Abstract
Amphiphilic homopolymer films have been immobilized onto substrates to study the interactions of these polymers with proteins. X-ray photoelectron spectroscopy (XPS) was utilized to measure the amount of protein adsorption. Amphiphilic homopolymers have been shown to reduce protein adsorption, despite the high affinity of the hydrophobic or hydrophilic functional groups by themselves toward proteins. This protein resistant property seems to arise from the unique molecular scale alternation of incompatible functionalities. The combination of incompatible functionalities with a pre-defined alternating pattern within monomer could provide a potential design for non-fouling materials.
Introduction
Controlling protein adsorption has always been an interesting research area, since the accumulation of proteins at interfaces could have beneficial and detrimental effects. Protein surface binding based on specific ligand-receptor interactions is often desired, because these have the potential for new opportunities in biotechnology, e.g. biosensing.1,2 On the other hand, the non-specific adsorption of proteins could lead to biofouling, which is considered to be a cause for failure of materials in several areas.3–7 Note that proteins are amphipathic macromolecules and therefore they contain surfaces to bind to most materials that are in continuous contact with aqueous biological environment. Therefore, controlling the protein adsorption has been a major challenge in the field of protein-material interactions.
Proteins are polyampholytes, i.e. they have both positively and negatively charged surfaces within a single protein. Therefore, most proteins can easily adhere to positively or negatively charged surfaces.8–10 Polyethyleneglycol (PEG), which is charge neutral and widely considered to be hydrophilic, has been extensively explored as a viable material to significantly reduce non-specific adsorption of proteins.11–15 If the charge neutrality of PEG is the primary driving force, it has also been hypothesized that zwitterionic polymers should also be able to afford considerably lower non-specific adsorption of proteins. Indeed, zwitterionic polymers with both charges balanced in every repeating unit show magnificent adsorption resistance.16,17 While the overall charge neutrality might significantly contribute to the protein binding, it is also possible that the proximity of the positively and negatively charged surfaces in each repeat unit contributes significantly to the reduced non-specific adsorption, since this proximity provides conflicting interactions with the charged surfaces of the polyampholytes, proteins. While it is difficult to imagine a system that delineates this subtlety with polyelectrolytes, we envisioned the possibility of using a combination of hydrophilic and lipophilic functionalities in polymers that provide conflicting interactions in a molecular scale on a surface.
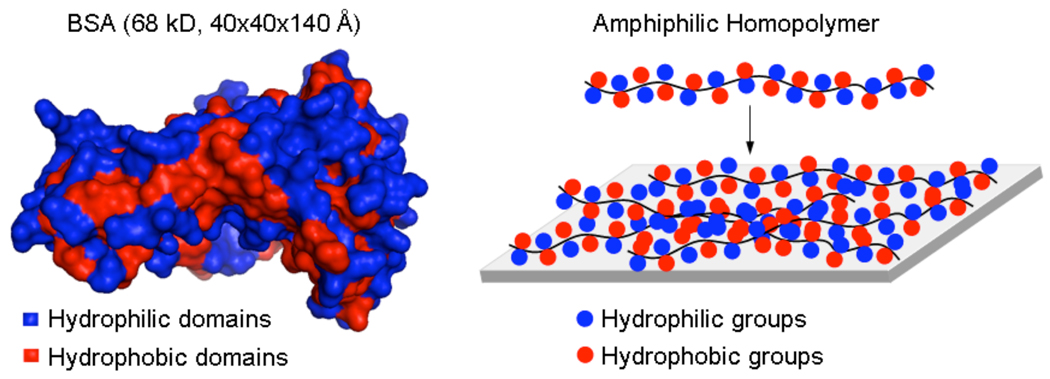
It is well known that both charged hydrophilic functionalities such as carboxylic acids and hydrophobic functionalities, such as alkyl groups, have significant binding affinity towards proteins.18–21 Our hypothesis is that if these incompatible functionalities were presented periodically within 2–3Å of each other, then the non-specific adsorption of proteins would be reduced. The premise for the hypothesis is that despite the fact that proteins have a diverse set of functionalized surfaces, the perfectly alternating presentation of hydrophilic and hydrophobic groups is not common, if not unavailable. An example of a protein structure is shown in Figure 1 with bovine serum albumin (BSA), where patches of hydrophilic and hydrophobic functionalities are distinctly highlighted. These patches are clearly much larger than a few angstroms. Therefore, we hypothesized that if a part of the surface in the protein has an affinity towards the charged hydrophilic domain of the polymer, the hydrophobic part of the polymer will be incompatible with the binding and vice versa. We used amphiphilic homopolymers to test this hypothesis and report the findings in this manuscript.
Figure 1.
Structure of BSA (PDB code: 1a06) and a hypothetical structure provided by an amphiphilic homopolymer containing hydrophilic and hydrophobic groups in every repeat unit.
Results and Discussion
We have recently introduced a new class of amphiphilic homopolymers, where the hydrophilic and hydrophobic functionalities are presented in the same monomer.22–24 These polymers are ideal architectures to obtain the proof-of-principle for the design hypothesis, since the placement of the hydrophilic and hydrophobic functionalities in the same monomer provides a pre-defined pattern of the functional group placement throughout the polymer surface (Figure 1). In our molecular design, the hydrophilic carboxylic acid functionality and the hydrophobic alkyl moiety are both located at meta-positions with respect to each other on the benzene ring of a polystyrene backbone. Thus we envision that these polymer films would be able to provide the desired periodic alternations for protein resistance. Solvents such as water and toluene have been shown to selectively solvate the hydrophilic and hydrophobic functionalities respectively. On the other hand, we have also shown that DMF is a non-selective solvent for this polymer.25 Since we are interested in presenting both of these functionalities on the surface in this study, DMF was used as the solvent to incorporate the polymers on surfaces in all our experiments.
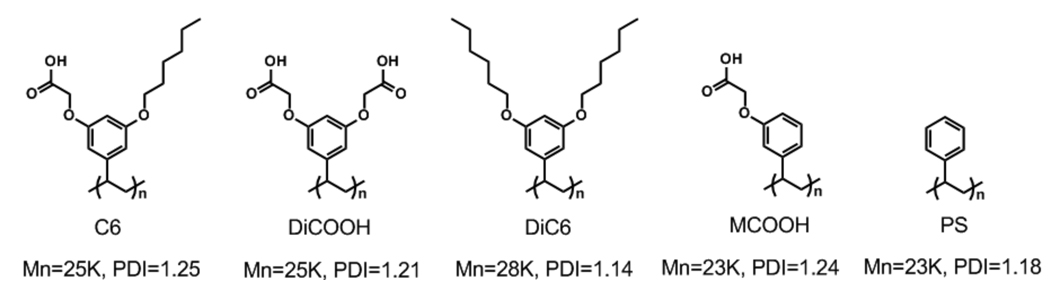
To obtain the initial proof-of-concept, we synthesized an amphiphilic homopolymer C6 containing a hexyl functionality as the hydrophobic chain and the carboxylic acid as the hydrophilic functionality (Figure 2). Note that our hypothesis is that the presentation of amphiphilic functionalities would result in reduced non-specific protein adsorption. We have synthesized three different polymers that are similar in structure, but do not present amphiphilic functionalities in their backbone. The polymer, DiCOOH, contains two hydrophilic carboxylic acid functionalities, while the polymer DiC6 contains two lipophilic hexyl functionalities. The third polymer, MCOOH, contains only one carboxylic acid functionality and also does not contain any lipophilic functionality. Polystyrene (PS) is used as the control polymer in all cases.
Figure 2.
Structures of the C6 amphiphilic polymer and the corresponding control polymers.
The polymer surfaces were prepared by spin-coating a solution of these molecules on to a silica or glass slide. Since the alkyl groups exhibit lower surface energy, it is possible that the optimal conformation of these polymers on surfaces would be such that the carboxylic acid functionalities are buried upon depositing the polymer on the silica surface. To analyze for this, we investigated the water contact angle (CA) measurements of the polymer-modified surfaces. The advancing CA (θa) for the amphiphilic polymer C6 is 52°, while the receding CA (θr) is 29°. The CAs obtained from the DiCOOH and MCOOH control polymers are similar to those for the amphiphilic polymers,26 while CAs of the DiC6 were significantly different (θa/θr=83°/64°). The CAs for the PS surface was found to be θa/θr=90°/74°. These results indicate that the hydrophilic carboxylic acid functionalities are indeed significantly exposed on the amphiphilic homopolymer surface.
Quantitative measurement of protein adsorption on a surface is difficult and every method, such as QCM27,28 and SPR29,30, has its own assumption to convert its response to the amount of adsorbed proteins. In our measurements, we used X-ray photoelectron spectroscopy (XPS)31–34 to analyze the polymer surfaces after 24 hours of continuous contact with a variety of proteins at a concentration of 1 mg/mL at 25 °C. As shown in Figure 2, none of the polymers used in this study contains any nitrogen atom. Therefore, we detect the protein adsorption using the 400 eV peak for N1s, the source of which could only be the protein.
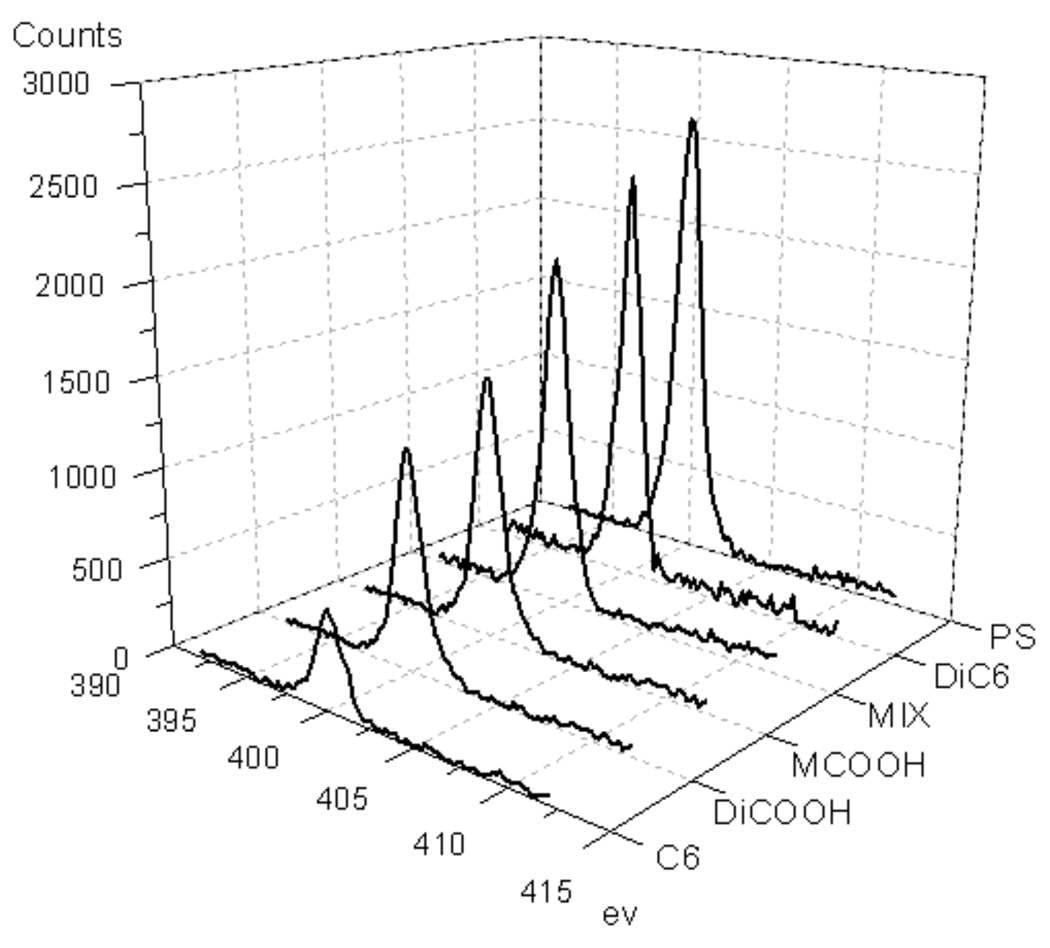
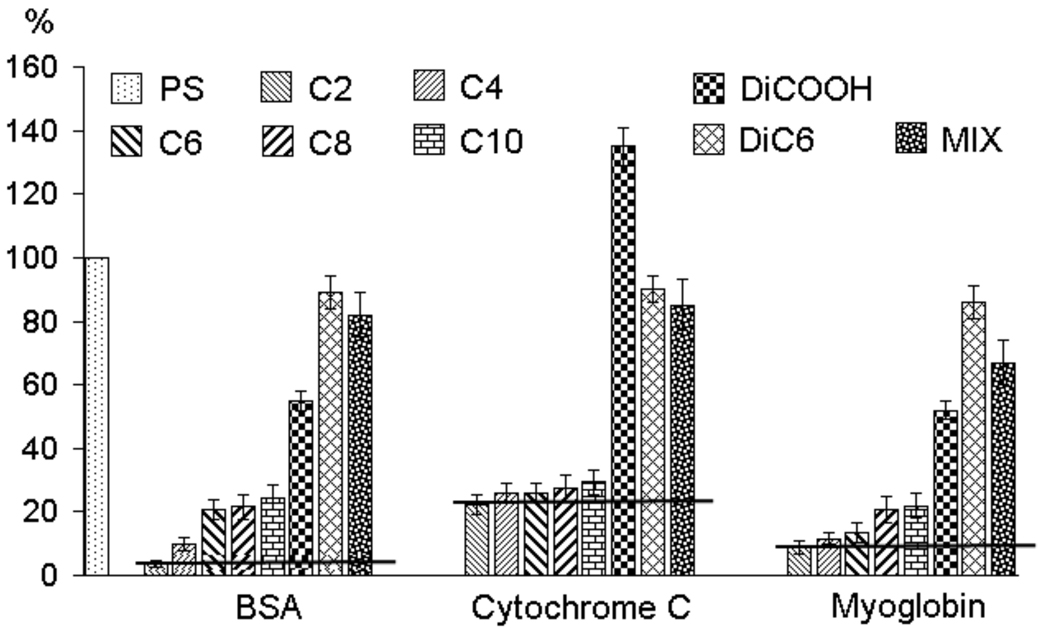
The relative intensities of the N1s XPS peak for different polymer surfaces are shown in Figure 3 using bovine serum albumin (BSA) as the protein. With PS as the control surface, the intensity observed for this surface is considered to have 100% adsorption and other surfaces can be quantified relative to this material. We were gratified to find that the amphiphilic homopolymer C6 exhibits a protein adsorption of only about 21%. Interestingly however, the polymers DiCOOH and DiC6 with only one of the functionalities exhibit much higher adsorption, 55% and 89%, respectively. Similarly, MCOOH surface also exhibits protein adsorption in between those of DiCOOH and DiC6. These results provide the initial evidence supporting our hypothesis that the presentation of the amphiphilic functionalities would indeed reduce non-specific adsorption of proteins. Next, we were interested in obtaining evidence whether the Angstrom scale presentation of the amphiphilic functionalities plays a role in the reduced protein adsorption on surfaces. To test this, we generated a surface using a physical mixture of the DiCOOH and DiC6 polymers (MIX). Again, the observed protein adsorption was found to be in between those of the individual DiCOOH and DiC6 surfaces. This result provides further support for our hypothesis.
Figure 3.
XPS spectra of N1s from surfaces (after immersion in BSA).
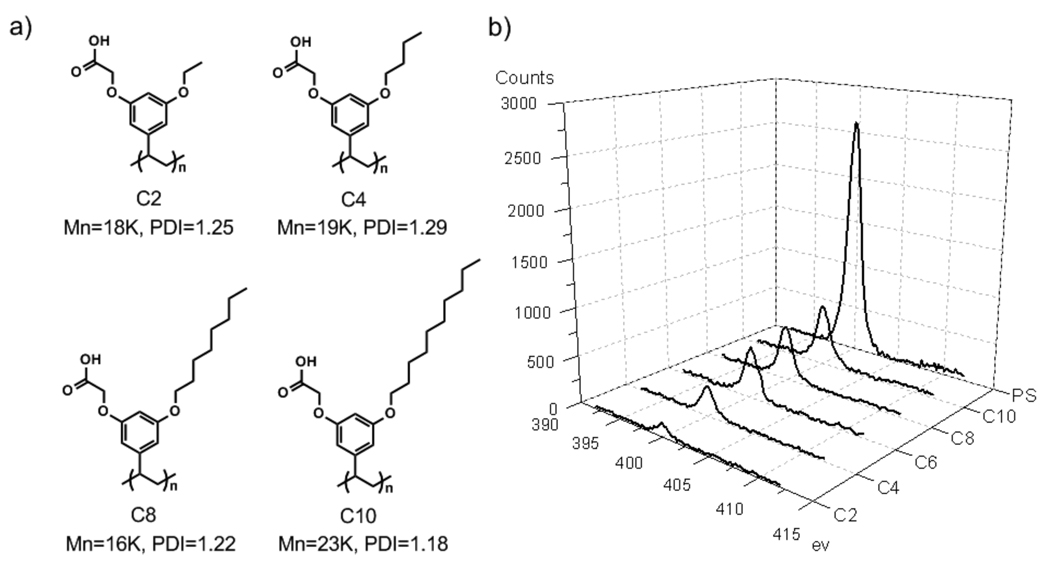
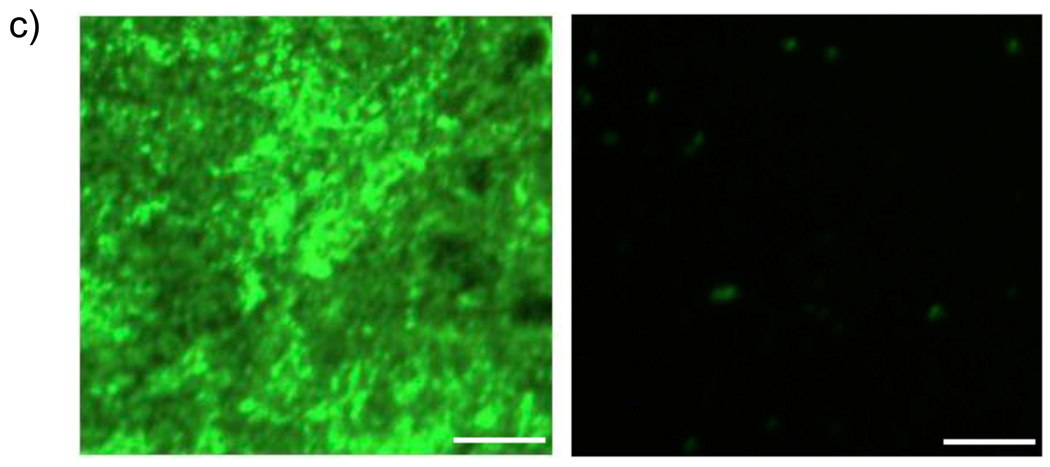
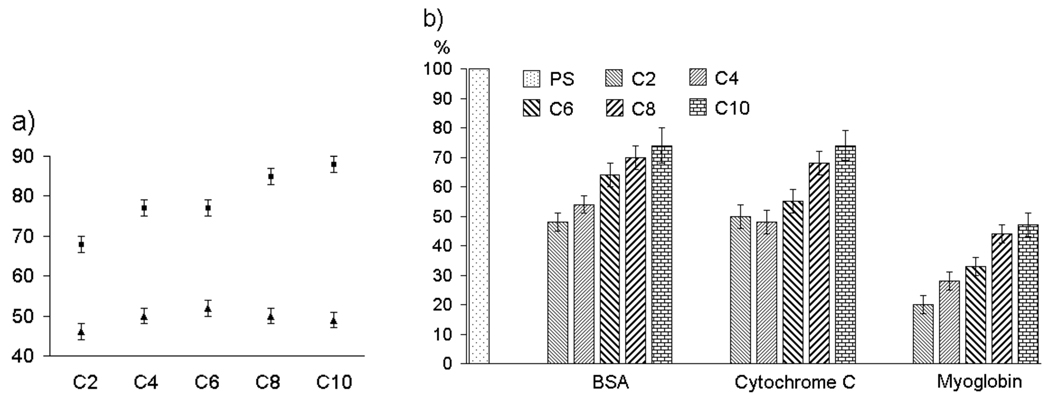
Among the amphiphilic polymers, we also hypothesized that the length of the alkyl chain would influence the extent of protein adsorption. We attribute this to the assumption that the hydrophilic and hydrophobic groups should be similar in length to achieve the optimized surface presentation, where neither hydrophilic nor hydrophobic functional groups dominate the presentation. For this purpose, we synthesized polymers with different alkyl chain lengths shown in Figure 4a. XPS spectra of N1s from these polymer surfaces are shown in Figure 4b. It is clear from this data that there is a dependence of hydrophobic group lengths. Polymer C2, where the hydrophobic alkyl chain is an ethyl functionality, seems to exhibit the best surface among the polymers investigated. The fact that the polymer MCOOH, which has no alkyl chain at all, exhibits higher adsorption of BSA provides further support for the hypothesis. It is also interesting to note that even the C10 polymer, where the hydrophobic chain is a decyl functionality neither exhibits a highly hydrophobic surface, from CA measurements, or has as high protein adsorption as the hydrophobic polymers, such as PS and DiC6. Considering that the C2 polymer provides a very low protein adsorption, we also wanted to at least qualitatively visualize this with a complementary method. For this purpose, we utilized fluorescein-conjugated BSA (FITC-BSA) for testing the protein adsorption using fluorescence microscopy. The results of these studies are shown in Figure 4c. The contrast in the extent of fluorescence from the surface provided by the C2 polymer and that of the PS surface indeed confirms that the surface provided by the amphiphilic homopolymer indeed exhibits much lower protein adsorption.
Figure 4.
a) Structures of amphiphilic polymers; b) XPS spectra of N1s from surfaces (after immersion in BSA); c) Fluorescence images of FITC-BSA adsorption on PS (left) and C2 (right). Scale bar: 50 µm.
Note that these polymers were simply spin-coated to the substrate. Therefore, we were interested in studying the integrity of the coating after immersion in aqueous media. After 24 hours of immersion, we found that there was about 20–30% loss of the polymer coatings. Although this seems like a significant loss, this is quite reasonable for a coating that is not covalently immobilized. Since all our polymers are polystyrene based, we immobilized the polymers by UV irradiation to achieve a robust attachment to surface and thus withstand the immersion in the buffer.26,35 This process provided very little change in the water contact angles of the polymer-modified surfaces and the protein adsorption characteristics were also fully retained.
If our approach were to have a broad repertoire and more importantly if our molecular design hypothesis were correct, it is also necessary that C2 exhibit consistently lower adsorption to proteins independent of their isoelectric points (pIs). All amphiphilic homopolymer surfaces displayed significantly lower non-specific adsorption of proteins, relative to the all hydrophilic or all hydrophobic control polymers, as shown in Figure 5. It should be noted, however, that the overall protein adsorption seems to be dependent on the pI. Positively charged protein cytochrome c (pI=10.6) does adsorb to the polymer-modified surfaces more than either the negatively charged BSA (pI=4.8) or the neutral myoglobin (pI=6.8). While this suggests that the nature of the hydrophilic functionality does influence the adsorption properties, the fact remains that systematic patterns of hydrophobic and hydrophilic functionalities at the molecular level indeed provide much lesser non-specific adsorption. We have also tested this with an additional set of positive, negative and neutral proteins and the results are consistent with the results in Figure 5.26
Figure 5.
Relative extent of protein adsorption (without solvent annealing treatment). PS was set to 100% as reference. The black scale bar was drawn for visual comparison between C2 and other polymers.
In order to further test whether the presentation of alternating hydrophilic and hydrophobic moieties on surfaces in the angstrom scale is indeed the reason, we envisaged the possibility of utilizing the same polymer but alter the presentation of the functionalities. We envisaged that if the amphiphilic polymer films were annealed with an apolar solvent, the presentation of the functionalities will be mostly hydrophobic.24 This presentation of the functionalities can be locked-in by immobilizing the polymer using the UV irradiation process. To identify, whether such a processing of the polymer films indeed resulted in the presentation of hydrophobic functionalities, we analyzed the contact angles of the polymer films after solvent annealing and UV-curing. As expected, there is a significant increase in the contact angle of the polymer film suggesting that the solvent annealing process indeed results in a hydrophobic surface, as shown in Figure 6a. Similarly, the protein adsorption of all these amphiphilic polymer films dramatically increased, as shown in Figure 6b. These results provide further support for our hypothesis that the equal presentation of the incompatible functionalities is an essential component for the anti-fouling properties observed with our amphiphilic homopolymers.
Figure 6.
(a) Water contact angle data in degrees for polymer coatings without (triangle) and with (square) solvent annealing; (b) Relative extent of protein adsorption (with solvent annealing treatment). PS was set to 100% as reference.
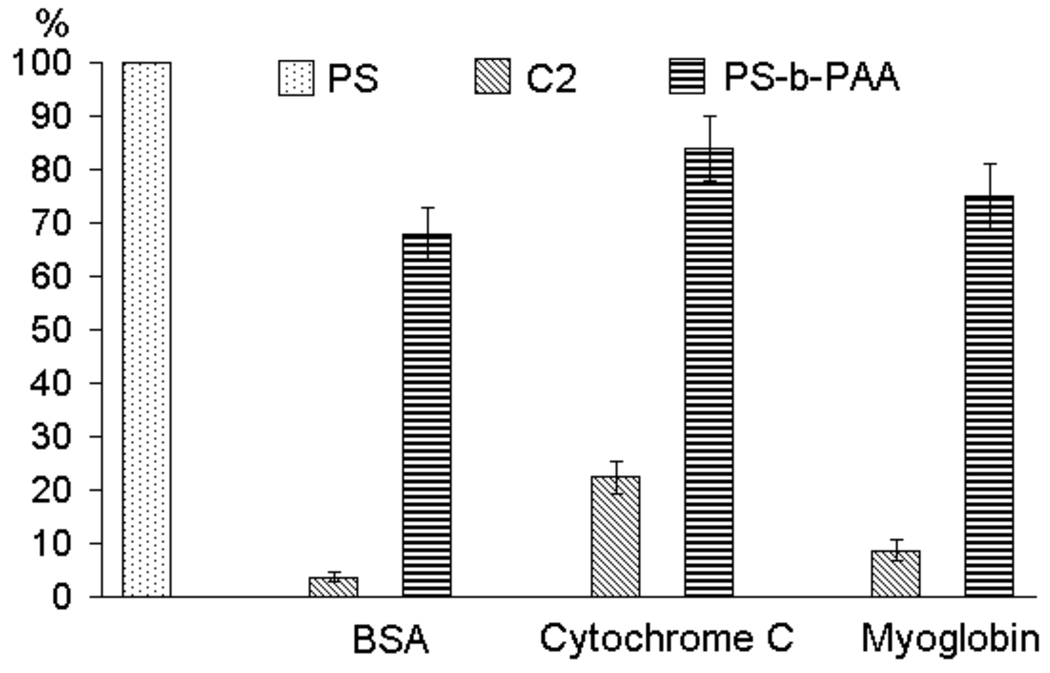
Considering our design requirement that needs alternations of the amphiphilic functionalities in Angstrom scale, it is interesting to ask: can block copolymers with the alternations in several nanometers scale exhibit similar reduction in protein adsorption? To address this question, we carried out experiments with an amphiphilic block copolymer, poly(styrene)-b-poly(acrylic acid) (PS-b-PAA). The results are shown in Figure 7 and it is readily apparent that block copolymers do not provide the extent of anti-fouling properties that our amphiphilic homopolymer C2 does. This is also an additional evidence that the presentation of both hydrophilic and hydrophobic functionalities alone is not sufficient. It is necessary that the molecular scale alternations be provided in the hydrophobic and hydrophilic functionalities for protein resistance.
Figure 7.
Comparison of protein adsorption between the amphiphilic homopolymer C2 and the amphiphilic block copolymer (PS-b-PAA).
Finally, we were also interested in identifying the effect of temperature upon protein binding.36 This is interesting, because increase in temperature results in enhanced conformational mobility in both protein and polymer. It is interesting then to probe the effect of these mobilities upon non-specific adsorption. We studied the extent of protein adsorption at 55 °C, since melting temperature of most of these proteins are ~ 60 °C. We observed very little difference between the results of 25 °C and 55 °C, indicating the versatility of our approach.26
Conclusion
In summary, we have shown that: (i) amphiphilic homopolymers, containing a hydrophilic and a hydrophobic functionality in every repeat unit, exhibit reduced non-specific protein adsorption. (ii) When the hydrophilic or lipophilic groups are presented alone, the surfaces show high affinity toward proteins. (iii) Loss of polymers from surfaces over time is obviated by crosslinking the polymers to the surfaces by UV-irradiation without compromising the anti-fouling properties of the surfaces. (iv) The protein-adsorption is dependent on the length of the lipophilic chain relative to the length of the hydrophilic functionality on the surface. (v) The simultaneous presentation of the hydrophilic and lipophilic functionalities is important for the reduced non-specific adsorption, as demonstrated by testing the surfaces after annealing them with an apolar solvent, toluene. (vi) Block copolymers, which present hydrophilic and lipophilic functionalities in nanoscale dimensions, do not provide the antifouling properties seen with the amphiphilic homopolymers with comparable functionalites. It is important to recognize that the surfaces shown here do not necessarily rival the PEG surfaces or the zwitterionic polymer surfaces for the anti-fouling technology. However, our studies here do provide a fundamentally different viewpoint at approaching the design of anti-fouling surfaces with a pre-defined pattern in the molecular scale. The styrenic polymer backbone also provides a facile way to immobilize these materials onto substrates. To achieve non-fouling polymers with better performance, exploring different combinations of incompatible functional groups based on this molecular design is underway in our laboratory.
Experimental Section
All chemicals and proteins were obtained from Sigma–Aldrich (St. Louis, MO, USA) unless otherwise noted. Polymers were synthesized according to the previously reported method.19 Briefly, the amphiphilic homopolymers were synthesized from the corresponding styrene monomers with a tert-butyl ester group and an alkyl group at each meta position relative to the olefinic group, followed by the hydrolysis of the tert-butyl ester group. The number average molecular weight (Mn) was determined by size exclusion chromatography (SEC), performed before the hydrolysis of tert-butyl ester group.
Polymer coatings preparation: All polymers were dissolved in DMF at the concentration of 10−4 M. Polymer coatings were prepared by spin-coating polymer solutions onto clean silicon wafers at 2000 rpm for 3 minutes. The polymer coatings were vacuumed at room temperature overnight before storing in argon filled vials. No residual DMF was observed in the polymer films as determined by XPS.
Polymer films immobilization: Polymer films were immobilized by UV irradiation (254 nm) 30 min at room temperature.
Contact angle (CA) measurements were made with a Ramé-Hart telescopic goniometer and a Gilmont syringe with a 24-gauge flat-tipped needle. The probe fluids used were Milli-Q water. Dynamic advancing (θa) and receding angles (θr) were recorded while the probe fluid was added to and withdrawn from the drop, respectively. The values reported are averages of 4–6 measurements made on different areas of each sample surface.
X-ray photoelectron spectra: XPS were recorded on a Physical Electronics Quantum 2000 spectrometer with Al KR excitation. Spectra were obtained at 45° takeoff angle. For each sample, three different sites were measured with a spot size of 100 µm diameter. Each measurement included a survey with 3 sweeps and a high resolution for N1s with 8 sweeps.
Protein adsorption studies: The polymer coatings (on silicon wafers) were immersed in deionized water for 10–15 min. The samples were then transferred into the vials containing 1 mg/mL of protein in PBS buffer (pH 7.4) and incubated at 25 °C for 24 hours. The samples were then taken out from the vials and rinsed with deionized water three times to remove the unbound proteins. Protein adsorption measurements of these samples were then immediately performed by X-ray photoelectron spectroscopy. The procedure for protein adsorption study at elevated temperature is the same as the one for 25 °C, except that the vials containing the samples and protein were placed in a pre-heated water bath of 55 °C. The protein adsorption was calculated by integration of the peak area of N1s from XPS. For each protein, the adsorption from PS was set to 100% as reference. Protein adsorptions of all polymer films with and without UV treatment have been measured. The results reported in this paper were data from films without UV irradiation. Data from polymer films immobilized by UV treatment are provided in the supporting information.
Supplementary Material
Acknowledgment
We thank NIGMS-NIH and NSF-MRSEC for support and Professor Francesco Stellaci for illuminating discussions during the conception of this work.
Footnotes
Supporting Information Available. Water contact angle data, XPS spectra and relative extent of protein adsorption. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Jelinek R, Kolusheva S. Chem. Rev. 2004;104:5987–6015. doi: 10.1021/cr0300284. [DOI] [PubMed] [Google Scholar]
- 2.Willner I, Katz E. Angew. Chem., Int. Ed. 2000;39:1180–1218. doi: 10.1002/(sici)1521-3773(20000403)39:7<1180::aid-anie1180>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 3.Ratner BD. J. Biomed. Mater. Res. 1993;27:283–288. doi: 10.1002/jbm.820270302. [DOI] [PubMed] [Google Scholar]
- 4.Bryers JD. Colloids Surf. B. 1994;2:9–23. [Google Scholar]
- 5.Schmidt S, Horch K, Normann R. J. Biomed. Mater. Res. 1993;27:1393–1399. doi: 10.1002/jbm.820271106. [DOI] [PubMed] [Google Scholar]
- 6.Bruinsma GM, van der Mei HC, Busscher HJ. Biomaterials. 2001;22:3217–3224. doi: 10.1016/s0142-9612(01)00159-4. [DOI] [PubMed] [Google Scholar]
- 7.Wisniewski N, Reichert M. Colloids Surf. B. 2000;18:197–219. doi: 10.1016/s0927-7765(99)00148-4. [DOI] [PubMed] [Google Scholar]
- 8.Roy R, Sandanaraj BS, Klaikherd A, Thayumanavan S. Langmuir. 2006;22:7695–7700. doi: 10.1021/la060496j. [DOI] [PubMed] [Google Scholar]
- 9.Renner C, Piehler J, Schrader T. J. Am. Chem. Soc. 2006;128:620–628. doi: 10.1021/ja0560229. [DOI] [PubMed] [Google Scholar]
- 10.Wagnera VE, Kobersteinb JT, Bryers JD. Biomaterials. 2004;25:2247–2263. doi: 10.1016/j.biomaterials.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Prime KL, Whitesides GM. J. Am. Chem. Soc. 1993;115:10714–10721. [Google Scholar]
- 12.Johnston EE, Bryers JD, Ratner BD. Langmuir. 2005;21:870–881. doi: 10.1021/la036274s. [DOI] [PubMed] [Google Scholar]
- 13.McPherson T, Kidane A, Szleifer I, Park K. Langmuir. 1998;14:176–186. [Google Scholar]
- 14.Zhu B, Eurell T, Gunawan R, Leckband D. J. Biomed. Mater. Res. 2001;56:406–416. doi: 10.1002/1097-4636(20010905)56:3<406::aid-jbm1110>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 15.Desai NP, Hubbell JA. Biomaterials. 1991;12:144–153. doi: 10.1016/0142-9612(91)90193-e. [DOI] [PubMed] [Google Scholar]
- 16.Holmlin RE, Chen X, Chapman RG, Takayama S, Whitesides GM. Langmuir. 2001;17:2841–2850. doi: 10.1021/la0015258. [DOI] [PubMed] [Google Scholar]
- 17.Cheng G, Xue H, Zhang Z, Chen S, Jiang S. Angew. Chem. Int. Ed. 2008;47:8831–8834. doi: 10.1002/anie.200803570. [DOI] [PubMed] [Google Scholar]
- 18.Roach P, Farrar D, Perry CC. J. Am. Chem. Soc. 2005;127:8168–8173. doi: 10.1021/ja042898o. [DOI] [PubMed] [Google Scholar]
- 19.Wagnera VE, Kobersteinb JT, Bryers JD. Biomaterials. 2004;25:2247–2263. doi: 10.1016/j.biomaterials.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Jung HW, Kang IK, Lee HB. Biomaterials. 1994;15:705–711. doi: 10.1016/0142-9612(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 21.Wertz CF, Santore MM. Langmuir. 2002;18:706–715. [Google Scholar]
- 22.Basu S, Vutukuri DR, Shyamroy S, Sandanaraj BS, Thayumanavan S. J. Am. Chem. Soc. 2004;126:9890–9891. doi: 10.1021/ja047816a. [DOI] [PubMed] [Google Scholar]
- 23.Basu S, Vutukuri DR, Thayumanavan S. J. Am. Chem. Soc. 2005;127:16794–16795. doi: 10.1021/ja056042a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savariar EN, Aathimanikandan SV, Thayumanavan S. J. Am. Chem. Soc. 2006;128:16224–16230. doi: 10.1021/ja065213o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim YJ, Shin D, Vutukuri D, Savariar EN, Kim SY, Thayumanavan S, Russell TP. Macromolecules. 2007;40:4267–4275. [Google Scholar]
- 26.See supporting information for details.
- 27.Ward MD, Buttry DA. Science. 1990;249:1000–1007. doi: 10.1126/science.249.4972.1000. [DOI] [PubMed] [Google Scholar]
- 28.Höök F, Kasemo B. Anal. Chem. 2001;73:5796–5804. doi: 10.1021/ac0106501. [DOI] [PubMed] [Google Scholar]
- 29.Sigal GB, Mrksich M, Whitesides GM. J. Am. Chem. Soc. 1998;120:3464–3473. [Google Scholar]
- 30.Green RJ, Frazier RA, Shakesheff KM, Davies MC, Roberts CJ, Tendler SJB. Biomaterials. 2000;21:1823–1835. doi: 10.1016/s0142-9612(00)00077-6. [DOI] [PubMed] [Google Scholar]
- 31.Ratner BD, Horbett TA, Shuttleworth D, Thomas HR. J. Colloid Interface Sci. 1981;83:630–642. [Google Scholar]
- 32.Sundgren J-E, Bodo P, Ivarsson B, Lundstrom I. J. Colloid Interface Sci. 1986;110:9–20. [Google Scholar]
- 33.Browne MM, Lubarsky GV, Davidson MR, Bradley RH. Surf. Sci. 2004;553:155–167. [Google Scholar]
- 34.Muir BW, Tarasova A, Gengenbach TR, Menzies DJ, Meagher L, Rovere F, Fairbrother A, McLean KM, Hartley PG. Langmuir. 2008;24:3828–3835. doi: 10.1021/la702689t. [DOI] [PubMed] [Google Scholar]
- 35.Yan M, Harnish B. Adv. Mater. 2003;15:244–247. [Google Scholar]
- 36.Schwendel D, Dahint R, Herrwerth S, Schloerholz M, Eck W, Grunze M. Langmuir. 2001;17:5717–5720. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.