Abstract
The generation of amyloid β-peptide (Aβ) by enzymatic cleavages of the β-amyloid precursor protein (APP) has been at the center of Alzheimer’s disease (AD) research. While the basic process of β- and γ-secretase-mediated generation of Aβ is text book knowledge, new aspects of Aβ and other cleavage products have emerged in recent years. Also our understanding of the enzymes involved in APP proteolysis has increased dramatically. All of these discoveries contribute to a more complete understanding of APP processing and the physiological and pathological roles of its secreted and intracellular protein products. Understanding APP processing is important for any therapeutic strategy aimed at reducing Aβ levels in AD. In this review we provide a concise description of the current state of understanding the enzymes involved in APP processing, the cleavage products generated by different processing patterns, and the potential functions of those cleavage products.
Keywords: Amyloid beta, α-secretase, β-secretase, γ-secretase, APP, AICD
Introduction
Excess amyloid β-peptide (Aβ) is believed to be a main contributor to the dysfunction and degeneration of neurons that occurs in Alzheimer’s disease (AD) (see Thinakaran and Koo, 2008 for review). Aβ is a 38 to 43 amino acid peptide that is derived from the β-amyloid precursor protein (APP) through sequential cleavages by β- and γ-secretase enzyme activities. The cleavage site for another APP processing enzyme, α-secretase lies within the Aβ sequence and thus precludes Aβ formation. The amino terminal fragment generated through α- or β-secretase is called secreted APP (sAPP) α or β, respectively. The carboxyterminal fragments (CTF) generated by α- and β-secretase are called CTF83 and CTF99, respectively. γ-Secretase cleavage of CTF83 and CTF99 will result in the generation of p3 and Aβ, respectively, as well as the amino-terminal APP intracellular domain (AICD) (Fig.1). α-Secretase activity is mediated by one or more enzymes from the family of disintegrin and metalloproteinase domain proteins (ADAM), with ADAM 9, 10, 17 and 19 being the most likely candidates (Fahrenholz et al., 2000; Asai et al., 2003; Tanabe et al., 2006). Beta-site APP cleaving enzyme 1 (BACE1) is the major β-secretase in the brain (Vassar et al., 1999). A multi-protein complex constitutes γ–secretase activity. Four proteins are minimally required for this complex: presenilin (PS) 1 or 2, nicastrin (Nct), presenilin enhancer 2 (Pen2) and anterior pharynx defective 1 (Aph-1) (Wolfe, 2008).
Figure 1.
APP processing and cleavage products. The non-amyloidogenic APP processing pathway (right/blue) involves cleavages by α- and γ-secretases resulting in the generation of a long secreted form of APP (sAPPα) and C-terminal fragments (CTF 83, p3 and AICD50). The amyloidogenic APP processing pathway (left/red) involves cleavages by β- and γ-secretases resulting in the generation of a long secreted form of APP (sAPPβ), C-terminal fragments (CTF 99 and CTF 89) and Aβs. Aβ fragments oligomerize and fibrillize leading to AD pathology (left and upper panel). ex extracellular, PM plasma membrane, cyt cytosol, DR6 death receptor 6.
Aβ is at the center of AD drug development research, and from this short introduction it is clear that at least paths can be followed to reduce Aβ production, namely inhibit β- or γ-secretase, or enhance α-secretase activity. The picture becomes more complex when subcellular localization of enzyme activities and amino acid modifications are considered, offering additional strategies for an Aβ-oriented therapy. On the other hand, over the recent years more functions of the other APP cleavage products have emerged as well as more substrates of APP-cleaving enzymes. It must be recognized therefore, that any intervention in APP processing is likely to affect more than just Aβ production. In this review we will have a look at the current state of knowledge of APP processing enzyme activities and the functions of various APP fragments. Interesting new findings have emerged recently, and it is therefore likely that more functions of APP fragments and APP processing enzymes will be discovered in the near future.
sAPPα
sAPPα is generated when α-secretase cleaves APP, a process also called shedding. The physiological functions of sAPPα are poorly understood, but its actions have been generally considered beneficial to neurons. Early in vitro studies have demonstrated that sAPPα protects cultured neurons against oxygen-glucose deprivation and excitotoxicity by inhibiting calcium currents and increasing potassium currents and thus stabilizing the resting membrane potential (Mattson et al., 1993; Furukawa et al., 1996). sAPPα also promotes neurite outgrowth, synaptogenesis and cell adhesion (Mattson 1997; Gakhar-Koppole et al., 2008). The biologically relevant site for these actions was located to the carboxy-terminus of sAPPα, spanning the region from just amino-terminal to the β-secretase cleavage site to the carboxy-terminal end with a heparin binding motif at the carboxy-terminus being of critical importance (Furukawa et al. 1996). The amino terminal end of sAPPα was not required for these effects. In vivo studies reported that sAPPα, when administered intracerebroventricularly, enhances learning and memory in mice and rats (Meziane et al., 1998; Taylor et al., 2008). Interestingly the latter study reported that this effect was accompanied by increased long term potentiation (LTP) and enhanced N-methyl D-aspartate (NMDA) receptor mediated currents, which is difficult to reconcile with the in vitro observations of increased potassium currents and reduced cytosolic calcium concentrations. However, Ishida et al. (1997) had reported that when applied to hippocampal slices recombinant sAPPα enhanced LTP and shifted the frequency dependence for induction of long term depression (LTD), apparently by increasing cyclic GMP production. sAPPα also has been shown to act as a growth factor for cells of epidermal origin (Herzog et al., 2004; Siemes et al., 2006) to induce increased proliferation rates of embryonic and adult neural stem cells (Ohsawa et al., 1999; Caille et al., 2004) as well as epidermal basal cells (Hoffmann et al., 2000) and increased migration rates in keratinocytes (Kirfel at al., 2002). sAPPα is able to reverse most of the deficits seen in APP knockout mice (Ring et al., 2007). We conclude that sAPPα likely has growth promoting functions in dividing cells of epithelial origin, including embryonic and adult neural stem cells and plays a role in brain development. In differentiated neurons sAPPα may have prosurvival functions, enhance neurite outgrowth and improve memory formation, although the exact mechanisms for these actions are not yet fully understood.
While sAPPα concentrations in cerebrospinal fluid (CSF) are reduced in carriers of the Swedish APP mutation (APP 670/671) (Lannfelt et al., 1995), in cases of sporadic AD they seem to be unaltered (Sennvik et al., 2000; Olsson et al., 2003) or increased (Lewczuk et al., 2008). The Dutch and Flemish mutations in the APP gene (APP E693Q and APP A692G, respectively) are likely to (negatively) affect the α-secretase cleavage site. Interestingly, families with these mutations display a somewhat different clinical picture, with congophilic amyloid angiopathy and in the case of the Flemish mutations also AD-like dementia and neuropathology. In these cases the altered Aβ sequence and reduced α-secretase cleavage of APP both may contribute to the clinical picture.
α-Secretase
α-Secretase activity is generally attributed to the ADAM family of proteases. Among this family ADAM 9, 10, 17 and 19 have been shown to exert α-secretase activity (Fahrenholz et al., 2000; Asai et al., 2003; Tanabe et al., 2006). At the moment it is unclear which of these is the relevant protease in AD patients. However, in a mouse model of AD ADAM 10 overexpression has been shown to reduce Aβ production and plaque deposition in addition to lessening cognitive deficits (Postina et al., 2004). Therefore ADAM 10 at the moment is a good candidate to be a relevant α-secretase in AD pathogenesis. However, given the existing redundancy of ADAMs, it is quite possible that more than one ADAM is involved in sAPPα generation. α-secretase activity has a constitutive and an inducible component that depends on its subcellular localization. α-secretase at the plasma membrane surface is considered constitutively active and it is believed to be the main proteolytic pathway for APP that reaches the plasma membrane (de Strooper and Annaert, 2000). On the other hand, α-secretase that resides in the trans-Golgi network is regulated by PKC and competes with β-secretase in the same location (Skovronsky et al., 2000). It is not clear which specific ADAM protein is associated with constitutive and inducible α-secretase activity, but ADAM 10 can be activated by calcium (Le Gall et al., 2009). Neuronal depolarization will lead to PKC activation and increased vesicle and membrane turnover and, accordingly, neuronal activity was shown to increase α-secretase activity in an NMDA receptor-dependent and calcium-sensitive fashion in mature neurons and neuron precursor cells (Gakhar-Koppole et al., 2008; Hoey et al., 2009). However, the opposite (i.e. reduced α-secretase activity) upon neuronal depolarization has been reported as well (Lesne et al., 2005). APP is only one of many substrates for ADAM family proteins; others include N-cadherin (Kohutek et al., 2009), EGFR ligands, TNF-α, TGF-α, notch and ephrin (Edwards et al., 2008; Le Gall et al., 2009). While a dominant negative mutant of ADAM 10 caused increased sensitivity to kainate-induced epileptic seizures in mice overexpressing the APP V717I (London) mutation, it reduced sensitivity to seizures in wild type mice. Similarly surprising results were obtained with overexpression of wild type ADAM 10, which was associated with an increased rate of seizures in both wild type and APP V717I mice (Clement et al., 2008). One possible interpretation is that in a context of increased Aβ production, reducing sAPPα (and possibly further increasing Aβ) is the dominant biological effect of dominant negative α-secretase, whereas other effects of α-secretase (i.e. cleavage of proteins other than APP) become dominant, when the dominant negative mutant is expressed in a wild type context, or when α-secretase is overexpressed. If this interpretation is correct, then it has to be assumed that one or more of α-secretase’s cleavage products has the potential to facilitate seizures, but Aβ has a greater potential to do so.
P3
No clear biological role has been established for the P3 fragment that is generated by α- and γ-secretase cleavage of APP.
sAPPβ
sAPPβ is the secreted amino-terminal fragment that is generated when β-secretase cleaves APP. sAPPβ lacks most of the neuroprotective effects that have been associated with sAPPα (Furukawa et al., 1996). A recent report demonstrated that sAPPβ is critically involved in pruning of synapses during development of both central and peripheral neurons (Nikolaev et al., 2009). In this study sAPPβ was found to act as a ligand for the death receptor 6 (DR6) (a member of the TNFα family), which, upon binding, leads to cleavage and activation of caspase 6 but not caspase 3. This caspase 6 activation was associated with disintegration of axons but not cell somata. Importantly, sAPPβ generation was induced by trophic factor withdrawal, and sAPPβ could also bind to p75 neurotrophin receptor (p75NTR), albeit with lower sensitivity and no clear biological relevance in the context that was studied. Other functions of sAPPβ may include suppression of neuronal stem cell differentiation in favor of glial differentiation (Kwak et al., 2006), but these actions are not well characterized.
β-Secretase
β-Secretase activity is mediated by BACE1 and cleaves APP to release sAPPβ and produce CTF99. BACE1 can also cleave APP at a more carboxy-terminal position, resulting in CTF89 (and Aβ 11–40 after γ-secretase cleavage) (Fig.2). A related protein, BACE2, also can exert β-secretase activity (Hussain et al., 2000), but it is expressed at very low levels in the brain and is mostly confined to glial cells (Laird et al., 2005); interestingly, BACE2 is a better α- than β-secretase (Farzan et al., 2000). BACE2 appears irrelevant to AD pathogenesis and will not be considered in this review. Genetic ablation of BACE1 completely prevents the amyloid pathology in a mouse model expressing the Swedish mutation of APP (APP670/671) together with mutated PS1 (Farzan et al., 2000; Laird et al., 2005) or in mice expressing only the Swedish APP mutation (Tg2576) (Ohno et al., 2004). BACE1-deficient mice lack β-secretase activity and Aβ formation in neurons (Cai et al. 2000; Roberds et al. 2001; Luo et al. 2001. Also, BACE1 expression and activity is increased in brains of AD patients (Holsinger et al. 2002; Yang et al. 2003).These studies provide the rationale for the current view that BACE1 is the physiologically relevant β-secretase in the brain that contributes to AD pathology. At the end of this section, however, we will also have a look at cathepsins as enzymes with potential β-secretase activity.
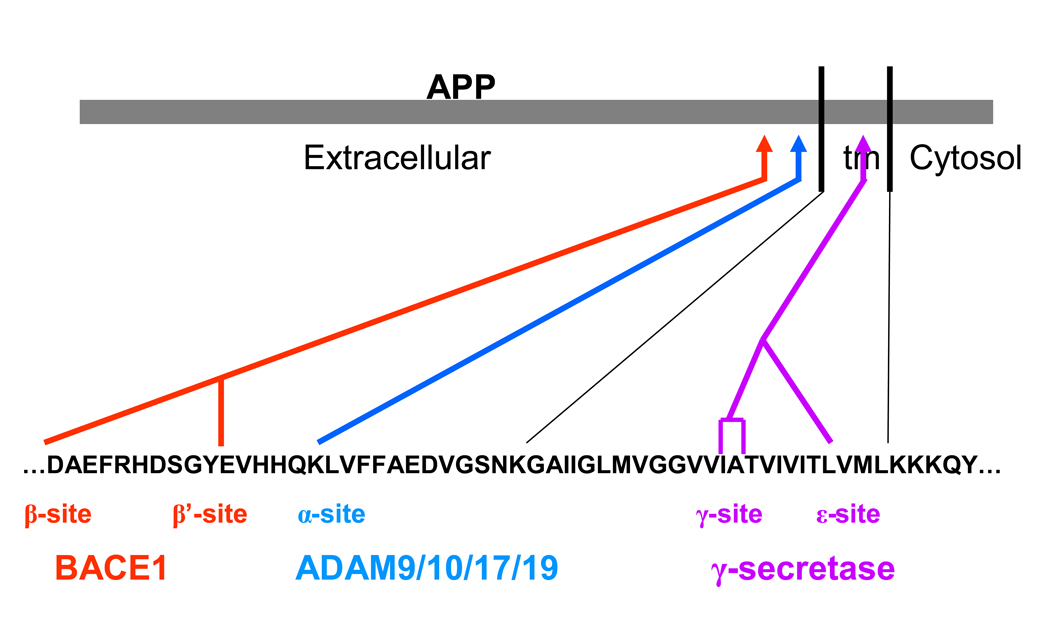
Figure 2.
APP processing enzymes and their cleavage sites. The amino acid sequence of Aβ and the carboxyterminal adjacent region are displayed in single letter amino acid code. Red lines indicate the β and β’ cleavage site of BACE1. The blue line indicate the cleavage site for α-secretase, which belongs to the ADAM family of proteases. Purple lines indicate cleavage site for γ-secretase, which is located within the transmembrane region, as indicated by the black lines.
BACE1 expression is increased in situations of cellular stress, e.g. energy deprivation, hypoxia and ischemia (O’Connor et al., 2008; Guglielmotto et al., 2009), and oxidative stress has been shown to increase BACE1 expression in a γ-secretase dependent fashion (Jo et al., 2008). BACE1 is synthesized as a proenzyme in the endoplasmic reticulum (that nonetheless possesses β-secretase activity). Homodimerization, cleavage of the prodomain in the trans-Golgi network by furin or furin-like proteases and complex glycosylation are further steps toward the fully active enzyme (Westmeyer et al., 2004; Cole and Vassar 2008; Hunt and Turner 2009). BACE1 can be further cleaved to be released from the plasma membrane (shedding). Shedding of BACE1 likely depends on proteases of the ADAM family which appears to be an interesting connection between α-secretase and BACE1. The function and relevance of BACE1 shedding remain unclear at the moment. Other posttranslational modifications occur and have an impact on BACE1 activity (see Cole and Vassar 2008 and Hunt and Turner, 2009 for review). The subcellular localization of BACE1 is predominantly within the trans-Golgi network and endosomal compartment (Huse et al., 2002). Although BACE1 reaches the plasma membrane due to vesicle traffic, it is recycled quickly, and very little BACE1-mediated APP cleavage occurs at the plasma membrane; instead APP is cleaved by BACE primarily in endocytic vesicles. Interestingly, within the secretory pathways of the endoplasmic reticulum and trans-Golgi network, APP and BACE1 are located in separate membrane microdomains, due to APP binding to X11/Munc18 proteins (Saito, 2008). Neuronal depolarization leads to Munc18 phosphorylation and causes APP to relocalize in BACE1-containing membrane microdomains (Sakurai 2008). Accordingly, neuronal depolarization and vesicle turnover have been shown to increase BACE1-mediated APP cleavage and Aβ production (Kamenetz et al., 2003). Thus, increased neuronal depolarization increases APP cleavage by both α-secretase and BACE1 in a competitive fashion (Kamenetz et al., 2003). It will therefore be of interest to determine if neuronal activity can be fine tuned in order to increase one activity over the other (Hoey et al., 2009). BACE1 is located in cholesterol rich lipid rafts (Abad-Rodriguez et al., 2004), but whether lowering brain cholesterol levels in AD patients will actually reduce Aβ generation and improve cognitive function remains to be seen (Abad-Rodriguez et al., 2004; Cheng et al., 2007; Vetrivel et al., 2009).
BACE1 has many other substrates apart from APP. They include APP-like proteins 1 and 2 (APLP1 and 2), voltage-gated sodium channel β2-subunit, low density lipoprotein receptor related protein, neuregulin 1, Pselectin glycoprotein ligand-1 and sialyltransferase ST6Gal I (Hunt and Turner 2009). BACE1 knockout (BACE1−/−) mice show some interesting phenotypes. They show a rather severe hypo-myelination of peripheral nerves (Willem et al., 2006), but only modest hypo-myelination in the CNS (Hu et al., 2006). They also have an increased rate of seizures that is even higher if BACE1−/− mice are crossed with PDAPP mice (Kobayashi et al., 2008). BACE1 genetic ablation also causes a reduction in spine density in hippocampal pyramidal neurons and alterations in behavior tests that are believed to reveal schizophrenia-like phenotypes, including reduced pre-pulse inhibition, novelty-induced hyperactivity, hypersensitivity to the NMDA receptor antagonist MK-801, as well as impairments in cognition and social recognition (Savonenko et al., 2008). It is possible that these effects are mediated through impaired processing of non-APP substrates, with neuregulin 1 being a very likely candidate. It is interesting to note, however, that the (modest) deficit that BACE1−/− mice display in the Morris water maze test was not seen when BACE1−/− mice were crossed with APPswe/PS1ΔE9 transgenic mice, while the deficits in social recognition remained unaltered (Laird et al., 2005). This leaves open the possibility that increased APP expression and or processing through α- and γ-secretase can compensate for some of the deficits of BACE1 deficiency.
The view that BACE1 is the only relevant β-secretase in AD has been challenged recently as other proteases, specifically cathepsins, have been shown to exert β-secretase activity (Hook et al., 2005; Hook et al., 2007a,b; Schechter and Ziv 2008; Hook et al., 2008, Böhme et al., 2008; Klein et al., 2009). A possible explanation for these findings and the seemingly discrepant results of Cai et al. (2000), Roberds et al. (2001) and Luo et al., (2001) is that cathepsins cleave wildtype (wt) human APP but not human APP that is mutated at the β-secretase cleavage site (mtAPP) as is the case in transgenic AD models that use an APP form containing the Swedish mutation (K670N/M671L). Accordingly, genetic ablation of cathepsin B has been reported to reduce Aβ 1–40 and Aβ 1–42 levels in the brains of mice that overexpress human wtAPP but not mtAPP with the Swedish and Indiana (V717F at the γ-secretase cleavage site) mutation (Hook et al. 2009). The finding that overexpression of BACE1 does not result in increased levels of endogenous (murine) Aβ may point to a similar direction (Hirata-Fukae et al. 2008). Also, the conversion of the aspartate residue at the β-secretase cleavage site to isoaspartate by a non-enzymatic reaction has been reported to be a frequent event resulting in loss of BACE1 but not cathepsin B activity at the β-secretase site (Böhme et al. 2008). However, the picture becomes more complex if it is considered that cathepsin B can also degrade Aβ 1–42 and genetic ablation of cathepsin B has been reported to lead to increased plaque load and Aβ 1–42 levels in mice with the Swedish and Indiana mutation of APP (Mueller-Steiner et al. 2006). Accordingly genetic ablation of the cathepsin B inhibitor cystatin C leads to reduced plaque load and Aβ 1–42 levels in the same mice (Sun et al., 2008). Thus the role of cathepsin B in AD needs to be further investigated as it may contribute to both Aβ generation and degradation. The seemingly conflicting results of some of these studies are a reminder that mouse models only imperfectly replicate the human disease and it is important to realize that unlike all mouse models the majority of sporadic AD cases neither contain mutations in the APP nor Presenilin genes. It is therefore important to further investigate potential differences in wtAPP versus mtAPP processing.
Aβ
Aβ is at the center of AD pathogenesis. It is generated through sequential cleavage of APP by β- and γ-secretases. The resulting peptide is predominantly 40 amino acids long but peptide length can range from 38 to 43 amino acids. Certain forms (e.g. Aβ 1–42, Aβ 3–40) are considered more amyloidogenic then others (e.g. Aβ1–40 or 1–38). Since both BACE1 and γ-secretase activity are located in the endosomal compartment and trans-Golgi network it is believed that most Aβ is generated in these subcellular localizations and subsequently secreted through exocytosis. Intracellular Aβ has been observed as well, both in animal models of AD and human patients (Wirths et al., 2001; Gómez-Ramos and Asunción Morán, 2007), the significance of which remains rather uncertain (Wegiel et al., 2007). Aβ can be cleaved by proteases such as insulin degrading enzyme (Kurochkin and Goto, 1994), neprilysin (Iwata et al., 2000), BACE1 (Huse et al., 2002) and cathepsin B (only Aβ 1–42) (Mueller-Steiner et al. 2006). While insulin degrading enzyme, neprilysin and cathepsin B render Aβ non-amyloidogenic, the significance of Aβ 11–40, which is generated by BACE1 cleavage of Aβ at the β’ site, has not yet been established. Other modifications include pyroglutamate formation of the amino-terminal glutamic acid residue through glutaminyl cyclase resulting in amino-terminal truncated pyroglutmate Aβ 3–40/42, which is highly amyloidogenic; inhibition of glutaminyl cyclase improves amyloidosis and cognition in mouse models of AD (Schilling et al., 2008). Conversion of aspartate to isoaspartate at residue 23 in the Aβ sequence also has been reported to increase Aβ aggregation (Shimizu et al. 2002). Aβ production is either increased or the ratio of Aβ42 to Aβ40 is increased in most hereditary cases and animal models of AD. In AD patients Aβ levels in the CSF are not increased, rather Aβ42 reduction in CSF is a good biomarker for AD (Shaw et al., 2009). This reduction in Aβ42 likely reflects reduced clearance of Aβ through the CSF and increased accumulation in amyloid plaques.
The currently predominant hypothesis states that Aβ forms soluble oligomers in the extracellular space and that these oligomers inhibit NMDA-mediated synaptic transmission and ultimately cause spine and synapse loss through mechanisms not yet fully understood (Selkoe 2000). In a study using extracts from brains of AD patients and hippocampal slice cultures Aβ dimers were shown to be the most potent form of Aβ oligomers to inhibit NMDA mediated synaptic transmission and higher molecular weight oligomers and insoluble aggregates were able to release Aβ dimers (Shankar et al., 2008). Most studies of Aβ toxicity have been performed with synthetic peptides of various lengths (e.g. 1–40, 1–42, 25–35). Generally caution must be exercised when translating such results to in vivo situations as oligomerization status and post translational modifications of endogenous Aβ are different than synthetic peptides. Notably, concentrations required to elicit Aβ toxicity in vitro with synthetic peptides are in the 5 to 10 µM range, which is higher than Aβ concentrations likely encountered by neurons in vivo. Also, in human primary neuron cultures 10 µM Aβ 1–38 and 25–35 are alone not toxic, but a pre-incubation for 3 days leads to increased sensitivity to glutamate toxicity (Mattson et al., 1992). One interesting interaction that has been reported recently is between Aβ and cellular prion protein (PrP) (Lauren et al., 2009). In this study Aβ oligomers of ~100 molecules would exert their inhibitory effect on NMDA-mediated synaptic transmission only when they could bind to the cellular from of PrP. In PrP deficient mice this interaction was absent and Aβ peptides were not inhibitory or toxic. Other interactions between APP or Aβ with PrP, and reciprocal modulation of AD or scrapie disease progression in mouse have been described as well (Baier et al., 2008, Tamguney et al., 2008); moreover, cellular PrP was shown to inhibit BACE1 mediated Aβ production (Parkin et al., 2007).
CTF83 and CTF99
No biologically relevant roles are currently established for the carboxyterminal fragments CTF83 and CTF99 generated by α- and β-secretase, respectively. A more carboxyterminal cleavage of APP by β-secretase cleavage results in a CTF89, the function of which also is unclear.
AICD
AICD is generated when γ-secretase cleaves CTF83 or CTF99. Although γ-secretase cleavage of APP to release Aβ results in an AICD fragment of 57 or 59 amino acids, another cleavage site (ε-cleavage site), just a few amino acids towards the carboxy-terminal end, will result in a 50 amino acid AICD fragment (Fig. 2). This is mostly considered the typical AICD, but more cleavage sites for γ-secretase and also for caspase 3 have been reported, resulting in even shorter peptides (e.g. AICD 31 after caspase 3 cleavage). Regardless of the cleavage site, AICD of various lengths still contains the consensus motif YENPTY that is thought to be crucial for AICD binding to adapter proteins such as Fe65 and subsequent biological actions. The term AICD has been coined by analogy to protease cleavage of the protein Notch, another γ-secretase substrate, which results in release of the Notch intracellular domain (NICD). γ-secretase mediated cleavage of Notch is a critical step in the Notch signaling cascade and this similarity has fostered much research into AICD. The AICD peptide can undergo more posttranslational modifications, including phosphorylation (see Müller et al., 2008 for review). The prototypical signaling pathway for AICD includes binding to the protein Fe65, then recruitment of the histone deacytelase TIP60, nuclear translocation and transcriptional activation of target genes such as p53, GSK3β, neprilysin, EGFR and others. Apart from this pathway many other AICD binding proteins and effects have been described. However, these pathways have been studied mainly in cell lines, sometimes in primary neurons, but hardly in vivo in the brain, making any extrapolation of existing results into the context of AD brains very speculative. Moreover, not all research groups can confirm even the prototypical pathway of AICD binding to Fe65 and nuclear translocation with target gene transcription (Waldron et al., 2008), leaving a fair amount of controversy in the debate. Transgenic mice that overexpressed both AICD and Fe65 have abnormal EEG recordings and increased susceptibility for seizures, features also seen in mice overexpressing APP with the Swedish mutation but not in mice with the Swedish and Indiana mutation as well as a disruption of the caspase cleavage site in the AICD sequence (D664A) (Vogt et al. 2009). Overexpressing AICD with Fe65 also leads to hyperphosphorylation of the microtubule associated protein tau and to neuronal cell loss in animals 18 months and older (Ghosal et al. 2009). Importantly transgenic mice overexpressing only Fe65 did not show any of these features. However since no data are available on the effect of AICD overexpression only we feel that as of now the role of AICD in AD pathogenesis remains to be firmly establishedt and future experiments using human APP constructs with mutations in the ε-cleavage site or the YENPTY domain should provide a clearer picture. In an elegant approach, wtAPP lacking the YENPTY domain was reintroduced into APP knockout mice (Ring et al., 2007). As a result Aβ generation was quite drastically reduced compared with wild type mice, probably due to the increased cell surface expression of APP and reduced entry into the endocytic pathway, pointing to a role for AICD in APP trafficking. The results indicate that a clear cut separate role for AICD may be difficult to establish as the domain may serve multiple roles in full length APP and after cleavage. This consideration applies of course not just to AICD but to other products of APP cleavage as well.
γ-Secretase
γ-secretase cleaves APP in its intra-membrane region at the γ-cleavage site to generate Aβ 1–40/42 or p3 and AICD59/57, a second cleavage at the ε-cleavage site results in AICD50 (Fig. 2). At least four proteins need to interact for γ-secretase activity to unfold: PS1 or 2, Nct, Aph-1 and Pen2 (Wolfe, 2008). Aph-1 exists in 2 isoforms, Aph-1a and b, encoded by 2 genes in humans; mice have a third isoform Aph-1c. PS1 and 2 have the actual protease activity and in the brain PS1 is the dominant presenilin. The full length PS proteins contain 9 transmembrane regions and are cleaved in the cytoplasmic loop between the 6th and 7th transmembrane region to generate an amino-terminal and carboxy-terminal fragments (Thinakaran et al., 1996). Both fragments are stable and stay in close association in the membrane and represent the active form of PS, whereas full length PS is rapidly degraded. In the current model of γ-secretase activity, both the amino-terminal and carboxy-terminal fragment of PS are required for the actual aspartyl protease activity, whereas Nct is attributed a substrate recognition function. Fully active γ-secretase matures in discrete steps: first Nct and Aph-1 form a complex, then PS binds to this complex and finally Pen2 will complete the complex and may facilitate auto-cleavage of PS. (Li et al., 2009). More proteins interact with γ-secretase and may modulate its function (Winkler et al., 2009). More than 50 γ-secretase substrates have been discovered (Beel and Sanders, 2008; Wakabayashi and de Strooper, 2008), including APP, Notch, the neuregulin binding partner ErbB4, N-cadherin, p75NTR and others. In a study using different knockouts of γ-secretase components heterozygous deletion of PS1, Aph-1a or Nct resulted in a reduction of γ-secretase activity ranging from 25 % (PS1+/−) to 64 % for PS1+/−; Nct+/− mice (Li et al., 2007). In this study a γ-secretase reduction of 30 % or lower (PS1+/− or Aph-1a+/− mice) was not associated with an increased rate of squamous cell carcinomas (that is caused by deficient Notch signaling), while Nct+/− and PS1+/−;Nct+/− mice with 50 % and 64 % reductions in γ-secretase activity, respectively, did have increased carcinogenesis. Interestingly a moderate reduction of γ-secretase activity by 30 % through Aph-1a heterozygous knockout was enough to reduce amyloid pathology in APPswe/PS1ΔE9 mice. A series of studies used a conditional knockout approach of γ-secretase proteins using the Cre recombinase system under a promoter that is expressed only in the postnatal forebrain (Yu et al., 2001; Saura et al., 2005; Tabuchi et al., 2009). From these studies it can be concluded that a complete inactivation of γ-secretase through conditional knockout of Nct (Tabuchi et al., 2009) in a normally developed brain leads to progressive neurodegeneration. Conditional knockout of PS1 does not lead to a progressive neurodegenerative phenotype, possibly because loss of PS1 can be partially compensated for by PS2. Nevertheless, these mice exhibited some cognitive deficits in behavior testing (Yu et al., 2001). Importantly, these deficits were unlikely to reflect a deficit in Notch signaling, as expression of Notch target genes was unaffected. When the conditional PS1 knockout mice were crossed with APP transgenic mice, Aβ production was clearly reduced, but the beneficial effect on memory and learning in behavior tests was statistically significant only in young (3 month) mice, while in older mice (15–17 month) the effect was reduced and not statistically significant (Saura et al., 2005). Another recent report indicates that genetic ablation of Aph-1b and c in mice reduces γ-secretase mediated APP cleavage (specifically the generation of the more amyloidogenic Aβ1–42) but does not affect Notch cleavage (Serneels et al., 2009). Genetic ablation of Aph-1b/c resulted in subtle behavioral abnormalities that were reminiscent of those seen in BACE1−/− mice (Laird et al., 2005, Savonenko et al., 2008) and may reflect a deficit in processing of neuregulin (which is also a BACE1 substrate) or its binding partner ErbB4. However, Aph-1b/c knockout resulted in a complete reversal of cognitive deficits in APP/PS1 transgenic mice together with a reduction in amyloid pathology. The oldest mice used in this study were 11 months old, and at the moment no data are published on whether the beneficial effects of Aph-1b/c knockout can be sustained in older APP/PS1 transgenic animals.
The orphan G-protein coupled receptor 3 (Gpr3) was recently shown to affect γ-secretase assembly and subcellular location (Thathiah et al., 2009). Gpr3, although not an essential part of the γ-secretase complex, facilitated the assembly of the 4 required proteins to the fully active protein complex and targeted γ-secretase preferentially to lipid rafts and the plasma membrane leading to increased Aβ 1–40 and 1–42 production in vitro and in vivo, whereas genetic ablation of Gpr3 led to a reduction of Aβ production in young mice. Notch signaling was not affected by Gpr3 knockout. The effects of Gpr3 expression on cognition and behavior have not been reported yet.
Taken together, good evidence has now been provided that γ-secretase activity exists as separate sub-species with different subunit composition and thus different structures, substrate specificities and binding partners. This provides a good rationale for the development of γ-secretase inhibitors with relative specificity for APP over Notch, and compounds with such a profile have been described already (Yang et al., 2008; Cole et al., 2009). However, results from the conditional knockout studies suggest that reduced γ-secretase activity is associated with detrimental side effects in the brain especially in older animals. It remains to be seen whether these effects reflect a deficit in APP cleavage and processing or are a due to reduced cleavage of other substrates (e.g. neuregulin or ErbB4) and how the human brain is affected by γ-secretase inhibition.
PS1 also has a role in endoplasmic reticulum-mediated calcium release. At the moment there is still some controversy about the exact mechanism, as PS1 may act as a calcium leak channel or it may enhance the opening of the inositol-3-phosphate receptor (IP3R); however, it does seem clear that PS1 causes an increase in cytosolic calcium concentrations (Tu et al., 2006, Nelson et al., 2007, Cheung et al., 2008). Interestingly although this function has been reported to be independent of γ-secretase activity, it is also affected by AD associated PS1 mutations, which cause higher cytosolic calcium concentrations when cells are stimulated (Guo et al., 1997; Chan et al., 2000). While γ-secretase activity is not required for PS1 mediated gating of (IP3Rs), IP3Rs gating through PS1 is important for γ-secretase function (Cheung et al., 2008). These data provide a further link between disturbed calcium homeostasis and AD, where increased cytosolic calcium concentrations may be associated with increased sensitivity to excitotoxicity, epileptiform discharges and seizures as well as skewed information processing, as relatively smaller amounts of synaptic input are required to elicit an action potential or calcium dependent signals, e.g. through calcium/calmodulin dependent kinases.
full length APP
The search for a physiological role of full length APP is inseparably linked to the functions of APP cleavage products. Overexpression of human APP (either wt or with the Swedish mutation) in mice is associated with increased size of cortical neurons, whereas overexpression of PS1 is not (Oh et al., 2009). At the moment it is not clear whether this is an effect of full length APP or one its cleavage products. Interestingly, increased size of neuron somata, nuclei and nucleoli in the hippocampus and cortex has also been observed in post mortem brains of humans that show AD like pathology but had no cognitive deficits (Riudavets et al., 2007, Iacono et al., 2008, Iacono et al., 2009).
APP deficient mice show a number of phenotypes including reduced body and brain seize, impaired learning and LTP, reduced grip strength, hypersensitivity to seizures and increased frequency of corpus callosum dysgenesis (Ring et al., 2007). These phenotypes mostly become apparent during early postnatal development, rather than in adult stages. By reintroducing only the sAPPα fragment into APP deficient mice it was demonstrated that lack of sAPPα is responsible for most of the phenotypes observed in APP mice (Ring et al., 2007). While single genetic ablation of APP or APP-like protein (APLP) 1 and 2 results in viable mice, deletion of APP and APLP2, APLP1 and APLP2, or APP and APLPs 1 and 2 results in perinatal lethality suggesting redundancy in some of the physiological functions of these proteins. Therefore it seems that there is an important role for APP in brain development that is in large part mediated by sAPPα and partly overlaps with functions of APLP2. Transient axonal glycoprotein l (TAG1) was recently discovered to act as a binding partner for full length APP (Ma et al., 2008). TAG1 binding to APP induced APP processing (preferentially through α- and γ-secretase), and through genetic manipulations it was shown that the AICD mediated signaling through Fe65 inhibited neuronal differentiation of embryonic stem cells and kept the stem cells in a mode of self renewal.
Conclusion
Great progress has been made in characterizing the molecules involved in APP processing and the functions of APP cleavage products. As a result a complex picture has emerged for the physiological and pathological roles of APP. A clearly defined role for sAPPβ in axon pruning has been demonstrated, and good evidence exists for sAPPα having important roles in brain development and synaptic plasticity, as well as growth factor-like properties. Whether AICD acts as a transcriptional activator remains controversial. Recent data indicate that it may play a role in embryonic neural stem cell development, but also regulate APP trafficking and thus modulate APP processing. Neuronal activity modulates APP processing pathways, suggesting important roles for activity induced APP cleavage products in regulating neuronal excitability and synaptic plasticity. The finding that cathepsin B can act as a β-secretase for wtAPP but not mtAPP is may have great impact on AD research and therefore merits further investigation and corroboration. Aβ remains the main suspect in AD pathogenesis and the molecular mechanisms and binding partners of soluble Aβ toxicity are beginning to be unraveled. Posttranslational modifications of Aβ may contribute to Aβ oligomerization and toxicity, as may the extent to which Aβ is cleared by Aβ-degrading proteases. With regards to APP processing enzymes, it has become very clear that all involved enzymes have multiple substrates and are not specific for APP, and that PS1 has functions beyond γ-secretase. While initially concerns were great that γ-secretase inhibitors inevitably would impair Notch processing, a better understanding of γ-secretase mechanics has helped to make the development of Notch sparing γ-secretase inhibitors seem a very feasible task. However, while Notch appears to become less of a problem to drug development, the sheer quantity of substrates for both β- and γ-secretase is reason for concern. Combining moderate β- and γ-secretase inhibition may be a tangible strategy to reduce potential side effects of secretase inhibition while additively reducing Aβ production. However, since γ-secretase requires a substrate to be cleaved first by a sheddase, β- and γ-secretase may in fact share multiple substrates, neuregulin/ErbB4 and the voltage-gated sodium channel β2-subunit being good examples. A detailed understanding of the molecular aspects of secretase activity may help to generate secretase inhibitors with high specificity for APP. Also, it is important to understand the physiological functions of secretases in the aged human brain to judge the potential for mechanism-based side effects of secretase inhibitors. The major challenge of course remains treating AD patients, who at the time of diagnosis are in an advanced disease stage if compared to AD mouse models. Therefore, improved diagnosis criteria and tools seem indispensable requirements for better efficacy of any anti-amyloid strategy. Considering all challenges that have to be met by anti-amyloid- or secretase-focused therapies, the development of non-amyloid based therapeutic strategies would be a very welcome addition to the portfolio of AD research.
Acknowledgment
This work was in part supported by the Intramural Research Program of the NIH, National Institute on Aging.
References
- Abad-Rodriguez J, Ledesma MD, Craessaerts K, Perga S, Medina M, Delacourte A, Dingwall C, De Strooper B, Dotti CG. Neuronal membrane cholesterol loss enhances amyloid peptide generation. J. Cell Biol. 2004;167:953–960. doi: 10.1083/jcb.200404149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai M, Hattori C, Szabó B, Sasagawa N, Maruyama K, Tanuma S, Ishiura S. Putative function of ADAM9, ADAM10, and ADAM17 as APP alpha-secretase. Biochem. Biophys. Res. Commun. 2003;301:231–235. doi: 10.1016/s0006-291x(02)02999-6. [DOI] [PubMed] [Google Scholar]
- Baier M, Apelt J, Riemer C, Gültner S, Schwarz A, Bamme T, Burwinkel M, Schliebs R. Prion infection of mice transgenic for human APPSwe: increased accumulation of cortical formic acid extractable Abeta(1–42) and rapid scrapie disease development. Int. J. Dev. Neurosci. 2008;26:821–824. doi: 10.1016/j.ijdevneu.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Beel AJ, Sanders CR. Substrate specificity of gamma-secretase and other intramembrane proteases. Cell. Mol. Life Sci. 2008;65:1311–1134. doi: 10.1007/s00018-008-7462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhme L, Hoffmann T, Manhart S, Wolf R, Demuth HU. Isoaspartate-containing amyloid precursor protein-derived peptides alter efficacy and specificity of potential beta-secretases. Biol. Chem. 2008;389:1055–1066. doi: 10.1515/BC.2008.125. [DOI] [PubMed] [Google Scholar]
- Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat. Neurosci. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- Caillé I, Allinquant B, Dupont E, Bouillot C, Langer A, Müller U, Prochiantz A. Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development. 2004;131:2173–2181. doi: 10.1242/dev.01103. [DOI] [PubMed] [Google Scholar]
- Chan SL, Mayne M, Holden CP, Geiger JD, Mattson MP. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J. Biol. Chem. 2000;275:18195–18200. doi: 10.1074/jbc.M000040200. [DOI] [PubMed] [Google Scholar]
- Cheng H, Vetrivel KS, Gong P, Meckler X, Parent A, Thinakaran G. Mechanisms of disease: new therapeutic strategies for Alzheimer's disease--targeting APP processing in lipid rafts. Nat. Clin. Pract. Neurol. 2007;3:374–382. doi: 10.1038/ncpneuro0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KH, Shineman D, Müller M, Cárdenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VM, Foskett JK. Mechanism of Ca2+ disruption in Alzheimer's disease by presenilin regulation of InsP3 receptor channel gating. Neuron. 2008;58:871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement AB, Hanstein R, Schröder A, Nagel H, Endres K, Fahrenholz F, Behl C. Effects of neuron-specific ADAM10 modulation in an in vivo model of acute excitotoxic stress. Neuroscience. 2008;152:459–468. doi: 10.1016/j.neuroscience.2007.10.060. [DOI] [PubMed] [Google Scholar]
- Cole SL, Vassar R. The role of amyloid precursor protein processing by BACE1, the beta-secretase, in Alzheimer disease pathophysiology. J. Biol. Chem. 2008;283:29621–29625. doi: 10.1074/jbc.R800015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DC, Stock JR, Kreft AF, Antane M, Aschmies SH, Atchison KP, Casebier DS, Comery TA, Diamantidis G, Ellingboe JW, Harrison BL, Hu Y, Jin M, Kubrak DM, Lu P, Mann CW, Martone RL, Moore WJ, Oganesian A, Riddell DR, Sonnenberg-Reines J, Sun SC, Wagner E, Wang Z, Woller KR, Xu Z, Zhou H, Jacobsen JS. (S)-N-(5-Chlorothiophene-2-sulfonyl)-beta,beta-diethylalaninol a Notch-1-sparing gamma-secretase inhibitor. Bioorg. Med. Chem. Lett. 2009;19:926–929. doi: 10.1016/j.bmcl.2008.11.116. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W. Proteolytic processing and cell biological functions of the amyloid precursor protein. J Cell Sci. 2000;113:1857–1870. doi: 10.1242/jcs.113.11.1857. [DOI] [PubMed] [Google Scholar]
- Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol. Aspects Med. 2008;29:258–289. doi: 10.1016/j.mam.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenholz F, Gilbert S, Kojro E, Lammich S, Postina R. Alpha-secretase activity of the disintegrin metalloprotease ADAM 10. Influences of domain structure. Ann. N. Y. Acad. Sci. 2000;920:215–222. doi: 10.1111/j.1749-6632.2000.tb06925.x. [DOI] [PubMed] [Google Scholar]
- Farzan M, Schnitzler CE, Vasilieva N, Leung D, Choe H. BACE2, a β-secretase homolog, cleaves at the βsite and within the amyloid-β region of the amyloid-β precursor protein. Proc. Natl. Acad. Sci. USA. 2000;97:9712–9717. doi: 10.1073/pnas.160115697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K, Barger SW, Blalock EM, Mattson MP. Activation of K+ channels and suppression of neuronal activity by secreted beta-amyloid-precursor protein. Nature. 1996;379:74–78. doi: 10.1038/379074a0. [DOI] [PubMed] [Google Scholar]
- Gakhar-Koppole N, Hundeshagen P, Mandl C, Weyer SW, Allinquant B, Müller U, Ciccolini F. Activity requires soluble amyloid precursor protein alpha to promote neurite outgrowth in neural stem cell-derived neurons via activation of the MAPK pathway. Eur. J. Neurosci. 2008;28:871–882. doi: 10.1111/j.1460-9568.2008.06398.x. [DOI] [PubMed] [Google Scholar]
- Ghosal K, Vogt DL, Liang M, Shen Y, Lamb BT, Pimplikar SW. Alzheimer's disease-like pathological features in transgenic mice expressing the APP intracellular domain. Proc. Natl. Acad. Sc.i U. S. A. 2009 doi: 10.1073/pnas.0907652106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Ramos P, Asunción Morán M. Ultrastructural localization of intraneuronal Abeta-peptide in Alzheimer disease brains. J. Alzheimer's Dis. 2007;11:53–59. doi: 10.3233/jad-2007-11109. [DOI] [PubMed] [Google Scholar]
- Guglielmotto M, Aragno M, Autelli R, Giliberto L, Novo E, Colombatto S, Danni O, Parola M, Smith MA, Perry G, Tamagno E, Tabaton M. The up-regulation of BACE1 mediated by hypoxia and ischemic injury: role of oxidative stress and HIF1alpha. J. Neurochem. 2009;108:1045–1056. doi: 10.1111/j.1471-4159.2008.05858.x. [DOI] [PubMed] [Google Scholar]
- Guo Q, Sopher BL, Furukawa K, Pham DG, Robinson N, Martin GM, Mattson MP. Alzheimer's presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: involvement of calcium and oxyradicals. J. Neurosci. 1997;17:4212–4222. doi: 10.1523/JNEUROSCI.17-11-04212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog V, Kirfel G, Siemes C, Schmitz A. Biological roles of APP in the epidermis. Eur. J. Cell Biol. 2004;83:613–624. doi: 10.1078/0171-9335-00401. [DOI] [PubMed] [Google Scholar]
- Hirata-Fukae C, Sidahmed EH, Gooskens TP, Aisen PS, Dewachter I, Devijver H, Van Leuven F, Matsuoka Y. Beta-site amyloid precursor protein-cleaving enzyme-1 (BACE1)-mediated changes of endogenous amyloid beta in wild-type and transgenic mice in vivo. Neurosci. Lett. 2008;435:186–189. doi: 10.1016/j.neulet.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoey SE, Williams RJ, Perkinton MS. Synaptic NMDA receptor activation stimulates alpha-secretase amyloid precursor protein processing and inhibits amyloid-beta production. J Neurosci. 2009;29:4442–4460. doi: 10.1523/JNEUROSCI.6017-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann J, Twiesselmann C, Kummer MP, Romagnoli P, Herzog V. A possible role for the Alzheimer amyloid precursor protein in the regulation of epidermal basal cell proliferation. Eur. J. Cell Biol. 2000;79:905–914. doi: 10.1078/0171-9335-00117. [DOI] [PubMed] [Google Scholar]
- Holsinger RM, McLean CA, Beyreuther K, Masters CL, Evin G. Increased expression of the amyloid precursor beta-secretase in Alzheimer's disease. Ann. Neurol. 2002;51:783–786. doi: 10.1002/ana.10208. [DOI] [PubMed] [Google Scholar]
- Hook V, Toneff T, Bogyo M, Greenbaum D, Medzihradszky KF, Neveu J, Lane W, Hook G, Reisine T. Inhibition of cathepsin B reduces beta-amyloid production in regulated secretory vesicles of neuronal chromaffin cells: evidence for cathepsin B as a candidate beta-secretase of Alzheimer's disease. Biol. Chem. 2005;386:931–940. doi: 10.1515/BC.2005.108. [DOI] [PubMed] [Google Scholar]
- Hook V, Kindy M, Hook G. Cysteine protease inhibitors effectively reduce in vivo levels of brain beta-amyloid related to Alzheimer's disease. Biol. Chem. 2007a;388:247–252. doi: 10.1515/BC.2007.027. [DOI] [PubMed] [Google Scholar]
- Hook G, Hook VY, Kindy M. Cysteine protease inhibitors reduce brain beta-amyloid and beta-secretase activity in vivo and are potential Alzheimer's disease therapeutics. Biol. Chem. 2007b;388:979–983. doi: 10.1515/BC.2007.117. [DOI] [PubMed] [Google Scholar]
- Hook VY, Kindy M, Hook G. Inhibitors of cathepsin B improve memory and reduce beta-amyloid in transgenic Alzheimer disease mice expressing the wild-type, but not the Swedish mutant, beta-secretase site of the amyloid precursor protein. J. Biol. Chem. 2008;283:7745–7753. doi: 10.1074/jbc.M708362200. [DOI] [PubMed] [Google Scholar]
- Hook VY, Kindy M, Reinheckel T, Peters C, Hook G. Genetic cathepsin B deficiency reduces beta-amyloid in transgenic mice expressing human wild-type amyloid precursor protein. Biochem. Biophys. Res. Commun. 2009;386:284–288. doi: 10.1016/j.bbrc.2009.05.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Hicks CW, He W, Wong P, Macklin WB, Trapp BD, Yan R. Bace1 modulates myelination in the central and peripheral nervous system. Nat. Neurosci. 2006;9:1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- Hunt CE, Turner AJ. Cell biology, regulation and inhibition of beta-secretase (BACE-1) FEBS J. 2009;276:1845–1859. doi: 10.1111/j.1742-4658.2009.06929.x. [DOI] [PubMed] [Google Scholar]
- Huse JT, Liu K, Pijak DS, Carlin D, Lee VM, Doms RW. Beta-secretase processing in the trans-Golgi network preferentially generates truncated amyloid species that accumulate in Alzheimer's disease brain. J. Biol. Chem. 2002;277:16278–16284. doi: 10.1074/jbc.M111141200. [DOI] [PubMed] [Google Scholar]
- Hussain I, Powell DJ, Howlett DR, Chapman GA, Gilmour L, Murdock PR, Tew DG, Meek TD, Chapman C, Schneider K, Ratcliffe FS, Dingwall C, Christie G. ASP1 (BACE2) cleaves the amyloid precursor protein at the β-secretase site. Mol. Cell. Neurosci. 2000;16:609–619. doi: 10.1006/mcne.2000.0884. [DOI] [PubMed] [Google Scholar]
- Iacono D, O'Brien R, Resnick SM, Zonderman AB, Pletnikova O, Rudow G, An Y, West MJ, Crain B, Troncoso JC. Neuronal hypertrophy in asymptomatic Alzheimer disease. J. Neuropathol. Exp. Neurol. 2008;67:578–589. doi: 10.1097/NEN.0b013e3181772794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono D, Markesbery WR, Gross M, Pletnikova O, Rudow G, Zandi P, Troncoso JC. The Nun Study. Clinically silent AD, neuronal hypertrophy, and linguistic skills in early life. Neurology. 2009 doi: 10.1212/WNL.0b013e3181b01077. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida A, Furukawa K, Keller JN, Mattson MP. Secreted form of beta-amyloid precursor protein shifts the frequency dependency for induction of LTD, and enhances LTP in hippocampal slices. Neuroreport. 1997;8:2133–2137. doi: 10.1097/00001756-199707070-00009. [DOI] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Watanabe K, Sekiguchi M, Hosoki E, Kawashima-Morishima M, Lee HJ, Hama E, Sekine-Aizawa Y, Saido TC. Identification of the major Abeta1-42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat. Med. 2000;6:143–150. doi: 10.1038/72237. [DOI] [PubMed] [Google Scholar]
- Jo DG, Arumugam TV, Woo HN, Park JS, Tang SC, Mughal M, Hyun DH, Park JH, Choi YH, Gwon AR, Camandola S, Cheng A, Cai H, Song W, Markesbery WR, Mattson MP. Evidence that gamma-secretase mediates oxidative stress-induced beta-secretase expression in Alzheimer's disease. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.07.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Kirfel G, Borm B, Rigort A, Herzog V. The secretory beta-amyloid precursor protein is a motogen for human epidermal keratinocytes. Eur. J. Cell Biol. 2002;81:664–676. doi: 10.1078/0171-9335-00284. [DOI] [PubMed] [Google Scholar]
- Klein DM, Felsenstein KM, Brenneman DE. Cathepsins B and L differentially regulate amyloid precursor protein processing. J. Pharmacol. Exp. Ther. 2009;328:813–821. doi: 10.1124/jpet.108.147082. [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Zeller M, Cole T, Buttini M, McConlogue L, Sinha S, Freedman S, Morris RG, Chen KS. BACE1 gene deletion: impact on behavioral function in a model of Alzheimer's disease. Neurobiol. Aging. 2008;29:861–873. doi: 10.1016/j.neurobiolaging.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kohutek ZA, diPierro CG, Redpath GT, Hussaini IM. ADAM-10-mediated N-cadherin cleavage is protein kinase C-alpha dependent and promotes glioblastoma cell migration. J Neurosci. 2009;29:4605–4615. doi: 10.1523/JNEUROSCI.5126-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurochkin IV, Goto S. Alzheimer's beta-amyloid peptide specifically interacts with and is degraded by insulin degrading enzyme. FEBS Lett. 1994;345:33–37. doi: 10.1016/0014-5793(94)00387-4. [DOI] [PubMed] [Google Scholar]
- Kwak YD, Brannen CL, Qu T, Kim HM, Dong X, Soba P, Majumdar A, Kaplan A, Beyreuther K, Sugaya K. Amyloid precursor protein regulates differentiation of human neural stem cells. Stem Cells Dev. 2006;15:381–389. doi: 10.1089/scd.2006.15.381. [DOI] [PubMed] [Google Scholar]
- Laird FM, Cai H, Savonenko AV, Farah MH, He K, Melnikova T, Wen H, Chiang HC, Xu G, Koliatsos VE, Borchelt DR, Price DL, Lee HK, Wong PC. BACE1, a major determinant of selective vulnerability of the brain to amyloid-beta amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J. Neurosci. 2005;25:11693–11709. doi: 10.1523/JNEUROSCI.2766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannfelt L, Basun H, Wahlund LO, Rowe BA, Wagner SL. Decreased alpha-secretase-cleaved amyloid precursor protein as a diagnostic marker for Alzheimer's disease. Nat. Med. 1995;1:829–832. doi: 10.1038/nm0895-829. [DOI] [PubMed] [Google Scholar]
- Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall SM, Bobé P, Reiss K, Horiuchi K, Niu XD, Lundell D, Gibb DR, Conrad D, Saftig P, Blobel CP. ADAMs 10 and 17 represent differentially regulated components of a general shedding machinery for membrane proteins such as transforming growth factor alpha, L-selectin, and tumor necrosis factor alpha. Mol. Biol. Cell. 2009;20:1785–1794. doi: 10.1091/mbc.E08-11-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesné S, Ali C, Gabriel C, Croci N, MacKenzie ET, Glabe CG, Plotkine M, Marchand-Verrecchia C, Vivien D, Buisson A. NMDA receptor activation inhibits alpha-secretase and promotes neuronal amyloid-beta production. J. Neurosci. 2005;25:9367–9377. doi: 10.1523/JNEUROSCI.0849-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewczuk P, Kornhuber J, Vanderstichele H, Vanmechelen E, Esselmann H, Bibl M, Wolf S, Otto M, Reulbach U, Kölsch H, Jessen F, Schröder J, Schönknecht P, Hampel H, Peters O, Weimer E, Perneczky R, Jahn H, Luckhaus C, Lamla U, Supprian T, Maler JM, Wiltfang J. Multiplexed quantification of dementia biomarkers in the CSF of patients with early dementias and MCI: a multicenter study. Neurobiol. Aging. 2008;29:812–818. doi: 10.1016/j.neurobiolaging.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Li H, Wolfe MS, Selkoe DJ. Toward structural elucidation of the γ-secretase complex. Structure. 2009;17:326–334. doi: 10.1016/j.str.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Bolon B, Kahn S, Bennett BD, Babu-Khan S, Denis P, Fan W, Kha H, Zhang J, Gong Y, Martin L, Louis JC, Yan Q, Richards WG, Citron M, Vassar R. Mice deficient in BACE1, the Alzheimer's beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat. Neurosci. 2001;4:231–232. doi: 10.1038/85059. [DOI] [PubMed] [Google Scholar]
- Ma QH, Futagawa T, Yang WL, Jiang XD, Zeng L, Takeda Y, Xu RX, Bagnard D, Schachner M, Furley AJ, Karagogeos D, Watanabe K, Dawe GS, Xiao ZC. A TAG1-APP signalling pathway through Fe65 negatively modulates neurogenesis. Nat. Cell Biol. 2008;10:283–294. doi: 10.1038/ncb1690. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Cheng B, Culwell AR, Esch FS, Lieberburg I, Rydel RE. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the beta-amyloid precursor protein. Neuron. 1993;10:243–254. doi: 10.1016/0896-6273(93)90315-i. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Cellular actions of beta-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol. Rev. 1997;77:1081–1132. doi: 10.1152/physrev.1997.77.4.1081. [DOI] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer's disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meziane H, Dodart JC, Mathis C, Little S, Clemens J, Paul SM, Ungerer A. Memory-enhancing effects of secreted forms of the beta-amyloid precursor protein in normal and amnestic mice. Proc. Natl. Acad. Sci. USA. 1998;95:12683–12688. doi: 10.1073/pnas.95.21.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Steiner S, Zhou Y, Arai H, Roberson ED, Sun B, Chen J, Wang X, Yu G, Esposito L, Mucke L, Gan L. Antiamyloidogenic and neuroprotective functions of cathepsin B: implications for Alzheimer's disease. Neuron. 2006;51:703–714. doi: 10.1016/j.neuron.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Müller T, Meyer HE, Egensperger R, Marcus K. The amyloid precursor protein intracellular domain (AICD) as modulator of gene expression, apoptosis, and cytoskeletal dynamics-relevance for Alzheimer's disease. Prog. Neurobiol. 2008;85:393–406. doi: 10.1016/j.pneurobio.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Nelson O, Tu H, Lei T, Bentahir M, de Strooper B, Bezprozvanny I. Familial Alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1. J. Clin. Invest. 2007;117:1230–1239. doi: 10.1172/JCI30447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- O'Connor T, Sadleir KR, Maus E, Velliquette RA, Zhao J, Cole SL, Eimer WA, Hitt B, Bembinster LA, Lammich S, Lichtenthaler SF, Hébert SS, De Strooper B, Haass C, Bennett DA, Vassar R. Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis. Neuron. 2008;60:988–1009. doi: 10.1016/j.neuron.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh ES, Savonenko AV, King JF, Fangmark Tucker SM, Rudow GL, Xu G, Borchelt DR, Troncoso JC. Amyloid precursor protein increases cortical neuron size in transgenic mice. Neurobiol. Aging. 2009;30:1238–1244. doi: 10.1016/j.neurobiolaging.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Sametsky EA, Younkin LH, Oakley H, Younkin SG, Citron M, Vassar R, Disterhoft JF. BACE1 deficiency rescues memory deficits and cholinergic dysfunction in a mouse model of Alzheimer's disease. Neuron. 2004;41:27–33. doi: 10.1016/s0896-6273(03)00810-9. [DOI] [PubMed] [Google Scholar]
- Ohsawa I, Takamura C, Morimoto T, Ishiguro M, Kohsaka S. Amino-terminal region of secreted form of amyloid precursor protein stimulates proliferation of neural stem cells. Eur. J. Neurosci. 1999;11:1907–1913. doi: 10.1046/j.1460-9568.1999.00601.x. [DOI] [PubMed] [Google Scholar]
- Olsson A, Höglund K, Sjögren M, Andreasen N, Minthon L, Lannfelt L, Buerger K, Möller HJ, Hampel H, Davidsson P, Blennow K. Measurement of alpha- and beta-secretase cleaved amyloid precursor protein in cerebrospinal fluid from Alzheimer patients. Exp Neurol. 2003;183:74–80. doi: 10.1016/s0014-4886(03)00027-x. [DOI] [PubMed] [Google Scholar]
- Parkin ET, Watt NT, Hussain I, Eckman EA, Eckman CB, Manson JC, Baybutt HN, Turner AJ, Hooper NM. Cellular prion protein regulates beta-secretase cleavage of the Alzheimer's amyloid precursor protein. Proc. Natl. Acad. Sci. USA. 2007;104:11062–11067. doi: 10.1073/pnas.0609621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postina R, Schroeder A, Dewachter I, Bohl J, Schmitt U, Kojro E, Prinzen C, Endres K, Hiemke C, Blessing M, Flamez P, Dequenne A, Godaux E, van Leuven F, Fahrenholz F. A disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. J. Clin. Invest. 2004;113:1456–1464. doi: 10.1172/JCI20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring S, Weyer SW, Kilian SB, Waldron E, Pietrzik CU, Filippov MA, Herms J, Buchholz C, Eckman CB, Korte M, Wolfer DP, Müller UC. The secreted beta-amyloid precursor protein ectodomain APPs alpha is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. J. Neurosci. 2007;27:7817–7826. doi: 10.1523/JNEUROSCI.1026-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riudavets MA, Iacono D, Resnick SM, O'Brien R, Zonderman AB, Martin LJ, Rudow G, Pletnikova O, Troncoso JC. Resistance to Alzheimer's pathology is associated with nuclear hypertrophy in neurons. Neurobiol. Aging. 2007;28:1484–1492. doi: 10.1016/j.neurobiolaging.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberds SL, Anderson J, Basi G, Bienkowski MJ, Branstetter DG, Chen KS, Freedman SB, Frigon NL, Games D, Hu K, Johnson-Wood K, Kappenman KE, Kawabe TT, Kola I, Kuehn R, Lee M, Liu W, Motter R, Nichols NF, Power M, Robertson DW, Schenk D, Schoor M, Shopp GM, Shuck ME, Sinha S, Svensson KA, Tatsuno G, Tintrup H, Wijsman J, Wright S, McConlogue L. BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: implications for Alzheimer's disease therapeutics. Hum. Mol. Genet. 2001;10:1317–1324. doi: 10.1093/hmg/10.12.1317. [DOI] [PubMed] [Google Scholar]
- Saito Y, Sano Y, Vassar R, Gandy S, Nakaya T, Yamamoto T, Suzuki T. X11 proteins regulate the translocation of amyloid beta-protein precursor (APP) into detergent-resistant membrane and suppress the amyloidogenic cleavage of APP by beta-site-cleaving enzyme in brain. J. Biol. Chem. 2008;283:35763–35771. doi: 10.1074/jbc.M801353200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Kaneko K, Okuno M, Wada K, Kashiyama T, Shimizu H, Akagi T, Hashikawa T, Nukina N. Membrane microdomain switching: a regulatory mechanism of amyloid precursor protein processing. J. Cell Biol. 2008;183:339–352. doi: 10.1083/jcb.200804075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura CA, Chen G, Malkani S, Choi SY, Takahashi RH, Zhang D, Gouras GK, Kirkwood A, Morris RG, Shen J. Conditional inactivation of presenilin 1 prevents amyloid accumulation and temporarily rescues contextual and spatial working memory impairments in amyloid precursor protein transgenic mice. J. Neurosci. 2005;25:6755–6764. doi: 10.1523/JNEUROSCI.1247-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savonenko AV, Melnikova T, Laird FM, Stewart KA, Price DL, Wong PC. Alteration of BACE1-dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. Proc. Natl. Acad. Sci. USA. 2008;105:5585–5590. doi: 10.1073/pnas.0710373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I, Ziv E. Kinetic properties of cathepsin D and BACE 1 indicate the need to search for additional beta-secretase candidate(s) Biol. Chem. 2008;389:313–320. doi: 10.1515/BC.2008.025. [DOI] [PubMed] [Google Scholar]
- Schilling S, Zeitschel U, Hoffmann T, Heiser U, Francke M, Kehlen A, Holzer M, Hutter-Paier B, Prokesch M, Windisch M, Jagla W, Schlenzig D, Lindner C, Rudolph T, Reuter G, Cynis H, Montag D, Demuth HU, Rossner S. Glutaminyl cyclase inhibition attenuates pyroglutamate Abeta and Alzheimer's disease-like pathology. Nat. Med. 2008;14:1106–1111. doi: 10.1038/nm.1872. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Toward a comprehensive theory for Alzheimer's disease. Hypothesis: Alzheimer's disease is caused by the cerebral accumulation and cytotoxicity of amyloid beta-protein. Ann. N.Y. Acad. Sci. 2000;924:17–25. doi: 10.1111/j.1749-6632.2000.tb05554.x. [DOI] [PubMed] [Google Scholar]
- Sennvik K, Fastbom J, Blomberg M, Wahlund LO, Winblad B, Benedikz E. Levels of alpha- and beta-secretase cleaved amyloid precursor protein in the cerebrospinal fluid of Alzheimer's disease patients. Neurosci. Lett. 2000;278:169–172. doi: 10.1016/s0304-3940(99)00929-5. [DOI] [PubMed] [Google Scholar]
- Serneels L, Van Biervliet J, Craessaerts K, Dejaegere T, Horré K, Van Houtvin T, Esselmann H, Paul S, Schäfer MK, Berezovska O, Hyman BT, Sprangers B, Sciot R, Moons L, Jucker M, Yang Z, May PC, Karran E, Wiltfang J, D'Hooge R, De Strooper B. gamma-Secretase heterogeneity in the Aph-1 subunit: relevance for Alzheimer's disease. Science. 2009;324:639–642. doi: 10.1126/science.1171176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat. Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ. Alzheimer's Disease Neuroimaging Initiative. (2009) Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann. Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Fukuda H, Murayama S, Izumiyama N, Shirasawa T. Isoaspartate formation at position 23 of amyloid beta peptide enhanced fibril formation and deposited onto senile plaques and vascular amyloids in Alzheimer's disease. J. Neurosci. Res. 2002;70:451–461. doi: 10.1002/jnr.10350. [DOI] [PubMed] [Google Scholar]
- Siemes C, Quast T, Kummer C, Wehner S, Kirfel G, Müller U, Herzog V. Keratinocytes from APP/APLP2-deficient mice are impaired in proliferation, adhesion and migration in vitro. Exp. Cell Res. 2006;312:1939–1949. doi: 10.1016/j.yexcr.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Skovronsky DM, Moore DB, Milla ME, Doms RW, Lee VM. Protein kinase C-dependent alpha-secretase competes with beta-secretase for cleavage of amyloid-beta precursor protein in the trans-golgi network. J. Biol. Chem. 2000;275:2568–2575. doi: 10.1074/jbc.275.4.2568. [DOI] [PubMed] [Google Scholar]
- Sun B, Zhou Y, Halabisky B, Lo I, Cho SH, Mueller-Steiner S, Devidze N, Wang X, Grubb A, Gan L. Cystatin C-cathepsin B axis regulates amyloid beta levels and associated neuronal deficits in an animal model of Alzheimer's disease. Neuron. 2008;60:247–257. doi: 10.1016/j.neuron.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Chen G, Südhof TC, Shen J. Conditional forebrain inactivation of nicastrin causes progressive memory impairment and age-related neurodegeneration. J. Neurosci. 2009;29:7290–7301. doi: 10.1523/JNEUROSCI.1320-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamgüney G, Giles K, Glidden DV, Lessard P, Wille H, Tremblay P, Groth DF, Yehiely F, Korth C, Moore RC, Tatzelt J, Rubinstein E, Boucheix C, Yang X, Stanley P, Lisanti MP, Dwek RA, Rudd PM, Moskovitz J, Epstein CJ, Cruz TD, Kuziel WA, Maeda N, Sap J, Ashe KH, Carlson GA, Tesseur I, Wyss-Coray T, Mucke L, Weisgraber KH, Mahley RW, Cohen FE, Prusiner SB. Genes contributing to prion pathogenesis. J. Gen. Virol. 2008;89:1777–1788. doi: 10.1099/vir.0.2008/001255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe C, Hotoda N, Sasagawa N, Sehara-Fujisawa A, Maruyama K, Ishiura S. ADAM19 is tightly associated with constitutive Alzheimer's disease APP alpha-secretase in A172 cells. Biochem. Biophys. Res. Commun. 2006;352:111–117. doi: 10.1016/j.bbrc.2006.10.181. [DOI] [PubMed] [Google Scholar]
- Taylor CJ, Ireland DR, Ballagh I, Bourne K, Marechal NM, Turner PR, Bilkey DK, Tate WP, Abraham WC. Endogenous secreted amyloid precursor protein-alpha regulates hippocampal NMDA receptor function, long-term potentiation and spatial memory. Neurobiol Dis. 2008;31:250–260. doi: 10.1016/j.nbd.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Thathiah A, Spittaels K, Hoffmann M, Staes M, Cohen A, Horré K, Vanbrabant M, Coun F, Baekelandt V, Delacourte A, Fischer DF, Pollet D, De Strooper B, Merchiers P. The orphan G protein-coupled receptor 3 modulates amyloid-beta peptide generation in neurons. Science. 2009;323:946–951. doi: 10.1126/science.1160649. [DOI] [PubMed] [Google Scholar]
- Thinakaran G, Borchelt DR, Lee MK, Slunt HH, Spitzer L, Kim G, Ratovitsky T, Davenport F, Nordstedt C, Seeger M, Hardy J, Levey AI, Gandy SE, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 1996;17:181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 2008;283:29615–29619. doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, et al. β-Secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Vetrivel KS, Meckler X, Chen Y, Nguyen PD, Seidah NG, Vassar R, Wong PC, Fukata M, Kounnas MZ, Thinakaran G. Alzheimer disease Abeta production in the absence of S-palmitoylation-dependent targeting of BACE1 to lipid rafts. J. Biol. Chem. 2009;284:3793–3803. doi: 10.1074/jbc.M808920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt DL, Thomas D, Galvan V, Bredesen DE, Lamb BT, Pimplikar SW. Abnormal neuronal networks and seizure susceptibility in mice overexpressing the APP intracellular domain. Neurobiol. Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.09.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi T, De Strooper B. Presenilins: members of the gamma-secretase quartets, but part-time soloists too. Physiology. 2008;23:194–204. doi: 10.1152/physiol.00009.2008. [DOI] [PubMed] [Google Scholar]
- Waldron E, Isbert S, Kern A, Jaeger S, Martin AM, Hébert SS, Behl C, Weggen S, De Strooper B, Pietrzik CU. Increased AICD generation does not result in increased nuclear translocation or activation of target gene transcription. Exp. Cell Res. 2008;314:2419–2433. doi: 10.1016/j.yexcr.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Wegiel J, Kuchna I, Nowicki K, Frackowiak J, Mazur-Kolecka B, Imaki H, Wegiel J, Mehta PD, Silverman WP, Reisberg B, Deleon M, Wisniewski T, Pirttilla T, Frey H, Lehtimäki T, Kivimäki T, Visser FE, Kamphorst W, Potempska A, Bolton D, Currie JR, Miller DL. Intraneuronal Abeta immunoreactivity is not a predictor of brain amyloidosis-beta or neurofibrillary degeneration. Acta Neuropathol. 2007;113:389–402. doi: 10.1007/s00401-006-0191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmeyer GG, Willem M, Lichtenthaler SF, Lurman G, Multhaup G, Assfalg-Machleidt I, Reiss K, Saftig P, Haass C. Dimerization of beta-site beta-amyloid precursor protein-cleaving enzyme. J. Biol. Chem. 2004;279:53205–53212. doi: 10.1074/jbc.M410378200. [DOI] [PubMed] [Google Scholar]
- Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, Rittger A, DeStrooper B, Saftig P, Birchmeier C, Haass C. Control of peripheral nerve myelination by the beta-secretase BACE1. Science. 2006;314:664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- Winkler E, Hobson S, Fukumori A, Dümpelfeld B, Luebbers T, Baumann K, Haass C, Hopf C, Steiner H. Purification, pharmacological modulation, and biochemical characterization of interactors of endogenous human gamma-secretase. Biochemistry. 2009;48:1183–1197. doi: 10.1021/bi801204g. [DOI] [PubMed] [Google Scholar]
- Wirths O, Multhaup G, Czech C, Blanchard V, Moussaoui S, Tremp G, Pradier L, Beyreuther K, Bayer TA. Intraneuronal Abeta accumulation precedes plaque formation in beta-amyloid precursor protein and presenilin-1 double-transgenic mice. Neurosci. Lett. 2001;306:116–120. doi: 10.1016/s0304-3940(01)01876-6. [DOI] [PubMed] [Google Scholar]
- Wolfe MS. Inhibition and modulation fo γ-secretase for Alzheimer's Disease. Neurotherapeutics. 2008;5:391–398. doi: 10.1016/j.nurt.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LB, Lindholm K, Yan R, Citron M, Xia W, Yang XL, Beach T, Sue L, Wong P, Price D, Li R, Shen Y. Elevated beta-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med. 2003;9:3–4. doi: 10.1038/nm0103-3. [DOI] [PubMed] [Google Scholar]
- Yang T, Arslanova D, Gu Y, Augelli-Szafran C, Xia W. Quantification of gamma-secretase modulation differentiates inhibitor compound selectivity between two substrates Notch and amyloid precursor protein. Mol. Brain. 2008;1:1–15. doi: 10.1186/1756-6606-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Saura CA, Choi SY, Sun LD, Yang X, Handler M, Kawarabayashi T, Younkin L, Fedeles B, Wilson MA, Younkin S, Kandel ER, Kirkwood A, Shen J. APP processing and synaptic plasticity in presenilin-1 conditional knockout mice. Neuron. 2001;31:713–726. doi: 10.1016/s0896-6273(01)00417-2. [DOI] [PubMed] [Google Scholar]