% at week 24.23,24
With regard to the feasibility of including and treating depressed youths at high risk for suicide in a treatment study, the retention rate in TASA at week 12 (85%) compares favorably to that of other treatment studies in adolescent depression, although it declined to 71% by week 24 (Table 2). One must consider, however, that treatment was mainly chosen by the participants in TASA, but randomly assigned in TADS and the other adolescent clinical trials. A greater attrition rate might be expected in randomized trials involving suicidal patients. As premature treatment discontinuation is a major obstacle to providing care to suicidal adolescents, future studies will need to develop effective strategies for patient retention.
The finding of a robust improvement in depressive symptomatology in TASA is consistent with the results of a smaller study of psychotherapy in adolescents with repeated self-harm. This study found that, by the end of the 7-month treatment, the depressive symptom scores had declined by about 50% over the baseline.25 The results are also consistent with the data from the Sequenced Treatment Alternatives to Relieve Depression study (STAR*D) in adults, where history of having attempted suicide did not affect the rate of remission.26
The decrease in depressive symptoms was accompanied by a decrease in suicidal ideation as indicated by the parallel decline of CDRS-R and SSI scores. The incidence of suicide attempts during the 6-month period of TASA, however, was higher than observed in studies of depressed adolescents who had not attempted suicide (Table 2). It should be noted that at least one-third of the attempts occurred in the first 4 weeks of treatment and the large majority (83%) in the first 12 weeks. This distribution of suicide attempts suggest that the risk of reattempt is highest in the first three months of treatment, when depressive symptoms, though improving, are still prominent, and then declines as depression abates. The suicide that occurred about 3 weeks after completing TASA points at treatment transition as another period of high risk. The fact that this patient had improved and reached remission from depression while in treatment confirms that other factors, beside depression, contribute to suicidal behavior.
A number of important limitations must be taken into account when interpreting these data. First, as already underscored, the lack of randomization and experimental control prevents drawing causal inferences about treatment effects, which cannot be disentangled from time effects. Because of self-selection of treatment by the large majority of the TASA participants, we cannot determine how CBT-SP, Med, and Comb compared with each other with regards to efficacy or safety. Second, the CBT-SP administered in TASA is a particular type of CBT that was modified to address the high suicidality risk, and, in this respect, was different from the standard CBT developed for non-suicidal depressed adolescents. This difference limits the comparisons with other clinical trials in depressed adolescents that used standard CBT. Finally, patients with psychotic symptoms or prominent substance abuse were excluded, thus limiting the generalizability of the findings. Given the prominence of substance abuse in increasing the risk for suicidal behavior, there is a need to identify methods for including youths with substance abuse in future studies.
In conclusion, in this sample of depressed youths who had recently attempted suicide, intensive treatment, mainly with a combination of CBT-SP and antidepressant medication, was associated with rates of improvement and remission of depression that appear comparable to those observed in non-suicidal depressed adolescents treated with combined medication and CBT, thus supporting the broad relevance of clinical trials conducted in depressed adolescents and the inclusion of this high risk population in future clinical trials.
Acknowledgments
Funded by the National Institute of Mental Health through cooperative agreement grants MH66750 (PI: K. Wells, Duke University Medical Center), MH66769 (PI; J. Walkup, Johns Hopkins University), MH66762 (PI: L Greenhill, New York State Psychiatric Institute), MH66775 (PI: D. Brent, University of Pittsburgh), and MH66778 (PI: G. Emslie, University of Texas Southwestern Medical Center). Data management was funded by an NIMH contract to KAI Research, Inc. Rockville, Maryland.
The authors would like to acknowledge Brian Knizner (project coordinator), Marcie McCullough (data entry), Travis Schermer (interviewer), Angela Aloise (interviewer), Kim Poling (therapy supervisor, therapist), Nancy Tormey (therapist), Brian McKain (pharmacotherapist and therapist), Sue Wesner (pharmacotherapist), Allen Chrisman (psychiatrist, pharmacotherapy supervisor), Rameshari Tumuluru (psychiatrist), John Campo (psychiatrist), Joanne Severe (operations), and the NIMH Data and Safety Monitoring Board.
Disclosure: Dr. Greenhill was a consultant to Pfizer and served as chairman of DSMB for pediatric ziprasidone trials; received research grants from NIMH, Otsuka/Bristol Myers-Squib, Johnson & Johnson.
Dr. Emslie received research support Biobehavioral Diagnostics Inc., Forest Laboratories, Shire, and Somerset; has been a consultant for Biobehavioral Diagnostics Inc., Eli Lilly, Forest Laboratories Inc, Pfizer Inc., Shire, Validus Pharmaceuticals, and Wyeth Pharmaceuticals.
Dr. Bukstein has consulted for Quintiles CME, has served on the speakers' bureau of Quintiles CME, has received research and/or education funding from Shire Pharmaceuticals, Ortho-McNeil-Janssen, and Quintiles CMS, and has received book royalties from Routledge.
Dr. Walkup receives research support from the NIH, SAMHSA, and the Tourette Syndrome Association. He received free medication and placebo from Eli Lilly, Pfizer and free medication from Abbott for NIMH funded clinical trials. He has received honoraria from Eli Lilly in 2006 and from Pfizer in 2003 and 2005 and from the Tourette Syndrome Association in 2007-8. He also provided consultation to defense council on behalf of GlaxoSmithKline (Paxil) in 2007.
Dr. Coffey has received research support from NIMH, Boehringer Ingelheim, Bristol Myers Squibb, Novartis, and Tourette Syndrome Association, and has been advisor to Novartis and Jazz Pharmaceuticals.
Dr. Posner has received only research support from the following pharmaceutical companies, as part of an effort to help execute the FDA suicidality mandates/requests: Amgen, Astra Zeneca Pharmaceuticals, Forest Laboratories, GlaxoSmithKline, i3 Research, Eli Lilly, Johnson & Johnson, H. Lundbeck A/S, Medtronic, Merck & Co., Inc., Next Wave Pharmaceuticals, Novo Nordisk A/S, Orexigen Therapeutics, Otsuka Pharmaceuticals, Pfizer, Roche, Sanofi-Aventis, Schering-Plough Corporation, Schwarz Biosciences, Inc./UCB, Sepracor, Inc., Takeda Pharmaceutical Company, Valeant Pharmaceuticals, Vivus, Inc., and Wyeth Research.
Dr. March receives research support from NIMH and NARSAD; is a consultant or scientific advisor to Pfizer, Lilly, Wyeth, GSK, and MedAvante; receives research support from Pfizer and Lilly; has equity holdings in MedAvante; receives study drug for NIMH-funded studies from Lilly and Pfizer.
Dr. Riddle receives research funding from NIMH, has served as DSMB member for NICHD and Johnson & Johnson, has been a consultant/scientific advisor to Shire and Jazz Pharmaceuticals.
Dr. Wagner held stock in Johnson & Johnson, within NIH limit, and has since divested.
Dr. Curry receives speaking fees from the REACH Institute.
Dr. Wells receives speaking fees from the REACH Institute.
Footnotes
This article was reviewed under and accepted by Ad Hoc Editor Garry Walter, MD, PhD.
The opinions and assertions contained in this report are the private views of the authors, and are not to be construed as official or as reflecting the views of the National Institute of Mental Health, the National Institutes of Health, or the Department of Health and Human Services.
This article is the subject of an editorial by Dr. Garry Walter in this issue.
Clinical Trials Registry: Treatment of Adolescent Suicide Attempters (TASA); http://clinicaltrials.gov; NCT00080158
Disclosure: The other authors report no conflicts of interest.
References
- 1.Shaffer D, Gould MS, Fisher P, Trautman P, Moreau D, Kleinman M, Flory M. Psychiatric diagnosis in child and adolescent suicide. Arch Gen Psychiatry. 1996;53:339–348. doi: 10.1001/archpsyc.1996.01830040075012. [DOI] [PubMed] [Google Scholar]
- 2.Bridge JA, Goldstein TR, Brent DA. Adolescent suicide and suicidal behavior. J Child Psychol Psychiatry. 2006;47:372–394. doi: 10.1111/j.1469-7610.2006.01615.x. [DOI] [PubMed] [Google Scholar]
- 3.Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297:1683–1696. doi: 10.1001/jama.297.15.1683. [DOI] [PubMed] [Google Scholar]
- 4.Weisz JR, McCarthy CA, Valeri SM. Effects of psychotherapy for depression in children and adolescents: a meta-analysis. Psychol Bull. 2006;132:132–149. doi: 10.1037/0033-2909.132.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.TADS Team Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression. JAMA. 2004;292:807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- 6.TADS Team The Treatment for Adolescents with Depression Study (TADS): long-term effectiveness and safety outcomes. Arch Gen Psychiatry. 2007;64:1132–1144. doi: 10.1001/archpsyc.64.10.1132. [DOI] [PubMed] [Google Scholar]
- 7.Brent D, Emslie G, Clarke G, et al. The Treatment of Adolescents with SSRI-Resistant Depression (TORDIA): a comparison of switch to venlafaxine or to another SSRI, with or without additional cognitive behavioral therapy. JAMA. 2008;299:901–913. doi: 10.1001/jama.299.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mann JJ, Apter A, Bertolote J, et al. Suicide prevention strategies: a systematic review. JAMA. 2005 Oct 26;294:2064–2074. doi: 10.1001/jama.294.16.2064. [DOI] [PubMed] [Google Scholar]
- 9.Brent D, Greenhill L, Compton S, et al. The Treatment of Adolescent Suicide Attempters (TASA): Predictors of suicidal events in an open treatment. doi: 10.1097/CHI.0b013e3181b5dbe4. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanley B, Brown G, Brent D, et al. Cognitive Behavior Therapy for Suicide Prevention (CBT-SP): treatment model, feasibility, and acceptability. doi: 10.1097/CHI.0b013e3181b5dbfe. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 12.Poznanski EO, Mokros HB. Manual for the Children's Depression Rating Scale-Revised, Manual. Western Psychological Services; Los Angeles, LA: 1996. [Google Scholar]
- 13.Hughes CW, Emslie GJ, Crismon ML, et al. Update from Texas Consensus Conference Panel on Medication Treatment of Childhood Major Depressive Disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:667–686. doi: 10.1097/chi.0b013e31804a859b. [DOI] [PubMed] [Google Scholar]
- 14.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 15.Jain S, Carmody T, Trivedi MH, et al. A psychometric evaluation of the CDRS and MADRS in assessing depressive symptoms in children. J Am Acad Child Adolesc Psychiatry. 2007;46:1204–1212. doi: 10.1097/chi.0b013e3180cc2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 17.Beck A, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicidal Ideation. J Cons Clin Psychol. 1979;47:343–352. doi: 10.1037//0022-006x.47.2.343. [DOI] [PubMed] [Google Scholar]
- 18.March JS, Parker JD, Sullivan K, Stallings P, Conners CK. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- 19.Guy W. ECDEU Assessment Manual for the Psychopharmacology. 2nd ed. Dept. of Health and Human Services; Washington, DC: 1976. pp. 91–338. Publication No. (ADM) [Google Scholar]
- 20.Shaffer D, Gould MS, Brasic J, et al. A children's global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 21.Weissman M, Orvschel H, Padian N. Children's symptoms and social functioning self-report scales: comparisons of mothers's and children's reports. J Nerv Ment Dis. 1980;168:736–740. doi: 10.1097/00005053-198012000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Goodyer I, Dubicka B, Wilkinson P, et al. Selective serotonin reuptake inhibitors (SSRIs) and routine specialist care with and without cognitive behaviour therapy in adolescents with major depression: randomized controlled trial. BMJ. 2007;335:142. doi: 10.1136/bmj.39224.494340.55. Epub doi: 10.1136/bmj.39224.494340.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennard BD, Silva S, Vitiello B, et al. Remission and residual symptoms after acute treatment of adolescents with major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2006;45:1404–1411. doi: 10.1097/01.chi.0000242228.75516.21. [DOI] [PubMed] [Google Scholar]
- 24.Kennard B, Silva SG, Tonev S, et al. Remission and recovery in the Treatment for Adolescents with Depression Study (TADS): acute and long-term outcomes. J Am Acad Child Adolesc Psychiatry. 2009 doi: 10.1097/CHI.0b013e31819176f9. In press, March issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood A, Trainor G, Rothwell J, Moore A, Harrington R. Randomized trial of group therapy for repeated deliberate self-harm in adolescents. J Am Acad Child Adolesc Psychiatry. 2001;40:1246–1253. doi: 10.1097/00004583-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]


