Abstract
Objective
To assess the frequency of vitamin D deficiency among men with prostate cancer, as considerable epidemiological, in vitro, in vivo and clinical data support an association between vitamin D deficiency and prostate cancer outcome.
Patients, subjects and methods
The study included 120 ambulatory men with recurrent prostate cancer and 50 with clinically localized prostate cancer who were evaluated and serum samples assayed for 25-OH vitamin D levels. Then 100 controls (both sexes), matched for age and season of serum sample, were chosen from a prospective serum banking protocol. The relationship between age, body mass index, disease stage, Eastern Cooperative Oncology Group performance status, season and previous therapy on vitamin D status were evaluated using univariate and multivariate analyses.
Results
The mean 25-OH vitamin D level was 25.9 ng/mL in those with recurrent disease, 27.5 ng/mL in men with clinically localized prostate cancer and 24.5 ng/mL in controls. The frequency of vitamin D deficiency (< 20 ng/mL) and insufficiency (20–31 ng/mL) was 40% and 32% in men with recurrent prostate; 28% had vitamin D levels that were normal (32–100 ng/mL). Among men with localized prostate cancer, 18% were deficient, 50% were insufficient and 32% were normal. Among controls, 31% were deficient, 40% were insufficient and 29% were normal. Metastatic disease (P = 0.005) and season of blood sampling (winter/spring; P = 0.01) were associated with vitamin D deficiency in patients with prostate cancer, while age, race, performance status and body mass index were not.
Conclusions
Vitamin D deficiency and insufficiency were common among men with prostate cancer and apparently normal controls in the western New York region.
Keywords: prostate cancer, 25-hydroxy vitamin D, deficiency, season
Introduction
25-OH vitamin D is the accepted measure of the adequacy of vitamin D body stores. Vitamin D deficiency is common [1–4]; populations particularly at risk are those living in northern latitudes, those whose cultural mores call for complete skin coverage (resulting in limited exposure to sunlight) and individuals with renal failure in whom vitamin D synthesis is impaired [5,6]. Thomas et al. [7] reported that 57% of patients admitted to the Massachusetts General Hospital were vitamin D-deficient and vitamin D deficiency was still common (42%) after individuals with factors known to lead to vitamin D deficiency were excluded. Vitamin D deficiency was defined as a serum 25-OH vitamin D concentration of < 15 ng/mL, and 22% of patients were `severely deficient', as defined by a 25-OH vitamin D level of < 8 ng/mL.
Substantial epidemiological data indicate that vitamin D deficiency is associated with an increased risk of many types of cancer [8–11]. Increasing data link vitamin D deficiency and cancer prognosis [12–14], and numerous studies suggest that vitamin D deficiency is associated with an increased risk of medical complications to which patients with cancer are already predisposed, i.e. infection, thromboembolism, muscle weakness and falls, and immune dysfunction [15–20].
Very few studies have called attention to the frequency of vitamin D deficiency among patients with cancer [21,22]. In this report we describe the frequency of vitamin D deficiency, assessed using levels of 25-OH vitamin D, which is the accepted surrogate measure for body vitamin D stores.
Patients, subjects and methods
The medical records of all patients treated in the Urologic Oncology Clinic at Roswell Park Cancer Institute between 1 January 2005 and 28 February 2007 were reviewed in accordance with a protocol approved by the institution's Biomedical Institutional Review Board (IRB). All patients in whom a 25-OH vitamin D level was determined were identified. Patients taking vitamin D replacement of > 400 IU per day were excluded. Clinical data, including age, sex, Gleason grade and stage of disease, body mass index (BMI), date of 25-OH vitamin D assay, and treatment history, were collected. The first available vitamin D result was used in patients with multiple test results. Three groups of individuals constitute those in whom 25-OH vitamin D levels were measured. Group A: ambulatory men with recurrent prostate cancer seen in the practice of one of the authors (D.L.T.). We have been assessing routinely the 25-OH vitamin D levels among ambulatory men with prostate cancer for > 3 years; the values were measured in the clinical laboratory and were warranted by the recognition that vitamin D deficiency is common and usually not addressed among patients with prostate cancer. Group B: samples were obtained from 50 men with newly diagnosed, clinically localized prostate cancer from whom samples were obtained and stored in the data bank and biospecimen repository (DBBR). Samples were obtained according to a protocol approved by the IRB. These men had had no therapy when samples were obtained. Group C: a control group was chosen from individuals in whom blood samples had been obtained under the IRB-approved protocol for the DBBR. All patients participating in this bio-repository had signed an approved consent form for enrolment in DBBR that covers the use of their stored serum/blood samples for research purposes. The DBBR maintains biological samples from individuals with cancer and from healthy volunteers with no history of cancer. These non-cancer `controls' were largely drawn from individuals who accompany patients with cancer to the ambulatory clinics at Roswell Park. Samples from 100 apparently healthy volunteers were selected from the DBBR, and matched for age and month of sample acquisition from the patients with prostate cancer. All prostate cancer cases and matched controls lived in Western New York, USA.
Among group A, 25-OH vitamin D was assayed using a standard commercially available immunochemiluminometric assay [23]. The lower limit of normal for this assay is 32 ng/mL, which is based on maximum suppression of parathyroid hormone [23]; the normal range is 32–100 ng/mL (80–250 nmol/mL). The samples from group B and C were assayed in the laboratory of Dr Bruce W. Hollis using a previously described radioimmunoassay [24]. Serum 25-OH vitamin D measurements by these two methods are comparable [25] and normal ranges in both laboratories are the same. For this study, vitamin D deficiency was defined as a 25-OH vitamin D level of < 20 ng/mL and insufficiency 20–31 ng/mL.
The percentages of vitamin D deficiency and insufficiency were calculated for the three groups of patients; for group A the effects of age, race, BMI, Eastern Cooperative Oncology Group performance status (ECOG PS), presence of castration-resistant cancer, and the season in which the serum sample was procured were investigated using univariate logistic regression (that regressed the logarithm of the chance of having vitamin D deficiency or insufficiency on the above covariates). Continuous covariates such age and BMI were dichotomized. The P values were calculated using Wald chi-square tests or exact Pearson chi-square test when frequencies were low. A similar analysis was used for group B. To investigate the effect of patient demographics on vitamin D status among groups A and B, univariate and multivariate logistic regression analyses were used with cancer status (recurrent prostate cancer or clinically localized cancer) as one of the covariates. Univariate and multivariate logistic regression analyses were used for cases and controls with vitamin D status as a covariate.
Results
Group A (120 patients) had an initial serum sample assayed for 25-OH vitamin D between 1 January 2005 and 28 February 2007 (Table 1); 56% of men had radiographic or physical examination evidence of metastatic disease, and recurrence was evident only by detectable PSA levels in 44%. Of the men, 70% had been castrated (medical or surgical), and cytotoxic chemotherapy had been administered to 18% of the 120 men. Sixty-four men (53%) had prostate cancer categorized as `androgen-dependent' disease (because of an ongoing response to androgen-deprivation therapy or having recurrent prostate cancer in whom androgen deprivation had not been initiated) and 56 (47%) had `androgen-independent' or `castration-resistant' disease.
Table 1.
The baseline patient demographics and vitamin D levels
| Group |
|||
|---|---|---|---|
| Variable | A | B | C |
| No. of patients | 120 | 50 | 100 |
| Median (range) age, years | 70 (46–88) | 62 (44–85) | 63 (30–92) |
| Race: Caucasian, % | 95 | 90 | 90 |
| Summer/autumn sampling | 54 | 40 | 25 |
| BMI, kg/m2, n(%) | |||
| < 25 | 20 (17) | 14 (34) | NA |
| 25–30 | 45 (37) | 19 (46) | |
| > 30 | 55 (46) | 8 (20) | |
| Mean 25-OH vitamin D, ng/mL | 25.9 | 27.5 | 24.5 |
| Vitamin D, n (%) | |||
| deficient | 48 (40) | 9 (18) | 31 (31) |
| insufficient | 38 (32) | 25 (50) | 40 (40) |
| normal | 34 (28) | 16 (32) | 29 (29) |
NA, not available.
The demographic details of age, distribution of BMI and race in the three groups are shown in Table 1. Most men in group A had a good ECOG PS (85% of 0 or 1). Of the 120 patients in group A, 54% had 25-OH vitamin D levels determined in summer/autumn and 46% of samples were obtained in winter/spring. The mean (median) 25-OH vitamin D concentration was 25.9 (22.5) ng/mL. Forty-eight men (40%) had deficient, 38 (32%) had insufficient and 34 (28%) had normal vitamin D levels.
On univariate analysis, neither age, race, BMI, ECOG PS nor castration had an influence on vitamin D status. Patients with castration-resistant disease or those in whom the 25-OH vitamin D level was assessed in winter/spring were more likely to have vitamin D insufficiency and deficiency on both univariate and multivariate analyses. Patients with previous radiotherapy more often had deficient or insufficient vitamin D levels on multivariate analyses (P = 0.03).
In group B data for calculating the BMI were available in 41 men (Table 1). The mean (median) 25-OH vitamin D concentration was 27.5 (27.1) ng/mL. Of these men, 18% were vitamin D deficient, 50% were vitamin D insufficient and 32% had normal vitamin D levels. Age, race and BMI had no influence on vitamin D insufficiency. African-American men were more likely to be vitamin D deficient (P = 0.04). Winter/spring sample acquisition was associated with vitamin D insufficiency (P = 0.006) and deficiency (P = 0.05).
Among the 100 apparently healthy volunteers selected from the DBBR, group C consisted of 71 men and 29 women. The 25-OH vitamin D levels were similar between men (71, mean 23.7 ng/mL) and women (29, mean 26.3 ng/mL; P = 0.189). Published evidence reports no difference between vitamin D levels in males and females, and no difference was found in group C, so this well characterized control group was valid. The mean (median, range) age of group C was 62.4 (63, 30–92) years; 90 were white (90%), nine were African-American (9%) and one was Native American. In group C, only 25% had their 25-OH vitamin D levels determined in summer/autumn rather than winter/spring. The mean (median) 25-OH vitamin D level was 24.5 (23.7) ng/mL; 31% were vitamin D deficient, 40% were vitamin D insufficient and 29% had normal vitamin D levels.
Patients in group A were more likely to have 25-OH vitamin D levels of < 20 ng/mL (48, 40%) than men in group B (nine, 18%; P = 0.006). The frequency of 25-OH vitamin D levels `below normal' (< 32 ng/mL) were similar in the two groups (72% and 68%). The season of blood sampling was associated with vitamin D status in groups A and B (170 men), and winter/spring was associated with lower vitamin D levels. Age, race, ECOG PS and BMI were not associated with vitamin D status in the 170 men in groups A and B on univariate analyses. On multivariate analyses, season of sampling (summer/autumn vs spring/winter; P = 0.01) and disease status (clinically localized vs recurrent; P = 0.006) were associated with vitamin D deficiency.
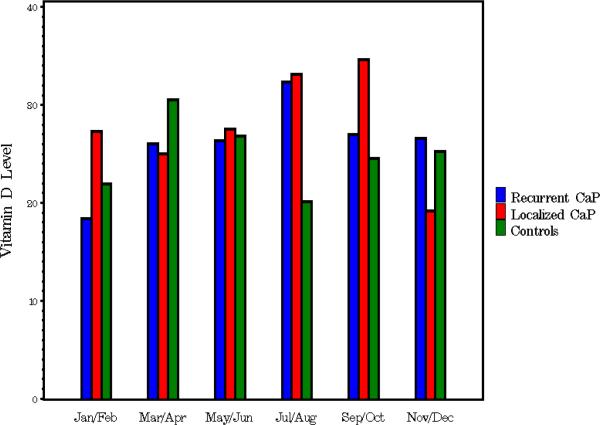
For the case-control study, 100 healthy controls were selected from the DBBR and matched by the same decade of age and to the season of blood sampling for comparison with the 120 men in group A. Controls were younger and had a lower BMI than cases. There was a similar frequency of vitamin D deficiency and insufficiency among men with prostate cancer (localized or recurrent) and controls in the study population. Samples were drawn more often in winter/spring among the available controls from the DBBR sample bank than among patients with cancer (P < 0.001). Figure 1 shows the 25-OH vitamin D levels in groups A, B and C in different months of the year; there was a similar trend in vitamin D levels by month of sampling among the three groups. There was some variation amongst the groups in January/February (P = 0.05) and July/August (P = 0.02), but the small sample size precludes any meaningful conclusion from this unplanned subgroup analyses.
Fig. 1.
The mean vitamin D levels amongst the three groups (A, recurrent prostate cancer; B, localized prostate cancer; and C, controls), drawn during different months.
The data indicate that low 25-OH vitamin D levels are common in all groups; patients in group A more often had 25-OH vitamin D levels of < 20 ng/mL but overall below-normal levels were similar in all groups. In this exploratory analysis abnormally low vitamin D levels in the western New York geographical area occurred with similar frequency in all three groups.
Discussion
Given that low vitamin D levels are associated with a higher risk of many cancers, we hypothesized that patients with prostate cancer might have lower vitamin D levels than controls. This does not appear to be the case. Vitamin D deficiency is common and this study shows that vitamin D deficiency also is common among patients with either clinically localized or recurrent prostate cancer and healthy controls in New York (≈□75%). A previous study found a similar frequency among individuals with colorectal cancer [26] and in a less formal analysis among individuals with lung, breast, head and neck cancers, lymphoma and leukaemia who were treated at the Roswell Park Cancer Institute. While such analyses provide no reasons for vitamin D deficiency, the most likely factors contributing to these findings are the latitude of residence of these patents (≈□44°N), the infrequent use of vitamin D supplements, the conservative level of vitamin D supplementation usually recommended, and the limited availability of vitamin D in foods. Limited outside activity due to illness and the avoidance of sun exposure through the use of sun screens and protective clothing might play a role; however, the diagnosis of prostate cancer itself seems an unlikely factor contributing to vitamin D deficiency, as patients with advanced disease, limited disease before treatment and controls had a similar frequency of 25-OH vitamin D levels below normal (< 32 ng/mL). There might be an effect of disease or its therapy; vitamin D `deficiency' (< 20 ng/mL) was more common among men in group A than among men in group B or C. This difference was unlikely to be due to a difference in sunlight exposure or poor dietary intake, as all men were ambulatory, of good PS and resided in the same geographical area. Li et al. [27] reported a similar finding in a prospective study of vitamin D levels in a cohort from Physicians Health Study, where lower vitamin D levels were associated with advanced disease. Seasonal variation of sample acquisition (summer/autumn vs winter/spring) was associated with lower levels, as expected. The study was limited by the lack of `true match' with the control population due to the limited availability of control samples. Despite this limitation, multivariate analyses confirmed a similar frequency of low vitamin D status in the present study population across the three groups. The use of two different vitamin D assays did not affect the analyses as the two assays are comparable [25]. There were no differences between the vitamin D status among groups C and B, the samples from which were assayed at Dr Hollis' laboratory using radioimmunoassay.
Vitamin D deficiency is relatively common (and largely not commented upon) in many reports in which bone health, and calcium and mineral balance, have been studied in patients with cancer [28–30]. The present study provides a formal analysis of the frequency of vitamin D deficiency among men with localized and advanced prostate cancer compared to a healthy control population.
Are there health consequences of vitamin D deficiency in patients with cancer? While it is widely recognized that vitamin D deficiency is associated with osteoporosis and osteomalacia, the measurement of 25-OH vitamin D concentration and use of vitamin D supplements in castrate men with prostate cancer is low, despite the likelihood that vitamin D deficiency would predispose to accelerated osteoporosis and might impair bone integrity in a disease where bone destruction is quite common [31,32]. Even less well recognized among practitioners are the numerous other medical conditions associated with vitamin D deficiency. These include increased risk of thromboembolism [33], infection [34–37], altered neuromuscular function [38,39], abnormal angiogenesis and wound healing [40–43], and immune dysfunction [44–48].
The current recommended daily allowance of D3 is 400 IU/day [49]. Considerable recent data suggest that this recommended daily allowance is inordinately low [50]. The current recommended normal serum level of 25-OH vitamin D is 32–100 ng/mL (80–250 nM) [1]. This recommendation is based on the suppression of serum parathyroid hormone as well as a reduced incidence of osteoporosis among large populations with serum 25-OH vitamin D levels of 32–100 ng/mL [51,52]. Whether this is the optimal blood concentration for patients with cancer or normal individuals is uncertain and debated [7,53]. Limited data suggest that administering vitamin D might slow the progress of prostate cancer. Vieth et al. [54] reported on a small group of men with prostate cancer who had increasing PSA levels despite local therapy; supplementation with 2000 IU per day of cholecalciferol reduced the rate of PSA increase. Gross et al. [55] showed, in a similar setting, that 1,25-D3 administration (1.5-2.0 μg/day) reduced the serum PSA level. Our group reported a 28% PSA response rate in men with castration-resistant disease treated with calcitriol (12 μg daily, three times weekly) and dexamethasone (4 mg daily, four times weekly) [56].
The present study clearly showed a high frequency of abnormally low 25-OH vitamin D levels among patients with prostate cancer, regardless of disease status or treatment, and in controls, in New York. These findings in patients with prostate cancer and in controls are similar to those reported in patients hospitalized in Boston [7]. Attention to 25-OH vitamin D levels and careful repletion appear warranted, as is the careful study of the consequences of vitamin D3 deficiency in patients with prostate cancer and in normal adults.
Acknowledgements
Supported in part by grants from the National Institutes of Health/National Cancer Institute CA67267, CA85142 and CA95045 and a grant from The Roswell Park Alliance Foundation.
Abbreviations
- IRB
Institutional Review Board
- BMI
body mass index
- DBBR
data bank and biospecimen repository
- ECOG PS
Eastern Cooperative Oncology Group performance status
Footnotes
Conflict of Interest None declared.
References
- 1.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–73. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 2.Holick M. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Chapuy MC, Preziosi P, Maamer M, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439–43. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–6S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 5.Nesby-O'Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age. Third National Health and Nutrition Examination Survey, 1988-94. Am J Clin Nutr. 2002;76:187–92. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 6.Lips P, Hosking D, Lippuner K, et al. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med. 2006;260:245–54. doi: 10.1111/j.1365-2796.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- 7.Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–83. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 8.Garland CF, Comstock GW, Garland FC, Helsing KJ, Shaw EK, Gorham ED. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. 1989;2:1176–8. doi: 10.1016/s0140-6736(89)91789-3. [DOI] [PubMed] [Google Scholar]
- 9.Hanchette CL, Schwartz GG. Geographic patterns of prostate cancer mortality: evidence for a protective effect of ultraviolet radiation. Cancer. 1992;70:2861–9. doi: 10.1002/1097-0142(19921215)70:12<2861::aid-cncr2820701224>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 10.Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol. 1980;9:227–31. doi: 10.1093/ije/9.3.227. [DOI] [PubMed] [Google Scholar]
- 11.Ahonen MH, Tenkanen L, Teppo L, Hakama M, Tuohimaa P. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland) Cancer Causes Control. 2000;11:847–52. doi: 10.1023/a:1008923802001. [DOI] [PubMed] [Google Scholar]
- 12.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–9. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 13.Zhou W, Heist RS, Liu G, et al. Circulating 25-hydroxyvitamin d levels predict survival in early-stage non-small-cell lung cancer patients. J Clin Oncol. 2007;25:479–85. doi: 10.1200/JCO.2006.07.5358. [DOI] [PubMed] [Google Scholar]
- 14.Grant WB. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer. 2002;94:1867–75. doi: 10.1002/cncr.10427. [DOI] [PubMed] [Google Scholar]
- 15.Manuel Quesada J, Solana R, Serrano I, et al. Immunologic effects of vitamin D. N Engl J Med. 1989;321:833–4. doi: 10.1056/NEJM198909213211215. [DOI] [PubMed] [Google Scholar]
- 16.Adams JS, Hewison M. Unexpected actions of vitamin D. new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beer TM, Ryan CW, Venner PM, et al. Double-blinded randomized study of high-dose calcitriol plus docetaxel compared with placebo plus docetaxel in androgen- independent prostate cancer: a report from the ASCENT Investigators. J Clin Oncol. 2007;25:669–74. doi: 10.1200/JCO.2006.06.8197. [DOI] [PubMed] [Google Scholar]
- 18.Broe KE, Chen TC, Weinberg J, Bischoff-Ferrari HA, Holick MF, Kiel D. A higher dose of vitamin D reduces the risk of falls in nursing home residents: a randomized, multiple-dose study. J Am Geriatr Soc. 2007;55:234–9. doi: 10.1111/j.1532-5415.2007.01048.x. [DOI] [PubMed] [Google Scholar]
- 19.Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003;78:1463–70. doi: 10.4065/78.12.1463. [DOI] [PubMed] [Google Scholar]
- 20.Visser M, Deeg DJH, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength an muscle mass (sarcopenia): the longitudinal aging study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766–72. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 21.Tangpricha V, Colon NA, Kaul H, et al. Prevalence of vitamin D deficiency in patients attending an outpatient cancer care clinic in Boston. Endocr Pract. 2004;10:292–3. doi: 10.4158/EP.10.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Everett PC. The prevalence of vitamin D deficiency and insufficiency in a hematology-oncology clinic. Clin J Oncol Nurs. 2008;12:33–5. doi: 10.1188/08.CJON.33-35. [DOI] [PubMed] [Google Scholar]
- 23. Available at http://www.labcorp.com/datasets/labcorp/hml/chapter/mono/sr004600.htm Accessed 18 November 2008.
- 24.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency; implications for establishing a new effective dietary intake recommendation for Vitamin D. J Nutr. 2005;135:317–22. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 25.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem. 1993;39:529–33. [PubMed] [Google Scholar]
- 26.Ersfeld DL, Rao DS, Body J, et al. Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON automated analyzer. Clin Biochem. 2004;37:867–74. doi: 10.1016/j.clinbiochem.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Fakih MG, Trump DL, Johnson CS, Tian L, Muindi J, Sunga AY. Chemotherapy is linked to severe vitamin D deficiency in patients with colorectal cancer. Int J Colorectal Dis. 2009;24:219–24. doi: 10.1007/s00384-008-0593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Stampfer MJ, Hollis JB, et al. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. Plos Med. 2007;4:e103. doi: 10.1371/journal.pmed.0040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adler RA. Cancer treatment-induced bone loss. Curr Opin Endocrinol Diabetes Obes. 2007;14:442–5. doi: 10.1097/MED.0b013e3282f169b5. [DOI] [PubMed] [Google Scholar]
- 30.Hoff AO, Gagel RF. Osteoporosis in breast and prostate cancer survivors. Oncology (Williston Park) 2005;19:651–8. [PubMed] [Google Scholar]
- 31.Guise TA. Bone loss and fracture risk associated with cancer therapy. Oncologist. 2006;11:1121–31. doi: 10.1634/theoncologist.11-10-1121. [DOI] [PubMed] [Google Scholar]
- 32.Morote J, Morin JP, Orsola A, et al. Prevalence of osteoporosis during long-term androgen deprivation therapy in patients with prostate cancer. Urology. 2007;69:500–4. doi: 10.1016/j.urology.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Greenspan SL, Coates P, Sereika SM, et al. Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab. 2005;90:6410–7. doi: 10.1210/jc.2005-0183. [DOI] [PubMed] [Google Scholar]
- 34.Beer TM, Venner PM, Ryan CW, et al. High dose calcitriol may reduce thrombosis in cancer patients. Br J Haematol. 2006;135:392–4. doi: 10.1111/j.1365-2141.2006.06322.x. [DOI] [PubMed] [Google Scholar]
- 35.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 36.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge. vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–3. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 37.Martineau AR, Honecker FU, Wilkinson RJ, Griffiths CJ. Vitamin D in the treatment of pulmonary tuberculosis. J Steroid Biochem Mol Biol. 2007;103:793–8. doi: 10.1016/j.jsbmb.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 38.Martineau AR, Wilkinson RJ, Wilkinson KA, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176:208–13. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 39.Endo I, Inoue D, Mitsui T, et al. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology. 2003;144:5138–44. doi: 10.1210/en.2003-0502. [DOI] [PubMed] [Google Scholar]
- 40.Bischoff HA, Säthelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18:343–51. doi: 10.1359/jbmr.2003.18.2.343. [DOI] [PubMed] [Google Scholar]
- 41.Simpson RU, Hershey SH, Nibbelink KA. Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse. J Steroid Biochem Mol Biol. 2007;103:521–4. doi: 10.1016/j.jsbmb.2006.12.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mantell DJ, Owens PE, Bundred NJ, et al. 1,25- Dihydroxyvitamin D (3) inhibits angiogenesis in vitro and in vivo. Circ Res. 2000;87:214–20. doi: 10.1161/01.res.87.3.214. [DOI] [PubMed] [Google Scholar]
- 43.Doetsch AM, Faber J, Lynnerup N, et al. The effect of calcium and vitamin D3 supplementation on the healing of the proximal humerus fracture: a randomized placebo- controlled study. Calcif Tissue Int. 2004;75:183–8. doi: 10.1007/s00223-004-0167-0. [DOI] [PubMed] [Google Scholar]
- 44.Chung I, Wong MK, Flynn G, et al. Differential antiproliferative effects of calcitriol on tumor-derived and matrigel-derived endothelial cells. Cancer Res. 2006;66:8565–73. doi: 10.1158/0008-5472.CAN-06-0905. [DOI] [PubMed] [Google Scholar]
- 45.Amson Y, Amital H, Schoenfeld Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann Rheum Dis. 2007;66:1137–42. doi: 10.1136/ard.2007.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–8. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 47.Munger KL, Zhang SM, O'Reilly E, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60–5. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 48.Garcion E, Sindji L, Nataf S, et al. Treatment of experimental autoimmune encephalomyelitis in rat by 1,25-dihydroxyvitamin D3 leads to early effects within the central nervous system. Acta Neuropathol. 2003;105:438–48. doi: 10.1007/s00401-002-0663-0. [DOI] [PubMed] [Google Scholar]
- 49.Pedersen LB, Nashold FE, Spach KM, Hayes CE. 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by inhibiting chemokine synthesis and monocyte trafficking. J Neuroscience Res. 2007;85:2480–90. doi: 10.1002/jnr.21382. [DOI] [PubMed] [Google Scholar]
- 50.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes Food and Nutrition Board Institute of Medicine . Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. National Academy Press; Washington, DC: 1999. Vitamin D; pp. 250–87. [Google Scholar]
- 51.Glerup H, Mikkelsen K, Poulsen L, et al. Commonly recommended daily intake of vitamin D is not sufficient if sunlight exposure is limited. J Intern Med. 2000;247:260–8. doi: 10.1046/j.1365-2796.2000.00595.x. [DOI] [PubMed] [Google Scholar]
- 52.Chapuy MC, Preziosi P, Maaner M, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporosis Int. 1997;7:439–43. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 53.Vieth R. Why the optimal requirement for vitamin D3 is probably much higher than what is officially recommended for adults. J Steroid Biochem Mol Biol. 2004;89-90:575–9. doi: 10.1016/j.jsbmb.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 54.Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 55.Woo TC, Choo R, Jamieson M, et al. Pilot study. potential role of vitamin D (Cholecalciferol) in patients with PSA relapse after definitive therapy. Nutr Cancer. 2005;51:32–6. doi: 10.1207/s15327914nc5101_5. [DOI] [PubMed] [Google Scholar]
- 56.Gross C, Stamey T, Hancock S, Feldman D. Treatment of early recurrent prostate cancer with 1,25-dihydroxyvitamin D3 (calcitriol) J Urol. 1998;159:2035–9. doi: 10.1016/S0022-5347(01)63236-1. [DOI] [PubMed] [Google Scholar]
- 57.Trump DL, Potter DM, Muindi J, Brufsky A, Johnson CS. Phase II trial of high-dose, intermittent calcitriol (1,25 dihydroxyvitamin D3) and dexamethasone in androgen- independent prostate cancer. Cancer. 2006;106:2136–42. doi: 10.1002/cncr.21890. [DOI] [PubMed] [Google Scholar]