Abstract
This study was designed to develop a zebrafish experimental model to examine defects in retinoic acid signaling caused by embryonic ethanol. Retinoic acid deficiency may be a causative factor leading to a spectrum of birth defects classified as fetal alcohol spectrum disorder (FASD). Experimental support for this hypothesis using Xenopus showed that effects of treatment with ethanol could be partially rescued by adding retinoids during ethanol treatment. Previous studies show that treating zebrafish embryos during gastrulation and somitogenesis stages with a pathophysiological concentration of ethanol (100 mM) produces effects that are characteristic features of FASD. We found that treating zebrafish embryos with retinoic acid at a low concentration (10−9 M) and 100 mM ethanol during gastrulation and somitogenesis stages significantly rescued a spectrum of defects produced by treating embryos with 100 mM ethanol alone. The rescue phenotype that we observed was quantitatively more similar to embryos treated with 10−9 M retinoic acid alone (retinoic acid toxicity) than to untreated or 100 mM ethanol treated embryos. Retinoic acid rescues defects caused by 100 mM ethanol treatment during gastrulation and somitogenesis stages that include early gastrulation cell movements (anterior-posterior axis), craniofacial cartilage formation and ear development. Morphological evidence also suggests that other characteristic features of FASD (e. g., neural axis patterning) are rescued by retinoic acid supplement.
Keywords: Fetal alcohol syndrome, zebrafish, embryogenesis, gastrulation, somitogenesis, ethanol, retinoic acid
Introduction
In humans, fetal ethanol exposure produces a spectrum of defects including facial abnormalities, mental retardation, stature reduction and other birth defects, collectively called fetal alcohol syndrome (FAS; Warren and Foudin, 2001). The diagnostic criteria for FAS include a characteristic pattern of facial abnormalities, growth retardation and neurodevelopmental abnormalities of the central nervous system (Warren and Foudin, 2001).
Fetal alcohol spectrum disorder (FASD) is a broad range of structural and functional developmental defects that can be simulated by ethanol exposure in animal models (Sulik, 2005). Indeed, Sulik and colleagues first showed that the best recapitulation of fetal alcohol syndrome morphology occurred if short-term exposure occurred during gastrulation stages (Sulik et al., 1981). Animal model research is employed to gain new mechanistic insight into the fundamental consequences of embryonic ethanol exposure. Mammalian models are extremely useful for FASD research, but embryonic fish and amphibian models permit more tightly controlled exposure regimens (ethanol and drug treatments) and direct observation of the highly conserved vertebrate early developmental stages (gastrulation and somitogenesis) due to external development. Our goal in this study was to adapt the zebrafish experimental model for experiments to determine whether retinoic acid supplement can compensate for embryonic exposure regimens of pathophysiological ethanol concentrations (e. g., 100 mM or 460 mg%).
Zebrafish embryos are very transparent, which allows direct microscopic observation and measurement of numerous embryonic developmental events (Kimmel et al., 1995). Zebrafish is an exemplary genetic model for early developmental genetics (Schier and Talbot, 2005). Early gastrulation cell movements in zebrafish include epiboly, the process of cell spreading over the yolk cell; involution, the inward movement of cells under outer surface (epiblast) to produce the hypoblast, which becomes mesodermal and endodermal cell layers; and convergent extension, polarized and directional cell movements converging on the embryonic midline and extending in the anterior-posterior axis (Kimmel et al., 1995).
Various investigators articulated the potential relationship between FAS and retinoic acid signaling (Duester, 1991; Pullarkat, 1991; Leo and Lieber, 1999; McCaffery et al., 2004; Sulik et al., 1981), suggesting that ethanol exposure during embryogenesis alters retinoic acid biosynthesis and signaling. The current consensus or `working model' is that ethanol decreases retinoic acid levels by ethanol or acetaldehyde competition for the alcohol or aldehyde dehydrogenase, but others (McCaffery et al., 2004) show that ethanol treatment actually increases retinoic acid levels in areas of the brain. Experimental support for this working model is growing using mouse and Xenopus models (Johnson et al., 2007; Yelin et al., 2005). Numerous investigators have studied effects of retinoic acid deficiency, which produces a spectrum of birth defects that include neural axis patterning, craniofacial development, heart development, limb induction, eye development and ear development (Collins and Mao, 1999). This spectrum of defects resembles the consequences of ethanol exposure during embryonic development, collectively defined as FASD (Begemann et al., 2001; Collins and Mao, 1999; Duester, 1991; Grandel et al., 2002).
Several observations form the basis of this retinoic acid hypothesis: (i) the similarity between FASD and effects of inhibiting retinoic acid biosynthesis (experimentally or genetically) in vertebrate embryos (Begemann et al., 2001; Collins and Mao, 1999; Duester, 1991; Grandel et al., 2002); (ii) exogenous retinoid exposure during embryogenesis, either environmental exposure or exposure to drugs like Accutane, produces a syndrome that resembles FASD, albeit less severe (Collins and Mao, 1999); (iii) enzymes that metabolize ethanol also catalyze retinol conversion to retinoic acid during development (Mark et al., 2006); and, (iv) recent studies using Xenopus and mouse models provide experimental support for this hypothesis (Johnson et al., 2007; Yelin et al., 2005) (see below).
Inductive signals during gastrulation include a role of retinoic acid during gastrulation and neural axis patterning (Lumsden and Krumlauf, 1996; Schier and Talbot, 2005). Retinoic acid signaling controls gastrulation morphogenetic movements and signaling of the prechordal plate (this embryonic tissue has head organizer activity) that induces forebrain identity, and retinoic acid controls hindbrain rhombomere patterning (Lumsden and Krumlauf, 1996; Begemann and Meyer, 2001; Dupe and Lumsden, 2001). An extension of the working model that embryonic ethanol exposure reduces retinoic acid signaling predicts that ethanol would interfere with early gastrulation retinoic acid mediated activities that control embryonic axis, including neuraxis developmental processes.
Two recent studies support the overall hypothesis that ethanol exposure in early embryos inhibits retinoic acid signaling, and reduced retinoic acid signaling is responsible for significant ethanol-induced developmental consequences. The Fainsod laboratory used the Xenopus model to study FASD, showing that high ethanol concentrations (ranging from 1.5 to 2.5% or 326 to 543 mM; concentrations that are fatal in humans) produce defects consistent with FASD, including small eyes, microcephaly, reduced body length and other effects, and acute effects of ethanol were partially rescued by retinoid (retinol or retinal) treatments, restoring eye size, brain size, brain regionalization marker expression and retinoic acid reporter gene expression (Yelin et al., 2005). Sulik and colleagues (Johnson et al., 2007) analyzed ethanol effects on mouse limb development, finding that early limb genes were suppressed by ethanol treatment, and reduced limb gene expression produced by ethanol treatment could be phenocopied (mimicked) using a drug that specifically blocks RXR transcription factors (retinoic acid receptors).
We hypothesized that ethanol-induced defects in anterior-posterior axis and neural axis formation result from reduced retinoic acid signaling during gastrulation and somitogenesis stages. We tested a high, but pathophysiological concentration of ethanol (100 mM, or 0.46 gram%) for effects on early zebrafish development, and co-treatment of 100 mM ethanol with retinoic acid (10−9 M; a low concentration that only produces mild defects; Collins and Mao, 1999) produces a striking rescue phenotype. The effectiveness of retinoic acid co-treatment with ethanol to reverse developmental defects was evaluated to validate a treatment regimen for future molecular studies.
Materials and Methods
Zebrafish husbandry
Zebrafish (Danio rerio; TL strain) were raised and housed under standard laboratory conditions (Westerfield, 2000) in accordance with Indiana University Policy on Animal Care and Use. For some experiments, 0.2 mM phenylthiourea was added to embryo medium to prevent melanization. Ages of the embryos are given as hours postfertilization (hpf) or days postfertilization (dpf).
Ethanol and Retinoic Acid Treatment
Embryos were maintained in embryo medium (Westerfield, 2000) until 3 hpf. At this time, embryos were transferred to one of four conditions: embryo medium (control); embryo medium containing 100 mM ethanol; embryo medium containing 100 mM ethanol and 10−9 M retinoic acid; and, embryo medium containing 10−9 M retinoic acid. Maier and colleagues (Bradfield et al. 2006) showed ethanol equilibrates within embryos after one hour of treatment. For epiboly measurements, embryos were fixed in 4% paraformaldehyde in PBS at 8 hpf. For other studies, treatments were extended until 24 hpf. At this time, embryos were transferred to embryo medium (without ethanol or retinoic acid). At various times, embryos or larvae were euthanized with Tricaine then fixed in 4% paraformaldehyde in PBS.
Alcian Blue Staining
Cartilage was stained with alcian blue using a modified previously published method (Loucks and Carvan, 2004). Larvae (5 dpf) were euthanized with Tricaine then fixed in 5% trichloroacetic acid for 10 min at room temperature followed by rinsing in 0.37% HCl in 70% ethanol (acid-alcohol) then incubated in 1% alcian blue in acid-alcohol overnight at room temperature. After staining, larvae were rinsed in acid-alcohol, then cleared with 0.25% KOH in 50% glycerol, followed by 1% H2O2 in H2O overnight at room temperature. Larvae were then rinsed with PBS, and mounted for DIC microscopy.
Microscopy and Image Analysis
All still images were digitally acquired with a color SPOT camera (Diagnostic Instruments Inc, Sterling Heights, MI). This camera was mounted on either a Leica MZ12 dissecting stereomicroscope (Leica Microsystems, Inc., Deerfield IL) or a Nikon Diaphot equipped with differential interference contrast (DIC) optics (Nikon, Inc., Melville, NY). Measurements were made using ImageJ software (Abramoff et al., 2004) calibrated using a stage micrometer. Video sequences in Supplemental Data Movie S1 were digitally acquired with the Leica DFC290 camera, driven by the Leica Application Suite software on the Leica MZ12 dissecting stereomicroscope. Video files were combined in a composite using Adobe Premier software (Adobe Systems Inc., San Jose CA) and compressed for publication (<10 Mb).
Statistical Methods
The effects of ethanol and retinoic acid were analyzed using two-way ANOVA. Bonferroni adjustment for multiple (six) comparisons was used to ensure an overall ninety five percent confidence. Thus, P-value≤0.008 was used to conclude that groups were statistically different.
Results
We exposed embryos to ethanol during gastrulation and somitogenesis stages. Various ethanol and retinoic acid concentrations were tested (data not shown) for their ability to consistently induce FASD-like phenotype (ethanol); to potentially reverse ethanol-induced developmental defects (ethanol plus retinoic acid); and have minimal retinoic acid-induced defects (retinoic acid alone). Optimal concentrations were selected: 100 mM ethanol and 10−9 M retinoic acid.
Pathophysiological ethanol concentrations produce FASD-like developmental defects in zebrafish, which are rescued by retinoic acid
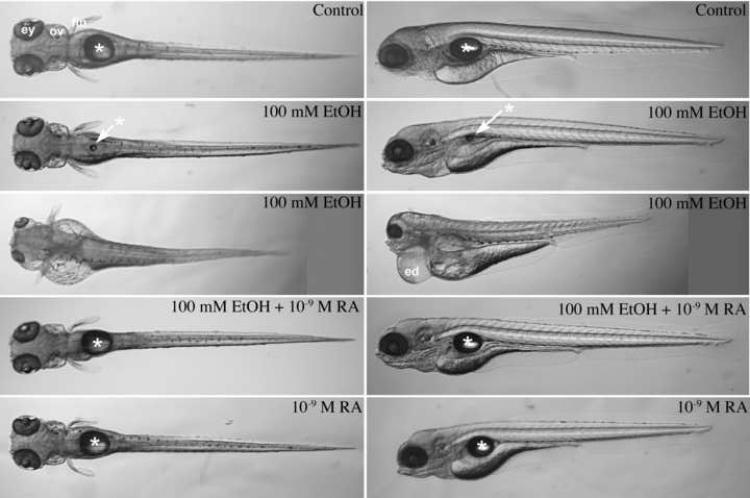
After embryos (TL strain, from Tubingen stock center) were treated from 3 to 24 hours postfertilization (hpf; during gastrulation and somitogenesis) with 100 mM ethanol, ethanol medium was rinsed and replaced with normal embryo medium without ethanol, and embryos were incubated in normal growth medium for 3 days. At 4 days postfertilization (dpf), larvae were anesthetized and photographed using a stereomicroscope (Figure 1). Ethanol treatment produced an array of defects, and developmental defects in individual larvae that varied in their degrees of severity. Less and more severe representative examples of 100 mM ethanol treated 4 dpf larvae are shown in the second and third row of Figure 1, respectively (left column, dorsal views; right column, lateral views of the same embryos shown in the corresponding row). In both ethanol treated embryos, significant developmental defects were observed, including reduced anterior-posterior axis, small fins, craniofacial defects, pericardial edema (indicating fluid homeostasis defects, like cardiovascular defects), and neural defects (including small eyes and small ears). In this and other experiments, nearly all embryos exposed to ethanol had recognizable developmental defects and uncoordinated movement (see Supplemental Data, Movie S1). A minority of these embryos showed severe defects like the severely affected embryo in Figure 1 and the two embryos in Movie S1. Retinoic acid treated embryos (with or without ethanol exposure) were more coordinated swimmers, similar to control embryos (see Supplemental Data, Movie S1).
Figure 1.
Ethanol treatment of zebrafish embryos during gastrulation and somitogenesis stages produces a FASD phenotype that was largely rescued by including retinoic acid during ethanol treatment. Dorsal (left column) and lateral (right column; dorsal is up) views of 4 dpf living larvae were imaged using a steromicroscope; anterior is left on each image. Control embryos were untreated (first, top row); treated with 100 mM ethanol (second and third row from top; note FASD phenotype is variable); treated with 100 mM ethanol and 10−9 M retinoic acid (fourth row from top); and treated with 10−9 M retinoic acid alone (fifth, bottom row) from 3 hpf until 24 hpf (see Materials and Methods). At 24 hpf, ethanol and/or retinoic acid treatments were discontinued, and embryos were incubated with normal embryo medium until 4 dpf. Asterisks and arrows indicate the swim bladder. Abbreviations: ed, edematous pericardium; ey, eye; fin, pectoral fin; and ov, otic vesicle.
Embryos treated with 100 mM ethanol show reduced or absent a swim bladder (Figure 1: asterisk symbols). Ethanol may block normal swim bladder development. Alternatively, a reduced or absent swim bladder may result from uncoordinated swimming behaviors due to neural and inner ear defects, and/or craniofacial (jaw) defects that make the embryo unable to swallow air effectively. We did not distinguish between these possibilities, but we observed that treated larvae swam less frequently and with less coordination (see Supplemental Data, Movie S1). Note that these are 4 dpf larvae and the ethanol exposure ended at 24 hpf. The swimming defect is not due to ongoing presence of ethanol.
To test the hypothesis that a zebrafish model for ethanol-induced developmental defects are due to reduced retinoic acid levels, we treated zebrafish embryos with low concentrations (10−9 M) retinoic acid during the ethanol treatment to determine whether this partially reverses or rescues detrimental effects of ethanol treatment. We found that ethanol-induced defects were partially rescued (Figure 1, fourth row). When embryos are treated with both 100 mM ethanol and 10−9 M retinoic acid, 5 dpf larvae swam actively (like the control larvae; see Supplemental Data, Movie S1) and were able to fill their swim bladders. We also examined effects of treating embryos with 10−9 M retinoic acid alone, and known effects of retinoic acid exposure (exogenous retinoid exposure during embryogenesis produces a defects similar to retinoid deficiency, albeit less severe; Collins and Mao, 1999) were detected in larvae treated with retinoic acid alone (Figure 1, fifth row). In fact, co-treatment with ethanol and retinoic acid produce larvae that more closely resembled larvae treated with retinoic acid alone, rather than control or ethanol treated embryos (Figure 1, compare fourth and fifth rows).
Craniofacial cartilage defects induced by ethanol were rescued by retinoic acid
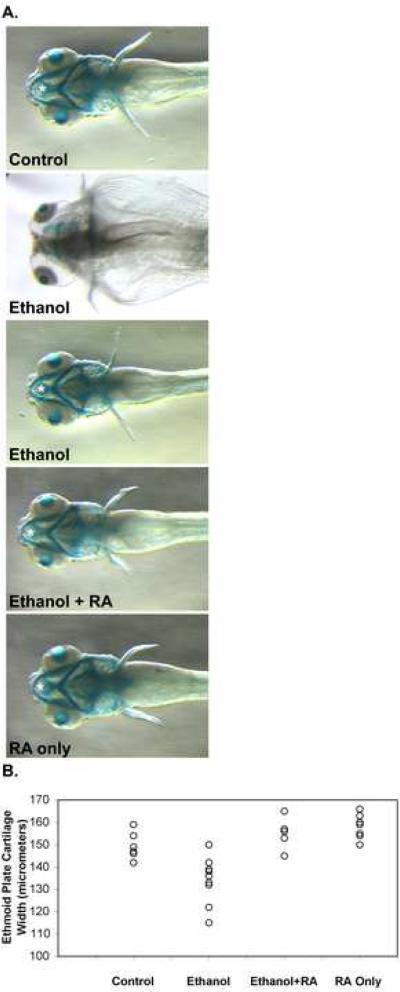
A dramatic effect on craniofacial cartilage formation was observed in embryos treated with a pathophysiological ethanol concentration (Figure 2A, compare ethanol treated embryos to control embryo), as shown previously (Loucks and Carvan, 2004). Treating embryos with 100 mM ethanol and 10−9 M retinoic acid restored nearly normal craniofacial cartilage formation (Figure 2A, Ethanol + RA). Gross effects of treating embryos with 10−9 M retinoic acid alone on craniofacial cartilage development were minimal (Figure 2A, RA).
Figure 2.
Ethanol treatment of zebrafish embryos during gastrulation and somitogenesis stages produces craniofacial defects that were improved by including retinoic acid during ethanol treatment. (A.) Dorsal views (anterior is left) of 5 dpf alcian blue stained larvae were imaged using a steromicroscope. Embryos were untreated in control embryos (top panel), and treated with 100 mM ethanol (second and third panels from top), 100 mM ethanol and 10−9 M retinoic acid (third panel from top), and 10−9 M retinoic acid alone (bottom panel) from 3 hpf until 24 hpf (see Materials and Methods). At 24 hpf, ethanol and/or retinoic acid treatments were discontinued, and embryos were incubated with normal embryo medium until 5 dpf. These 5 dpf larvae were processed for alcian blue staining to label craniofacial cartilages. White asterisks mark the ethmoid cartilage measured (in other embryos) for Table I. The ethmoid cartilage cannot be seen in the more severely affected ethanol treated embryo (second panel from top), and consequently, when we could not detect the cartilage, we did not include these embryos in our measurements. Note that the ethmoid cartilage extends beyond the hyoid arch (the most anterior structure in the ventral jaw cartilages). (B.) Scatter plot representation of ethmoid cartilage width variation in embryos treated from 3 hpf until 24 hpf (during gastrulation and somitogenesis) with 100 mM ethanol, 10−9 M retinoic acid, or 100 mM ethanol and 10−9 M retinoic acid together, and compared to untreated control embryos. Ethmoid plate cartilage width was measured in alcian blue stained 4.5 dpf larvae (see Table I for statistical analysis).
Caravan and colleagues showed that the width of the ethmoid plate (Figure 2A, see asterisk symbols), a dorsal cartilage structure was significantly affected in ethanol treated 3 and 10 dpf larvae (Carvan et al., 2004). We examined the ethmoid plate cartilage width in control and treated 4.5 dpf larvae (Figure 2B and Table I). Ethanol treatment significantly reduced the average ethmoid plate cartilage width from 148.4 (±6.2) μm in the control group to 134.1 (±10.4) μm. The average ethmoid plate cartilage width in larvae treated with both ethanol and retinoic acid, 154.8 (±6.5) μm, or retinoic acid alone, 158.1 (±5.5) μm, were greater than, but not statistically different from the control group. Treating embryos with both 100 mM ethanol and 10−9 M retinoic acid increased the average ethmoid plate cartilage width as compared to ethanol treated embryos, which was statistically different. The average ethmoid plate cartilage width in larvae treated with retinoic acid alone was also statistically different from ethanol treatment. The average ethmoid plate cartilage width in retinoic acid alone was not statistically different from that of the group treated with the combination ethanol and retinoic acid. It is important to note that retinoic acid treatment increased the average ethmoid plate cartilage width (Figure 2B and Table I), showing that ethanol and retinoic treatment can have opposite effects on the same structure. Furthermore, ethanol-by-retinoic acid interaction was tested using by twoway ANOVA, showing that the interaction was significant for all measurements (see Tables I-IV legends).
Table I. Ethmoid plate cartilage width (in micrometers) at 4.5 dpf in ethanol and retinoic acid treated embryos.
Embryos were treated from 3 hpf until 24 hpf (during gastrulation and somitogenesis) with 100 mM ethanol, 10−9 M retinoic acid, or 100 mM ethanol and 10−9 M retinoic acid together, and compared to untreated control embryos. At 4.5 dpf larvae were fixed using paraformaldehyde to stop development, and processed for alcian blue staining to visualize developing craniofacial cartilages (see Materials and Methods). Differential interference contrast (DIC) microscopy images focused on the dorsal craniofacial cartilage structures were used to measure the width of the ethmoid plate using ImageJ image analysis software (see Materials and Methods). Two-way ANOVA was performed to compare treatment groups. Ethanol-by-retinoic acid interaction was significant (P=0.0703), and pairwise comparison P-values are shown. Bonferroni adjustment for multiple (six) comparisons was used to ensure an overall ninety five percent confidence. Thus, P-value≤0.008 was used to conclude that groups were statistically different.
| Control | Ethanol | Ethanol+RA | RA only | |
|---|---|---|---|---|
| Mean | 148.4 | 134.1 | 154.8 | 158.1 |
| SD | 6.2 | 10.4 | 6.5 | 5.5 |
| n | 7 | 9 | 6 | 7 |
| P (vs Control) | 0.0012 | 0.1503 | 0.0274 | |
| P (vs Ethanol) | <0.0001 | <0.0001 | ||
| P (vs RA) | 0.4503 |
Table IV. Ear circumference (otic vesicle; in μm) in 30 hpf in ethanol, DEAB and retinoic acid treated embryos.
Upper part of table: TL strain embryos were treated from 3 hpf until 24 hpf (during gastrulation and somitogenesis) with 100 mM ethanol, 10−9 M retinoic acid, or 100 mM ethanol and 10−9 M retinoic acid together, and compared to untreated control embryos At 30 hpf embryos were fixed using paraformaldehyde to stop development, and differential interference contrast (DIC) microscopy images focused on the otic vesicle. Circumference was measured using ImageJ image analysis software (see Materials and Methods). Two-way ANOVA was performed to compare treatment groups. Ethanol-by-retinoic acid interaction was significant (P=0.0109), and pairwise comparison P-values are shown. Bonferroni adjustment for multiple (six) comparisons was used to ensure an overall ninety five percent confidence. Thus, P-value≤0.008 was used to conclude that groups were statistically different.
| Control | Ethanol | Ethanol+RA | RA only | |
|---|---|---|---|---|
| Mean | 298.6 | 259.5 | 316.8 | 304.1 |
| SD | 24.7 | 20.3 | 17.2 | 6.4 |
| n | 5 | 6 | 4 | 4 |
| P (vs Control) | 0.0042 | 0.1775 | 0.6743 | |
| P (vs Ethanol) | 0.0003 | 0.0026 | ||
| P (vs RA) | 0.3642 |
Ethanol induces epiboly, gastrulation and neural axis defects
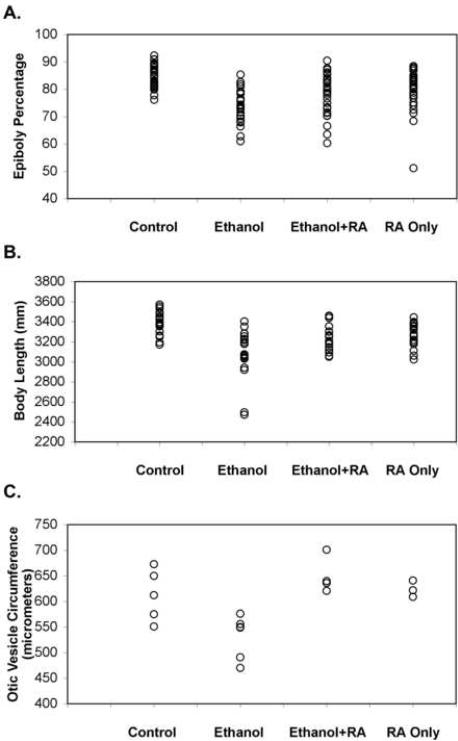
Retinoic acid signaling and ethanol treatment affects early morphogenetic cell movements in the early gastrulation stage vertebrate embryo (Lumsden and Krumlauf, 1996; Blader and Strahle, 1998; Yelin et al., 2007). Embryos were treated from 3 hpf (midblastula transition) until 8 hpf (near the end of gastrulation stages but prior to somitogenesis) (Kimmel et al., 1995) with: 100 mM ethanol; 10−9 M retinoic acid; and 100 mM ethanol plus 10−9 M retinoic acid; and these embryos were compared to untreated control embryos (Figure 3A and Table II). Percent epiboly (the amount of the yolk cell covered by the growing embryo) is an indirect, but reliable measure of early gastrulation progression (Kimmel et al., 1995). On the average, ethanol treatment reduced the percentage of the yolk cell covered by the embryo to 74.0% (±5.7) from 85.4% (±4.2) in the control group. Treating embryos with both 100 mM ethanol and 10−9 M retinoic acid increased the percent epiboly to 78.1% (±7.5), but this was not statistically different as compared to ethanol treated embryos (using Bonferroni adjustment for multiple comparisons: significance threshold P-value≤0.008; P=0.0163, Figure 3A and Table II). Retinoic acid alone reduced the percent epiboly to 79.2% (±7.5), which was statistically different from control and ethanol treatments, but retinoic acid alone was not statistically different from embryos treated with both ethanol and retinoic acid.
Figure 3.
Scatter plot representation of ethmoid cartilage width variation in embryos treated from 3 hpf until 24 hpf (during gastrulation and somitogenesis) with 100 mM ethanol, 10−9 M retinoic acid, or 100 mM ethanol and 10−9 M retinoic acid together, and compared to untreated control embryos. Ethmoid plate cartilage width was measured in alcian blue stained 4.5 dpf larvae (see Table I for statistical analysis).
Table II. Epiboly percentage in ethanol and retinoic acid treated embryos.
Embryos were treated from 3 hpf until 8 hpf (during gastrulation) with 100 mM ethanol, 10−9 M retinoic acid, or 100 mM ethanol and 10−9 M retinoic acid together, and compared to untreated embryos (normal embryo medium: Control). At 8 hpf embryos were fixed using paraformaldehyde to stop development, and these embryos were imaged using a stereo microscope. The percentage of the yolk cell that is covered by the embryo proper was measured using ImageJ image analysis software (see Materials and Methods). Two-way ANOVA was performed to compare treatment groups. Ethanol-by-retinoic acid interaction was significant (P=0.0001), and pairwise comparison P-values are shown. Bonferroni adjustment for multiple (six) comparisons was used to ensure an overall ninety five percent confidence. Thus, P-value≤0.008 was used to conclude that groups were statistically different.
| Control | Ethanol | Ethanol+RA | RA only | |
|---|---|---|---|---|
| Mean | 85.4 | 74.0 | 78.1 | 79.8 |
| SD | 4.2 | 5.7 | 7.5 | 7.5 |
| n | 29 | 28 | 29 | 29 |
| P (vs Control) | <0.0001 | <0.0001 | 0.0012 | |
| P (vs Ethanol) | 0.0163 | 0.0009 | ||
| P (vs RA) | 0.3268 |
We next determined whether defects induced by 100 mM ethanol on early epiboly and gastrulation progression leads to reduced anterior-posterior axis of embryos at the end of somitogenesis. Embryos were treated from 3 hpf until 24 hpf with 100 mM ethanol, 10−9 M retinoic acid, or 100 mM ethanol and 10−9 M retinoic acid together, and body length of these embryos were compared to untreated control embryos (Figure 3B and Table III). Average embryo body length for ethanol treatment was reduced to 3.10 mm (±0.25) from 3.39 mm (±0.12) in the control group, which was significantly different. Treating embryos with both 100 mM ethanol and 10−9 M retinoic acid increased the average embryo body length to 3.23 mm (±0.12) as compared with ethanol treated embryos (3.10 mm ±0.25), and this was statistically different from ethanol treated embryos (Figure 3B and Table III). Treating embryos with retinoic acid alone reduced body length to 3.26 mm (±0.12) as compared with control embryos. Retinoic acid treated embryos average body length was not statistically different from embryos treated with both ethanol and retinoic acid.
Table III. Body length (in mm) at 30 hpf in ethanol and retinoic acid treated embryos.
Embryos were treated from 3 hpf until 24 hpf (during gastrulation and somitogenesis) with 100 mM ethanol, 10−9 M retinoic acid, or 100 mM ethanol and 10−9 M retinoic acid together, and compared to untreated control embryos. At 30 hpf embryos were fixed using paraformaldehyde to stop development, and these embryos were imaged using a stereo microscope. Embryo length was measured along the dorsal surface of the embryos from the most anterior point where the embryo proper touches the yolk to the tip of the tail using ImageJ image analysis software (see Materials and Methods). Two-way ANOVA was performed to compare treatment groups. Ethanol-by-retinoic acid interaction was significant (P=0.0005), and pairwise comparison P-values are shown. Bonferroni adjustment for multiple (six) comparisons was used to ensure an overall ninety five percent confidence. Thus, P-value≤0.008 was used to conclude that groups were statistically different.
| Control | Ethanol | Ethanol+RA | RA only | |
|---|---|---|---|---|
| Mean | 3.39 | 3.10 | 3.23 | 3.26 |
| SD | 0.12 | 0.25 | 0.12 | 0.12 |
| n | 19 | 19 | 21 | 19 |
| P (vs Control) | <0.0001 | 0.0026 | 0.0206 | |
| P (vs Ethanol) | 0.0068 | 0.0011 | ||
| P (vs RA) | 0.4895 |
Ethanol effects on the early embryo development resemble prechordal plate signaling defects, namely, gastrulation defects, producing dorsalized embryos (reduced forebrain, ventral neural structures, like eyes) (Blader and Strahle, 1998; Yelin et al., 2007). We also noted that neural axis defects were most severe caudally (forebrain), and hindbrain defects were also apparent (Figure 4). Treating embryos with ethanol and retinoic acid partially rescued brain development defects (Figure 4; for example, forebrain: see asterisk symbols: eye, hindbrain and otic vesicle development was restored). To quantify an ethanol-induced neural defect, ear circumference (a direct measure of ear development) was measured. Ear circumference was reduced by 100 mM ethanol as compared with untreated controls, and ear development was restored when treated with 100 mM ethanol plus 10−9 M retinoic acid (Figure 3C and Table IV). Retinoic acid treatment alone at this early developmental stage had very little or no effect on ear circumference (Figure 3C and Table IV).
Figure 4.
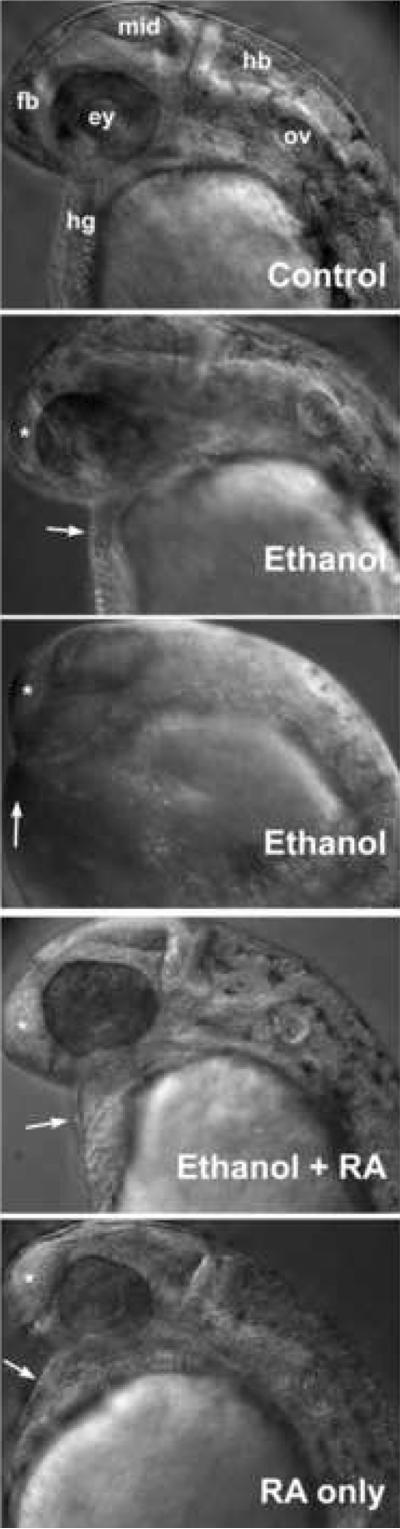
Ethanol treatment of zebrafish embryos during gastrulation and somitogenesis stages reduces neural axis patterning (particularly forebrain) and reduces hatching gland formation (a prechordal plate derivative), which was improved by including retinoic acid during ethanol treatment. Lateral views (anterior is left) of 30 hpf living embryos were imaged using DIC microscopy. Embryos were untreated in control embryos (top panel), and treated with 100 mM ethanol (second and third panels from top, note reduced forebrain, white asterisks, and reduced hatching gland, white arrows, which was variable), 100 mM ethanol and 10−9 M retinoic acid (fourth panels from top), and 10−9 M retinoic acid alone (bottom panel) from 3 hpf until 24 hpf (see Materials and Methods). At 24 hpf, ethanol and/or retinoic acid treatments were discontinued, and embryos were incubated with normal embryo medium until 30 dpf, anesthetized and imaged. Abbreviations: ey, eye; fb, forebrain; hb, hindbrain; hg, hatching gland; mid, midbrain; and ov, otic vesicle. In the four lower panels, asterisks indicate forebrains, and the hatching glands are indicated by arrows.
Discussion
The zebrafish has emerged as a powerful developmental genetics model organism, particularly for studies of early developmental processes. Comparing ethanol and retinoic treatment with embryos treated with both chemicals during gastrulation and somitogenesis showed a rescue phenotype. This experimental model was evaluated to determine the extent of the rescue phenotype. We contend that our findings are a necessary foundation that validate our experimental approach and will lead to a more detailed understanding of the ethanol effects on early vertebrate development.
Sulik and colleagues showed that an animal (mouse) model could recapitulate developmental defects seen in human patients (Sulik et al., 1981). They showed that short-term ethanol treatment during gastrulation affects craniofacial development and neural axis patterning. Detailed studies of ethanol-induced defects during mouse gastrulation are difficult because early development in mammalian systems occurs in utero. Non-mammalian vertebrate developmental model systems, like Xenopus and zebrafish, are better adapted to examine mechanistic information about early embryogenesis (Duncan and Su, 2004; Schier and Talbot, 2005). However, high ethanol concentrations (1–3% or 220–650 mM) were generally used in these experiments, which make it difficult to know whether pathophysiological effects are being modeled using these experimental models. The Carvan laboratory showed that pathophysiological ethanol concentrations (10–100 mM or 46–460 mg%) produce the FASD phenotype in zebrafish (Carvan et al., 2004; Loucks and Carvan, 2004).
Our current findings support previous findings (Blader and Strahle, 1998; Johnson et al., 2007; Yelin et al., 2007; Yelin et al., 2005): (i) that retinoic acid signaling was inhibited by ethanol exposure during gastrulation; (ii) that ethanol exposure to the early embryo inhibited extension of the anterior-posterior axis; and (iii) that ethanol treatment affected early gastrulation cell migration and resembles effects seen when prechordal plate signaling is reduced.
Ethanol-induced developmental defects could be quantitatively, but partially rescued by including retinoic acid during the ethanol treatment. It is significant that embryos rescued by the combined treatment of ethanol and retinoic acid quantitatively and morphologically resembled embryos treated with retinoic acid alone, suggesting that ethanol-induced defects are rescued to the level of retinoic acid toxicity. Additional experiments will be required to determine specific molecular events during early gastrulation and somitogenesis that are affected by ethanol treatment.
It is possible that retinoic acid is only masking effects from another, parallel pathway. However, average ethmoid plate cartilage width was larger than control embryos in embryos treated with retinoic acid alone and in embryos co-treated with ethanol and retinoic acid, but ethanol treated embryos had reduced ethmoid plate cartilage width as compared to control embryos, showing opposite effects. Additionally, raldh2 mutant and DEAB (retinaldehyde dehydrogenase specific retinoic acid biosynthesis inhibitor) treatment phenotypes can be rescued by retinoic acid treatment (Begemann et al., 2001; Perz-Edwards et al., 2001; Grandel et al., 2002), which is similar to the retinoic acid rescue of ethanol defects that we observed. Retinoid rescue of ethanol defects, first experimental support reported by Fainsod and colleagues using Xenopus (Yelin et al., 2005), and the similarity between effects of ethanol treatment and mutations affecting retinoic signaling (Duester, 1991; Leo and Lieber, 1999; Wang, 2005; Zachman and Grummer, 1998) can be most parsimoniously explained within a retinoic acid signaling model of embryonic ethanol exposure. We propose that the zebrafish model described here will be useful to study a variety of molecular hypotheses for embryonic ethanol toxicity.
Supplementary Material
Movie S1. Ethanol treatment of zebrafish embryos during gastrulation and somitogenesis stages produces a motility phenotype that was improved by including retinoic acid during ethanol treatment. One minute duration video sequences were collected of 4.5 dpf larvae, using a steromicroscope. Control embryos were untreated (control, upper left panel); treated with 100 mM ethanol (ethanol, upper right panel; note FASD phenotype is variable); treated with 100 mM ethanol and 10−9 M retinoic acid (ethanol+RA, lower left panel); and treated with 10−9 M retinoic acid alone (RA, lower right panel) from 3 hpf until 24 hpf (see Materials and Methods). At 24 hpf, ethanol and/or retinoic acid treatments were discontinued, and embryos were incubated with normal embryo medium until 4.5 dpf. For control, ethanol+RA, and RA, three randomly selected larvae were placed in a drop of embryo medium on a depression slide. For ethanol samples, two severely affected embryos and one slightly affected embryo were selected to visualize the spectrum of defects. Both severely affected, ethanol-treated larvae in this video sequence were tested before and after the video recording, and they responded to a probe touching their tails, by swimming rapidly, but uncoordinatedly away from the probe. In higher magnification observations, the two severely affected embryos had beating hearts before and after the video recording.
Acknowledgments
The authors thank Jeremy Jerford, Patrick Gentry, David Southern, Brad Poteat and Alice Nakatsuka for technical assistance, preliminary experiments and helpful discussions. This work was supported by NIH grant R21AA015938 to WFB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Begemann G, Meyer A. Hindbrain patterning revisited: timing and effects of retinoic acid signalling. Bioessays. 2001;23:981–986. doi: 10.1002/bies.1142. [DOI] [PubMed] [Google Scholar]
- Begemann G, Schilling TF, Rauch GJ, Geisler R, Ingham PW. The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development. 2001;128:3081–3094. doi: 10.1242/dev.128.16.3081. [DOI] [PubMed] [Google Scholar]
- Blader P, Strahle U. Ethanol impairs migration of the prechordal plate in the zebrafish embryo. Dev. Biol. 1998;201:185–201. doi: 10.1006/dbio.1998.8995. [DOI] [PubMed] [Google Scholar]
- Bradfield JY, West JR, Maier SE. Uptake and elimination of ethanol by young zebrafish embryos. Neurotoxicol. Teratol. 2006;28:629–633. doi: 10.1016/j.ntt.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Carvan MJ, 3rd, Loucks E, Weber DN, Williams FE. Ethanol effects on the developing zebrafish: neurobehavior and skeletal morphogenesis. Neurotoxicol. Teratol. 2004;26:757–768. doi: 10.1016/j.ntt.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Collins MD, Mao GE. Teratology of retinoids. Annu. Rev. Pharmacol. Toxicol. 1999;39:399–430. doi: 10.1146/annurev.pharmtox.39.1.399. [DOI] [PubMed] [Google Scholar]
- Duester G. A hypothetical mechanism for fetal alcohol syndrome involving ethanol inhibition of retinoic acid synthesis at the alcohol dehydrogenase step. Alcohol. Clin. Exp. Res. 1991;15:568–572. doi: 10.1111/j.1530-0277.1991.tb00562.x. [DOI] [PubMed] [Google Scholar]
- Duncan T, Su TT. Embryogenesis: coordinating cell division with gastrulation. Curr. Biol. 2004;14:R305–307. doi: 10.1016/j.cub.2004.03.050. [DOI] [PubMed] [Google Scholar]
- Dupe V, Lumsden A. Hindbrain patterning involves graded responses to retinoic acid signalling. Development. 2001;128:2199–2208. doi: 10.1242/dev.128.12.2199. [DOI] [PubMed] [Google Scholar]
- Grandel H, Lun K, Rauch GJ, Rhinn M, Piotrowski T, Houart C, Sordino P, Kuchler AM, Schulte-Merker S, Geisler R, et al. Retinoic acid signalling in the zebrafish embryo is necessary during pre-segmentation stages to pattern the anterior-posterior axis of the CNS and to induce a pectoral fin bud. Development. 2002;129:2851–2865. doi: 10.1242/dev.129.12.2851. [DOI] [PubMed] [Google Scholar]
- Johnson CS, Zucker RM, Hunter ES, 3rd, Sulik KK. Perturbation of retinoic acid (RA)-mediated limb development suggests a role for diminished RA signaling in the teratogenesis of ethanol. Birth Defects Res. A Clin. Mol. Teratol. 2007;79:631–641. doi: 10.1002/bdra.20385. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Leo MA, Lieber CS. Alcohol, vitamin A, and beta-carotene: adverse interactions, including hepatotoxicity and carcinogenicity. Am. J. Clin. Nutr. 1999;69:1071–1085. doi: 10.1093/ajcn/69.6.1071. [DOI] [PubMed] [Google Scholar]
- Loucks E, Carvan MJ., 3rd Strain-dependent effects of developmental ethanol exposure in zebrafish. Neurotoxicol. Teratol. 2004;26:745–755. doi: 10.1016/j.ntt.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Lumsden A, Krumlauf R. Patterning the vertebrate neuraxis. Science. 1996;274:1109–1115. doi: 10.1126/science.274.5290.1109. [DOI] [PubMed] [Google Scholar]
- Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu. Rev. Pharmacol. Toxicol. 2006;46:451–480. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- McCaffery P, Koul O, Smith D, Napoli JL, Chen N, Ullman MD. Ethanol increases retinoic acid production in cerebellar astrocytes and in cerebellum. Brain Res. Dev. Brain Res. 2004;153:233–241. doi: 10.1016/j.devbrainres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Perz-Edwards A, Hardison NL, Linney E. Retinoic acid-mediated gene expression in transgenic reporter zebrafish. Dev. Biol. 2001;229:89–101. doi: 10.1006/dbio.2000.9979. [DOI] [PubMed] [Google Scholar]
- Pullarkat RK. Hypothesis: prenatal ethanol-induced birth defects and retinoic acid. Alcohol. Clin. Exp. Res. 1991;15:565–567. doi: 10.1111/j.1530-0277.1991.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Schier AF, Talbot WS. Molecular genetics of axis formation in zebrafish. Annu. Rev. Genet. 2005;39:561–613. doi: 10.1146/annurev.genet.37.110801.143752. [DOI] [PubMed] [Google Scholar]
- Sulik KK. Genesis of alcohol-induced craniofacial dysmorphism. Exp. Biol. Med. (Maywood) 2005;230:366–375. doi: 10.1177/15353702-0323006-04. [DOI] [PubMed] [Google Scholar]
- Sulik KK, Johnston MC, Webb MA. Fetal alcohol syndrome: embryogenesis in a mouse model. Science. 1981;214:936–938. doi: 10.1126/science.6795717. [DOI] [PubMed] [Google Scholar]
- Wang XD. Alcoho l, vitamin A, and cancer. Alcohol. 2005;35:251–258. doi: 10.1016/j.alcohol.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Warren KR, Foudin LL. Alcohol-related birth defects--the past, present, and future. Alcohol Res. Health. 2001;25:153–158. [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book. The University of Oregon Press; Eugene, OR: 2000. [Google Scholar]
- Yelin R, Kot H, Yelin D, Fainsod A. Early molecular effects of ethanol during vertebrate embryogenesis. Differentiation. 2007;75:393–403. doi: 10.1111/j.1432-0436.2006.00147.x. [DOI] [PubMed] [Google Scholar]
- Yelin R, Schyr RB, Kot H, Zins S, Frumkin A, Pillemer G, Fainsod A. Ethanol exposure affects gene expression in the embryonic organizer and reduces retinoic acid levels. Dev. Biol. 2005;279:193–204. doi: 10.1016/j.ydbio.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Zachman RD, Grummer MA. The interaction of ethanol and vitamin A as a potential mechanism for the pathogenesis of Fetal Alcohol syndrome. Alcohol. Clin. Exp. Res. 1998;22:1544–1556. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1. Ethanol treatment of zebrafish embryos during gastrulation and somitogenesis stages produces a motility phenotype that was improved by including retinoic acid during ethanol treatment. One minute duration video sequences were collected of 4.5 dpf larvae, using a steromicroscope. Control embryos were untreated (control, upper left panel); treated with 100 mM ethanol (ethanol, upper right panel; note FASD phenotype is variable); treated with 100 mM ethanol and 10−9 M retinoic acid (ethanol+RA, lower left panel); and treated with 10−9 M retinoic acid alone (RA, lower right panel) from 3 hpf until 24 hpf (see Materials and Methods). At 24 hpf, ethanol and/or retinoic acid treatments were discontinued, and embryos were incubated with normal embryo medium until 4.5 dpf. For control, ethanol+RA, and RA, three randomly selected larvae were placed in a drop of embryo medium on a depression slide. For ethanol samples, two severely affected embryos and one slightly affected embryo were selected to visualize the spectrum of defects. Both severely affected, ethanol-treated larvae in this video sequence were tested before and after the video recording, and they responded to a probe touching their tails, by swimming rapidly, but uncoordinatedly away from the probe. In higher magnification observations, the two severely affected embryos had beating hearts before and after the video recording.