Abstract
Induction of terminal differentiation is a conceptually attractive approach for the therapy of neoplastic diseases. Although vitamin D derivatives (deltanoids) can induce differentiation of AML cells in vitro, so far deltanoids have not been successfully brought to the clinic, due to the likelihood of life-threatening hypercalcemia. Here, we incubated freshly obtained blood cells from patients with AML with a plant antioxidant (PAOx), silibinin (SIL), alone or together with a deltanoid. Twenty patients with AML (all subtypes except M3) were available for this study, and in 14 (70%), SIL (60 μM) either induced differentiation ex vivo, or enhanced differentiation induced by deltanoids, or both. Interestingly, SIL acting alone induced differentiation only in cases in which chromosome aberrations could not be detected. In eleven samples sufficient material was available for a limited analysis of the underlying events. Quantitative RT-PCR showed that differentiation markers were upregulated at the mRNA level by both SIL and deltanoids, suggesting that intracellular signaling pathways upstream of transcription factors (TFs) were activated by these agents. Western analysis for proteins which function as TFs in deltanoid-induced monocytic differentiation, such as members of jun and C/EBP families, surprisingly demonstrated that SIL upregulated all these TFs in the cases tested. This suggests that although the presence of SIL may not always be sufficient to induce differentiation, it can serve as a differentiation enabling factor for blasts obtained from a large proportion of patients with AML. Thus, SIL/deltanoid combinations warrant further consideration as preventive / therapeutic regimens in human leukemia.
Keywords: Acute myeloid leukemia; 1α,25-dihydroxyvitamin D3; silibinin; differentiation; c-jun; C/EBP
Introduction
Acute myeloid leukemia (AML) is a result of blocked differentiation of hematopoietic stem and/or progenitor cells, due to a variety of genetic and epigenetic errors. Since the affected cells are unable to undergo terminal differentiation and have associated defects in the regulation of growth and survival mechanisms, they continue to proliferate and replace the normal components of the hematopoietic system (1). The standard treatment of patients with this disease utilizes cytotoxic agents, which kill the bulk of proliferating cells, but even after aggressive cytotoxic treatment about 75% of AML patients have a recurrence within 2 years of remission (2). Recently, there has been enthusiasm for the use of the second-generation deoxyadenosine analog Clofarabine in the treatment of patients with AML. However, although this appears to be a promising new agent to improve outcome, recent clinical experience indicates that even with the addition of Clofarabine to Cytarabine, a significant portion of patients still do not achieve a complete remission, and the overall survival was not improved (3).
While attempts to target the bcr/c-abl fusion gene, the molecular lesion underlying the majority of cases of chronic myelogeneous leukemia, using specific kinase inhibitors such as imatinib have been highly successful, such strategy is difficult to apply to AML, since the molecular lesions in AML are highly heterogeneous. Other mechanism-based therapeutic approaches, for instance activation of the intrinsic apoptotic pathways (4), and targeting known mutations such as FLT-3 (5), or signal transduction pathways (6, 7), are being explored, and may lead to further advances in targeted therapy of AML.
However, there is a third therapeutic approach, based on the by-pass of the block to differentiation caused by the mutations and/or epigenetic changes, and thus inducing differentiation by brute force, or by directing differentiation to an alternate hematopoietic lineage (8). An example of the former approach is to force acute promyelocytic leukemia (APL) cells to differentiate to granulocytes using supra-physiological concentrations of retinoic acid, a normal morphogen (1, 9-11), and an example of the latter is to direct AML blasts to monocytic differentiation by elevated levels of the physiological form of vitamin D (1,25D) (12-14), or other agents (15). Although retinoic acid is successfully used for treatment of APL, induction of differentiation of AML cells by 1,25D, highly effective in vitro, has not been brought to the clinic due to the danger of life threatening hypercalcemia caused by 1,25D. Principally for this reason, the earlier clinical trials of vitamin D derivatives in a variety of malignant diseases did not demonstrate significant therapeutic effects, eg (16-18).
One potential solution to this problem is to combine 1,25D with agents that enhance its effect. While there are many such agents (19), plant antioxidants have the appeal of being natural compounds and of being already used for human consumption (20-22), and in animal models of AML have not increased the calcemic activity of the deltanoids so that these can be used at lower concentrations (23, 24). In this study we have focused on silibinin, a nontoxic polyphenolic component of the extract of milk thistle. Silibinin has been found to exhibit a wide spectrum of anticancer activity in various solid tumor models in vitro and in vivo (25-28), likely due its ability to target multiple cellular regulatory pathways. Indeed, this polyphenol has been shown to inhibit growth factor- and cytokine-induced signaling cascades (29, 30), to modulate the activation of mitogen-activated protein kinases (31-33) and mTOR (34, 35), and to suppress different transcription factors, such as NFκB, AP-1, STAT1, STAT3 and STAT5 (30, 32, 36) in tumor cell and tissue preparations. Recently, we and others have demonstrated that silibinin is capable of potentiating the differentiation effects of 1,25D in HL60 myeloid leukemia cell line (37-39). Here, using an ex vivo system, we show that silibinin has a modest differentiating effect on blasts from some patients with AML, and/or enhances the action of 1,25D or its analogs on such cells. In aggregate, a large proportion of blasts in primary culture show enhanced differentiation in the presence of silibinin. This effect is accompanied by an increased expression of nuclear transcription factors which have a known involvement in monocytic differentiation, providing a mechanistic clue to the action of silibinin on human AML cells.
Materials and Methods
Chemicals and antibodies
1,25D, its analogs 1,25-dihydroxy-16-ene-20-cyclopropyl-cholecalciferol (BXL-0062) and 1,25-dihydroxy-16-ene-20-cyclopropyl-24-oxo-cholecalciferol (BXL-0143) were synthesized in the BioXell laboratory (40). Silibinin was purchased from Sigma-Aldrich (St. Louis, MO), and the stock was prepared by dissolving in DMSO. VDR (C-20), RXRα (D-20), c-Jun (N), Jun B (210), PU.1 also known as Spi-1 (T-21), C/EBPβ (C-19), C/EBPα (14AA) and calregulin (H-170) antibodies were purchased from Santa Cruz Co (Santa Cruz, CA).
Patients for ex vivo studies
The study population comprised seven male subjects and thirteen female subjects with a median age of 59 years. These were consecutive patients who presented to the UMDNJ- Robert Wood Johnson University Hospital between December 2005 and April 2009 with AML and gave informed consent for this study according to the institutional IRB protocol. There were one M0, three M1, five M2, five M4, one M5, and one M7, classified according to French-American-British (FAB) classification of AML (41), and four specimens not sub-typed. In nine patients an abnormal karyotype was detected (Table 1).
Table 1.
Summary of ex vivo cases
| Sample | Diagnosis | Age (years) | Gender | Ethnicity | Karyotype / Mutation | Effects of Silibinin on cell differentiation | |||
|---|---|---|---|---|---|---|---|---|---|
| FAB | Induces | Enhances D** | Inhibits D | No effect on D | |||||
| 1 | AML-M2 | 67 | F | W | 46, XX; del(7) | − | + | ||
| 2 | AML | 79 | M | W | 46, XY | + | + | ||
| 3 | AML-M4eo | 62 | M | H | 46, XY; inv (16) | − | + | ||
| 4 | AML-M2 | 35 | F | A | 46, XX | + | + | ||
| 5 | AML-M0 | 43 | F | W | 46, XX; inv(3) | − | + | ||
| 6 | AML-M4eo | 51 | F | W | 46, XX; inv(16) | − | + | ||
| 7 | AML-M7 | 68 | F | B | 46, XX | − | + | ||
| 8 | AML-M4eo | 70 | M | W | 46, XY, inv(1) | − | + | ||
| 9 | AML-M2 | 51 | F | W | 46, XX | # N/A | + | ||
| 10 | AML-M2 | 34 | F | W | 46, XX | + | + | ||
| 11 | AML-M2 | 69 | F | W | 46, XX; Trisomy 11, del (16) | − | + | ||
| 12 | AML-M1 | 73 | M | W | 46, XY | − | + | ||
| 13 | AML | 68 | F | W | 46, XX; Trisomy 11 | N/A | + | ||
| 14 | AML-M4 | 72 | M | W | 46, XY; Trisomy 8q | − | + | ||
| 15 | AML-M1 | 50 | F | W | 46, XX; FLT3 internal tandem duplication | + | + | ||
| 16 | AML-M4 | 25 | F | W | 46, XX; mut NPM1, mut FLT-3 (D835) | + | + | ||
| 17 | AML-M5 | 69 | M | W | 46, XY | + | + | ||
| 18 | AML-M1 | 71 | F | H | 46, XX | + | + | ||
| 19 | AML | 61 | M | W | 46, XY | + | + | ||
| 20 | AML-M4 | 63 | F | W | 46,XX; Ph+, t(9,22) | + | + | ||
FAB-French-American-British classification of AML
W - White, H - Hispanic, A - Asian, B -Afro-American
D=Deltanoids
N/A = sufficient material not available
Isolation and culture of mononuclear cells from patients' bone marrow and peripheral blood
Blasts were separated from the specimens of peripheral blood by Ficoll (Histopaque ®-1077, Sigma-Aldrich) gradient centrifugation, and cultured with silibinin (added at time zero) and deltanoids (added at 30 min dissolved in ethanol) for periods of time ranging from 5-9 days in RPMI plus 10% bovine calf serum. Control groups received equivalent amount of DMSO and ethanol. The duration varied depending on maintenance of culture viability, monitored by Trypan Blue exclusion. Before harvesting, the cultures were observed under an inverted microscope for adherence of the cells to the bottom of the flask. When adherence was noted, the cells were harvested by scraping with a rubber “policeman”.
Determination of markers of differentiation
Monocytic differentiation was monitored by the expression of differentiation markers CD11b and CD14, determined by flow cytometry, as described previously (42). Because the numbers of available cells in patient specimens were not sufficient for replicate assays, we standardized the procedure using HL60 cells, and determined that the variability from the mean was < 5% between identically treated cell samples cultured separately. The upregulation of these flow cytometry markers on cells in patient samples was further confirmed by qRT-PCR.
Cell extracts and Western blotting
These procedures were carried out as previously described (42). Briefly, 40 μg protein extracts were boiled in 3× sample buffer (125 mM Tris–HCl, pH 6.8, 1% SDS, 2% β-mercaptoethanol, and 0.01% bromophenol blue) for 5 min, loaded onto a 10% SDS-polyacrylamide gel, then transferred to nitrocellulose membranes (Amersham, Piscataway, NJ). The membranes were blocked with 5% non-fat milk in Tris-buffered saline (TBS) with 0.1% (w/v) Tween 20 for 1 h at room temperature, probed for 1 h with the specified primary antibodies, and incubated with a horseradish peroxidase-linked secondary antibody for 1 h. Proteins on the membrane were visualized using the chemiluminescence assay system (ECL™, Amersham). The membranes were then stripped according to the manufacturer's protocol (Amersham), and reprobed with a control antibody, usually anti-calregulin.
RNA extraction and Real-time PCR (qRT-PCR)
RNA was extracted from cells using TRIzol® Reagent (Invitrogen, Carlsbad, CA), which is a mono-phasic solution of phenol and guanidine isothiocyanate, an improvement to the single-step RNA isolation method developed by Chomczynski and Sacchi (43). The procedures were done as previously described. Briefly, 5-10 × 106 cells were homogenized in 1 ml Trizol and incubated for 5 min at RT. After addition of chloroform followed by centrifugation at 14,000 rpm, the aqueous phase was transferred to a fresh tube. The RNA was precipitated by mixing with 500 μl isopropyl alcohol, and incubated 10 min at R. RNA pellet was obtained by centrifugation, the supernatant discarded, and the pellet washed with 75% ethanol in DEPC water, then briefly dried for 10 min. The RNA pellet was then dissolved in 50-100 μl DEPC water. The forward and reverse primers sequences used, respectively, were: CD11b, 5′-GCAAGTGTCTGTGTGCAAGTGTGT-3′ and 5′-TCAGTGGAGAGAAGCTGCTGTGTT-3′, CD14, 5′-AACTCCCTCAATCTGTCGTTCGCT-3′ and 5′-GGGCAAAGGGTTGAATTGGTCGAA-3′, VDR, 5′-AGAAAGGCGTTCTTCGAGGTGGAT-3′ and 5′-AGGCTCTTGCAGTGTGAAGTCTGA-3′, RXRα, 5′-GCAAGCTGGTGTGTCATCAGCAAA-3′ and 5′-ACCAGAACACCAAGGCAGAGAGAA-3′, ARP0, 5′-AGATGCAGCAGATCCGCAT-3′ and 5′-GTGGTGATACCTAAAGCCTG-3′. For reverse transcription, samples were incubated in an Eppendorf PCR system at 42°C for 15 min, then 99°C for 5 min, and 5°C for 5 min. For real-time PCR, relative quantification of target cDNA was performed using a Roche LightCycler instrument with Faststart DNA Masterplus Syber Green I kit (Hoffmann-La Roche) and gene-specific primers. The parameters for PCR were as follows: 45 cycles of 1) 95°C for 15 s, 2) 58°C for 10 s, and 3) 72°C for 15 s followed by an incremental increase (0.1°C/s) of temperature from 65 °C to 95°C to analyze the melting curve of the products. A mixture of forward and reverse primers at a final concentration of 1.5 μM was used to detect VDR, RXRα and ARP0 (acidic ribosomal protein p0, internal control). The Ct value, the cycle number at which signal fluorescence surpassed fluorescence background noise, was recorded, and relative fold values were calculated based on the equation 2-ΔΔCt, where ΔΔCt=ΔCt treated-ΔCt calibrator (untreated samples) and ΔCt= Ct target gene-Ct ARP0.
Statistical analysis
Significance of the differences between mean values of two compared groups was assessed by a two tailed student's test. P < 0.05 was considered statistically significant. The computations were performed with an IBM-compatible personal computer and used Microsoft EXCEL.
Results
Silibinin can initiate and enhance 1,25D-induced differentiation of AML cells ex vivo
Silibinin has been reported to have a modest differentiating effect as a sole agent, and to potentiate the action of 1,25D in HL60 cells, an established AML cell line (44, 45), but whether this reflects an effect of silibinin on human AML cells ex vivo has not been known. We have therefore studied the differentiation effects of SIL and deltanoids, alone and in combination, in samples of blasts obtained from 20 patients with all subtypes of AML except M3, for which differentiation therapy with retinoic acid is well established.
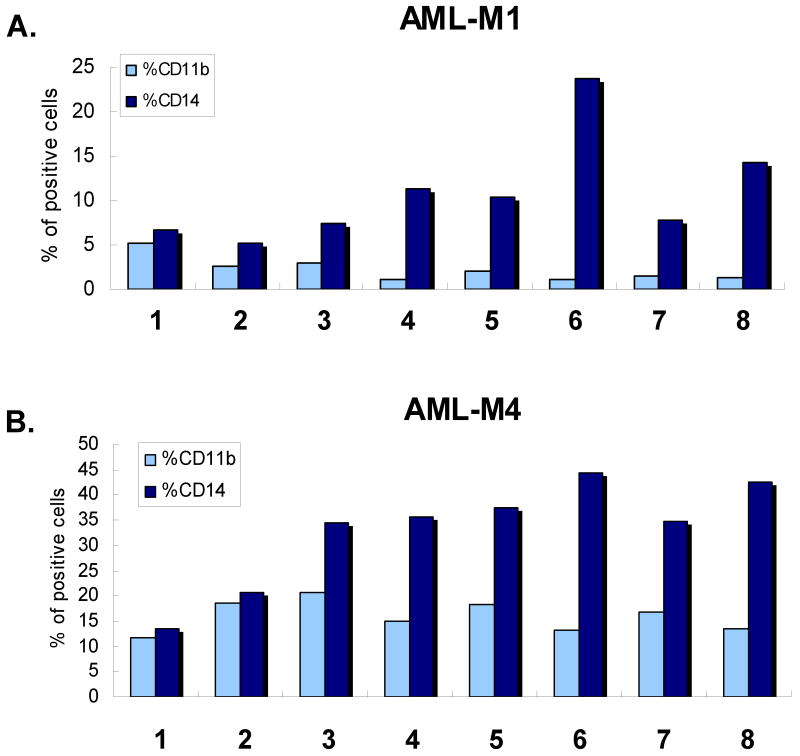
Not surprisingly, given the heterogeneity of molecular lesions that result in this disease, the results were variable. As detailed in Table 1 and illustrated in Fig 1 and 2, silibinin can induce and/or enhance differentiation induced by 1,25D in a large proportion of the cases available to us, though not all, with 11 samples (55%) showing some degree of enhancement. No definitive correlation was noted between differentiation and FAB subtypes or specific cytogenetic characteristics in this series of patients, but it may prove to be significant that silibinin as a sole agent had no effect on differentiation in all seven cases in which trisomy (patient # 14) or chromosome inversion or deletion was detected : patients #1 with del (7), patients #3 and # 6 with inv (16), #5 with inv(6), #8 with inv (1), and #11 with del(16). Thus, normal karyotype may be a prerequisite for silibinin-induced differentiating effects. Also of note, the M1 cases, which by definition lack evidence of differentiation, were induced (#15, and #18) or effect of 1,25D was enhanced (#12) by silibinin, indicating that differentiation mechanisms can be activated by this PAOx even if there is minimal endogenous differentiation.
Fig. 1. Silibinin can induce and enhance monocytic differentiation of AML blasts induced ex vivo by 1,25D.
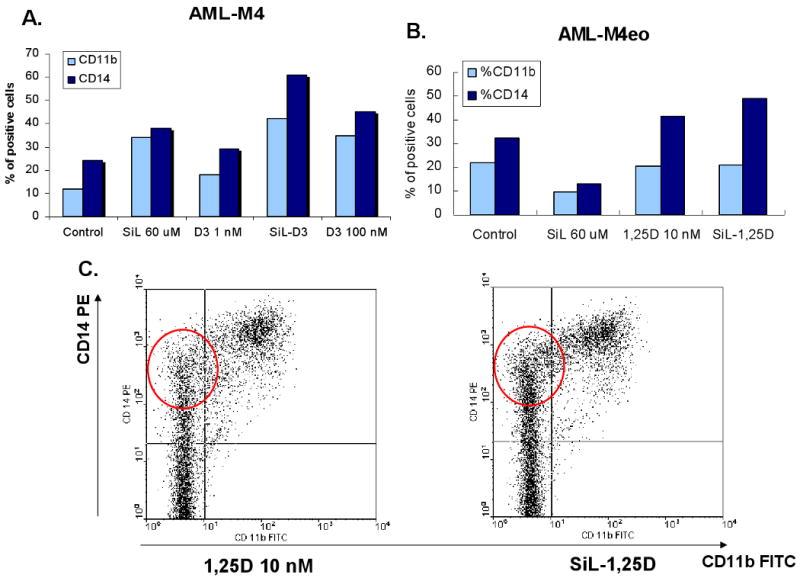
A. Quantitation of two monocytic markers, CD11b and CD14, following 5 days of simultaneous exposure of AML blasts to 60 μM silibinin (SIL)and/or 1 nM 1,25 dihydroxyvitamin D3 (1,25D), and to 100 nM 1,25D alone (last lane). Patient #20. B. Silibinin can enhance monocytic differentiation of AML blasts. Patient # 8 (M4eo, the subtype of M4 with bone marrow eosinophilia). C. Primary data obtained by flow cytometry for the results shown in panel B, showing greater density of high intensity signals for CD14 (circled area) when SIL was added to 1,25D.
Fig. 2. Illustration of primary data showing enhancement of 1,25D-induced differentiation by silibinin of differentiation of AML blasts from patient #13 in Table 1.
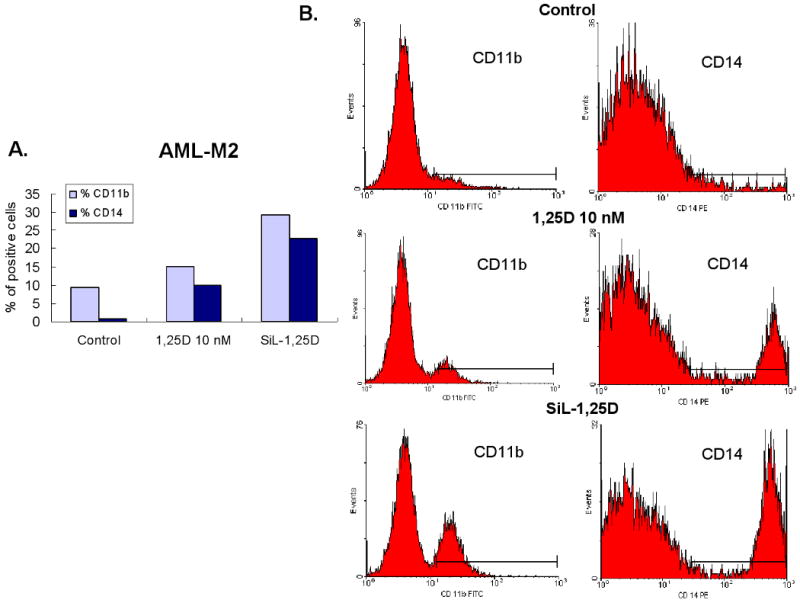
Interestingly, enhancement of deltanoid action was also observed in some cases in which silibinin inhibited the basal level of differentiation, as illustrated in Fig 1, B and C. In the patient specimen shown in this figure, examination of the flow cytometry dot-plotted data indicated that silibinin increased the effect of 1,25D by promoting higher intensity of CD14 expression (circled area), along with a weak promotion of CD11b expression (Fig. 1C). In total, silibinin either modestly induced differentiation, enhanced deltanoid-induced differentiation, or both, in 14 (70%) patient samples.
Silibinin cooperates with cyclopropyl analogs of 1,25D more effectively than with non-modified 1,25D
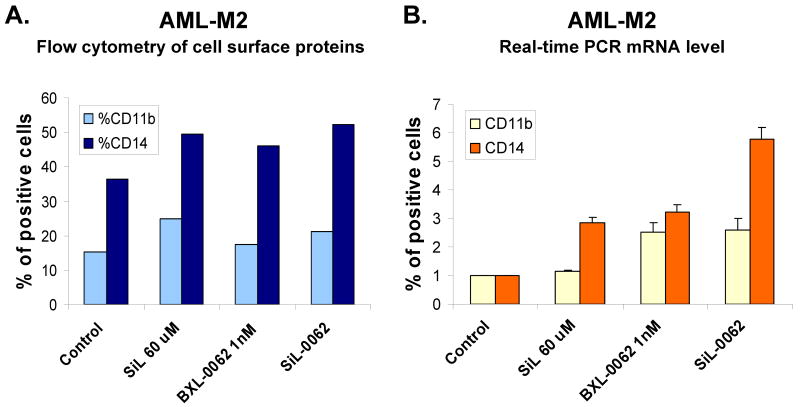
When sufficient numbers of cells were available after diagnostic procedures and analysis of differentiation induction, we set up more extensive experiments. One such investigation centered on a comparison of the ability of silibinin to enhance the induction of differentiation by 1,25D and its less calcemic analogs. In a series of these experiments we found that the analog BXL-0062 was more effectively enhanced by silibinin than its derivative BXL-0143 or the unmodified 1,25D (Fig 3). In this series we were able to demonstrate statistical significance (p<0.05) for the enhancement of CD14 expression in these patient samples, but the increases in CD11b were too small to achieve such significance.
Fig. 3. Silibinin enhances the effect of cyclopropyl analogs of 1,25D more effectively than of the parent compound.
A-C. Results from three different large samples (patients # 12, #16, #11, respectively) show similarity of differentiation outcomes when AML blasts were exposed to the indicated compounds or their combinations for 9, 5, and 7 days, respectively. Lane 1= vehicle- treated controls, lane 2=SIL 60 μM, lane 3=1,25D 10 nM, lane 4=SIL-1,25D, lane 5=BXL-0062 10 nM, lane 6=SIL-BXL -0062, lane 7=BXL-0143 10 nM, lane 8=SIL-BXL-0143 D. Mean values (+/- SD) of the three experiments shown in panels A-C. * signifies p< 0.05 for CD14 increase when SIL was added.
Silibinin can induce differentiation as a single agent, acting at pre-translational level
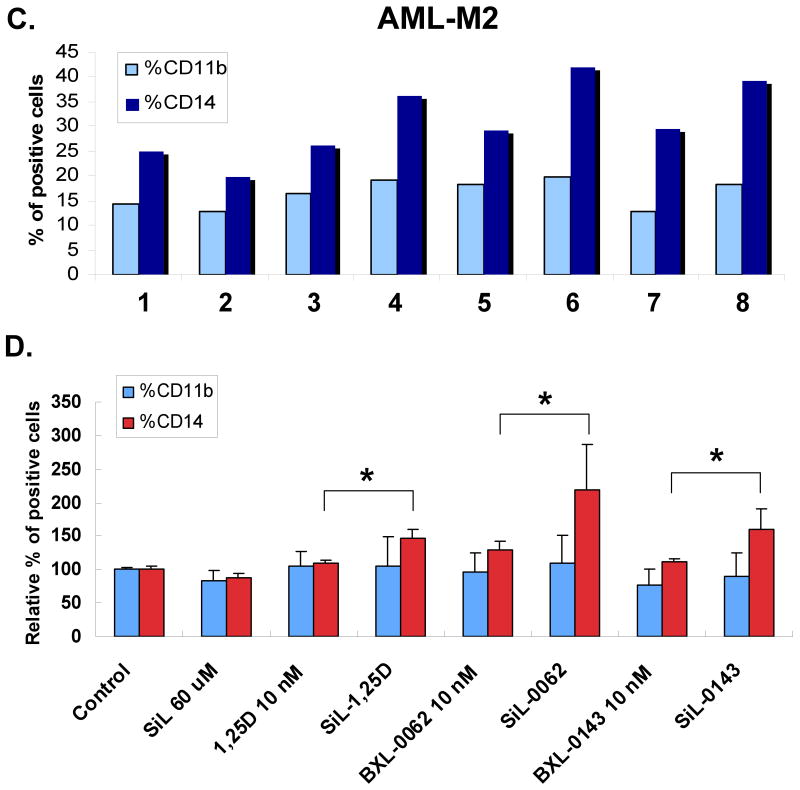
In 45% of samples studied here silibinin induced differentiation, albeit modestly, without the presence of co-inducers. An example, with further documentation of the induction of differentiation by qRT-PCR of the expression of monocytic markers CD11b and CD14, is shown in Fig 4. Again, silibinin also enhanced the effects of the analog BXL-0062, evident at both mRNA and cell surface protein level, notable even at a low (1 nM) level of the analog.
Fig. 4. Silibinin, acting as a single agent, can induce monocytic differentiation of AML blasts acting at mRNA level.
A. An example of differentiation induced by SIL alone, as determined by two cell surface differentiation markers CD11b and CD14 using flow cytometry. B. Enhanced expression of CD11b and CD14 mRNAs determined by quantitative RT-PCR, showing the action of SIL at transcriptional level. Mean values (+/-SD) of three measurements are shown here. Patient # 10.
Exposure to silibinin upregulates the levels of vitamin D receptor (VDR) and its co-receptor Retinoid Receptor α (RXRα)
Differentiation induction by 1,25D requires the presence of VDR, and it is enhanced by RXRα. We therefore tested if silibinin effects on differentiation can be explained on the basis of an upregulation of the expression of these nuclear TFs. Indeed, silibinin can, though usually moderately, increase the expression of VDR and RXRα (Fig 5A and C), the latter being particularly apparent at the mRNA level (Fig 5B), and enhance their upregulation by deltanoids (Fig 5C and D). However, this was not noted in all the patient specimens studied. Interestingly, in some specimens only one (VDR or RXRα) component was increased by such treatment of the cells (not shown). Also, when silibinin increased VDR or RXRα abundance, addition of 1,25D or analog did not always further increase its level. Thus, if the expression of VDR and/or RXRα contributes to the regulation of AML cell differentiation in primary culture, this is not a constant occurrence.
Fig. 5. The levels of vitamin D receptor (VDR) and its co-receptor RXRα are altered in silibinin-treated cells.
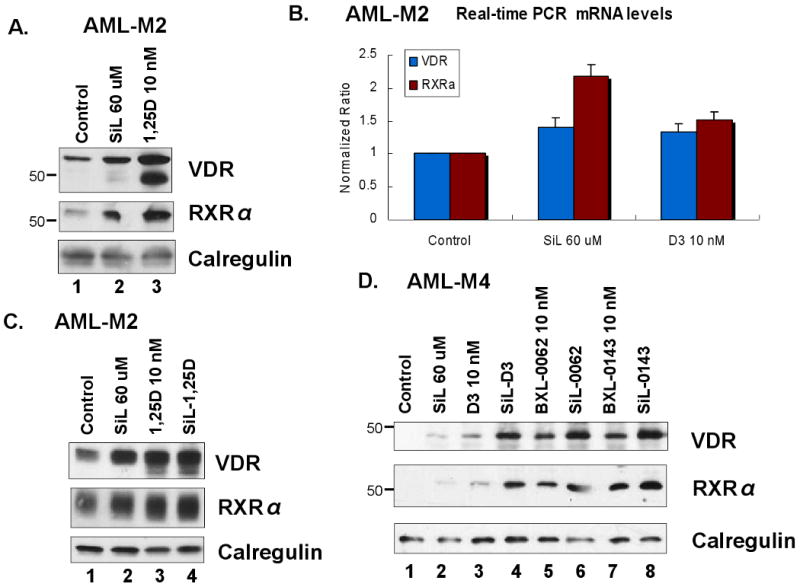
A. Western blots showing a comparison of the relative upregulation of the receptors by SIL (60 μM) and 1,25D (10 nM), 5 days treatment. B. Transcriptional upregulation of the receptors, conditions as in panel A, same patient, (#10), differentiation was both induced and enhanced by SIL. Note the prominent increase in RXRα mRNA. Mean values (+/-SD) of three measurements are shown here. C. Western blots from another patient (#1) showing a comparison of the relative upregulation of the receptors by SIL, 1,25D alone, and their combination. D. Western blots from another patient (#16) showing a correlation of receptor upregulation with increased differentiation, illustrated in Fig 3, panel B. Calregulin signal in these and subsequent Westerns presented served as loading and transfer controls.
Silibinin regulates the expression of general TFs previously linked to 1,25D-induced differentiation
Several ubiquitous TFs such as jun/AP-1, C/EBPs, and PU.1 are known to be upregulated when HL60 and other established AML cell lines such as U937 cells are induced to monocytic differentiation by 1,25D (39, 46-50). We therefore tested if members of the jun family of proteins, which are key components of the AP-1 transcription complex, and other monocytic differentiation-related TFs are regulated by silibinin. As shown in Fig 6, A-C, exposure to silibinin alone can upregulate all TFs tested, but the intensity of the upregulation in comparison to the effects of 1,25D varies in different patient specimens. However, although the effects of silibinin and 1,25D on TF levels can be additive (e.g. Fig 6C), this is not apparent in all cases, and does not always correlate with differentiation. For instance, while there is a correlation between differentiation and TF expression in some cases, eg patient #16 (panel B in Fig 3 and panel C in Fig 6), this was less obvious for the specimen from patient #15 (data not shown).
Fig. 6. The levels of downstream differentiation-related transcription factors are altered by silibinin.
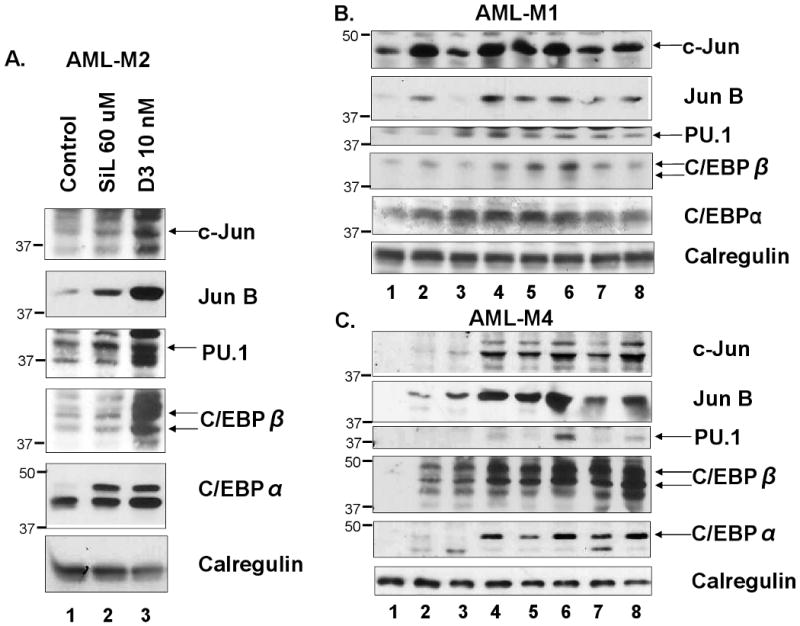
A. Western blots showing a comparison of the relative upregulation of TFs by SIL (60 μM) and 1,25D (10 nM), 5 days treatment. Patient #10. B and C. Western blots showing that while upregulation of TFs protein levels by SIL is a constant feature, the extent of the upregulation is variable. Note also that upregulation of TFs by SIL and deltanoids can be additive (e.g. panel C, lanes 4, 6 and 8), but this is not a constant feature (Panel B). The lanes were: Lane 1= vehicle treated controls, lane 2=SIL 60 μM, lane 3=1,25D 10 nM, lane 4=SIL-1,25D, lane 5=BXL-0062 10 nM, lane 6=SIL-BXL-0062, lane 7=BXL-0143 10 nM, lane 8=SIL-BXL-0143. Patient #15 (panel B) and # 16 (panel C).
Thus, there appears to be a complex relationship between regulation of TFs and the potentiation of differentiation by silibinin, and it is possible the relationship may not be causative. However, the ability of silibinin to increase the abundance of differentiation-related TFs in leukemia cells is noteworthy and potentially important.
Discussion
While it is well recognized that established cell lines do not necessarily mimic biological behavior in vivo, human cell lines have provided valuable insights regarding mechanisms which operate during the neoplastic change. The present study underscores, however, that disease manifestations of AML represent the final common pathway initiated by diverse errors during hematopoiesis and dysfunctional signaling resulting from these errors may result in different TF responses in different patients. Thus, we found here that although sibilinin provokes an increase in the levels of several differentiation-related TFs which may facilitate or be positively associated with differentiation, this effect of silibinin is not uniform on the cells of different patients. This is quite similar to the findings of Koschmieder et al (50), who used a demethylating agent decitabine and 1,25D on 17 samples of human AML blasts in primary culture, and found a variability in cellular responses as exemplified by the induction of individual markers of differentiation. For instance, CD11b expression was increased by 1,25D in 53% of the samples, by decitabine in 29%, and by both agents in 59%. Thus, available studies demonstrate the heterogeneity of AML blast responses to differentiating agents.
Such complexity is not easily resolved, but the present study is consistent with the “by-pass of the lesion” hypothesis, which posits that differentiation of myeloid leukemia cells by vitamin derived compound-based regimens depends on the increased levels of differentiation-related TFs, irrespective of the nature of the molecular lesions that produce the differentiation block, and thus the disease (8, 50). The silibinin-induced increases in VDR/RXR and general TFs and in differentiation found here appear to be specific to the hematopoietic cells, as in the cells derived from solid tumors silibinin has been reported to inhibit translation and has an anti-proliferative effect not associated with differentiation (34, 51), or when used at high concentrations (>150 μM) to result in apoptosis (52). While in our experiments silibinin was used at 60 μM concentration and produced differentiation but not apoptosis, this concentration–dependence and cell type-specificity may prove to have a therapeutic benefit, allowing different approaches to diseases with different sites of origin.
Our study is expected to facilitate the determination of which of the existing AML cell lines best approximate to the different patterns of responses to differentiation-enhancers, such as silibinin, in patient blood or bone marrow. This will then provide unlimited material for further studies of the molecular mechanisms that allow 1,25D and enhancers to by-pass the block to myeloid cell maturation imposed by mutations and other genetic changes.
Acknowledgments
We thank Dr Xuening Wang for comments on the manuscript.
Supported by NIH grants R01-CA 11794 (to GPS and MD) and R01-CA 044722 (to GPS), both from the National Cancer Institute.
References
- 1.Spira AI, Carducci MA. Differentiation therapy. Curr Opin Pharmacol. 2003 Aug;3(4):338–43. doi: 10.1016/s1471-4892(03)00081-x. [DOI] [PubMed] [Google Scholar]
- 2.Redaelli A, Botteman MF, Stephens JM, Brandt S, Pashos CL. Economic burden of acute myeloid leukemia: a literature review. Cancer Treat Rev. 2004 May;30(3):237–47. doi: 10.1016/j.ctrv.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Faderl S, Ravandi F, Huang X, Garcia-Manero G, Ferrajoli A, Estrov Z, et al. A randomized study of clofarabine versus clofarabine plus low-dose cytarabine as frontline therapy for patients aged 60 years and older with acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood. 2008 Sep 1;112(5):1638–45. doi: 10.1182/blood-2007-11-124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W, Konopleva M, Ruvolo VR, McQueen T, Evans RL, Bornmann WG, et al. Sorafenib induces apoptosis of AML cells via Bim-mediated activation of the intrinsic apoptotic pathway. Leukemia. 2008 Apr;22(4):808–18. doi: 10.1038/sj.leu.2405098. [DOI] [PubMed] [Google Scholar]
- 5.Griffin JD. The use of monoclonal antibodies in the characterization of myeloid leukemias. Hematol Pathol. 1987;1(2):81–91. [PubMed] [Google Scholar]
- 6.Nishioka C, Ikezoe T, Yang J, Gery S, Koeffler HP, Yokoyama A. Inhibition of mammalian target of rapamycin signaling potentiates the effects of all-trans retinoic acid to induce growth arrest and differentiation of human acute myelogenous leukemia cells. Int J Cancer. 2009 Apr 6; doi: 10.1002/ijc.24472. [DOI] [PubMed] [Google Scholar]
- 7.Lancet JE, Karp JE. Novel postremission strategies in adults with acute myeloid leukemia. Curr Opin Hematol. 2009 Mar;16(2):105–11. doi: 10.1097/MOH.0b013e3283257b04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Studzinski GP, Wang X, Ji Y, Wang Q, Zhang Y, Kutner A, et al. The rationale for deltanoids in therapy for myeloid leukemia: role of KSR-MAPK-C/EBP pathway. J Steroid Biochem Mol Biol. 2005 Oct;97(1-2):47–55. doi: 10.1016/j.jsbmb.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flynn PJ, Miller WJ, Weisdorf DJ, Arthur DC, Brunning R, Branda RF. Retinoic acid treatment of acute promyelocytic leukemia: in vitro and in vivo observations. Blood. 1983 Dec;62(6):1211–7. [PubMed] [Google Scholar]
- 10.Fontana JA, Rogers JS, 2nd, Durham JP. The role of 13 cis-retinoic acid in the remission induction of a patient with acute promyelocytic leukemia. Cancer. 1986 Jan 15;57(2):209–17. doi: 10.1002/1097-0142(19860115)57:2<209::aid-cncr2820570204>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 11.Degos L, Dombret H, Chomienne C, Daniel MT, Miclea JM, Chastang C, et al. All-trans-retinoic acid as a differentiating agent in the treatment of acute promyelocytic leukemia. Blood. 1995 May 15;85(10):2643–53. [PubMed] [Google Scholar]
- 12.Abe E, Miyaura C, Sakagami H, Takeda M, Konno K, Yamazaki T, et al. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4990–4. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munker R, Kobayashi T, Elstner E, Norman AW, Uskokovic M, Zhang W, et al. A new series of vitamin D analogs is highly active for clonal inhibition, differentiation, and induction of WAF1 in myeloid leukemia. Blood. 1996 Sep 15;88(6):2201–9. [PubMed] [Google Scholar]
- 14.Wang Q, Harrison JS, Uskokovic M, Kutner A, Studzinski GP. Translational study of vitamin D differentiation therapy of myeloid leukemia: effects of the combination with a p38 MAPK inhibitor and an antioxidant. Leukemia. 2005 Oct;19(10):1812–7. doi: 10.1038/sj.leu.2403916. [DOI] [PubMed] [Google Scholar]
- 15.Waxman S. Differentiation therapy in acute myelogenous leukemia (non-APL) Leukemia. 2000 Mar;14(3):491–6. doi: 10.1038/sj.leu.2401714. [DOI] [PubMed] [Google Scholar]
- 16.Koeffler HP, Hirji K, Itri L. 1,25-Dihydroxyvitamin D3: in vivo and in vitro effects on human preleukemic and leukemic cells. Cancer Treat Rep. 1985 Dec;69(12):1399–407. [PubMed] [Google Scholar]
- 17.Wieder R, Novick SC, Hollis BW, Bryan M, Chanel SM, Owusu K, et al. Pharmacokinetics and safety of ILX23-7553, a non-calcemic-vitamin D3 analogue, in a phase I study of patients with advanced malignancies. Invest New Drugs. 2003 Nov;21(4):445–52. doi: 10.1023/a:1026203418976. [DOI] [PubMed] [Google Scholar]
- 18.Chan JS, Beer TM, Quinn DI, Pinski JK, Garzotto M, Sokoloff M, et al. A phase II study of high-dose calcitriol combined with mitoxantrone and prednisone for androgen-independent prostate cancer. BJU Int. 2008 Dec;102(11):1601–6. doi: 10.1111/j.1464-410X.2008.08017.x. [DOI] [PubMed] [Google Scholar]
- 19.Danilenko M, Studzinski GP. Enhancement by other compounds of the anti-cancer activity of vitamin D(3) and its analogs. Exp Cell Res. 2004 Aug 15;298(2):339–58. doi: 10.1016/j.yexcr.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 20.Chu YF, Sun J, Wu X, Liu RH. Antioxidant and antiproliferative activities of common vegetables. J Agric Food Chem. 2002 Nov 6;50(23):6910–6. doi: 10.1021/jf020665f. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Velez M, Martinez-Martinez F, Del Valle-Ribes C. The study of phenolic compounds as natural antioxidants in wine. Crit Rev Food Sci Nutr. 2003;43(3):233–44. doi: 10.1080/10408690390826509. [DOI] [PubMed] [Google Scholar]
- 22.Mann CD, Neal CP, Garcea G, Manson MM, Dennison AR, Berry DP. Phytochemicals as potential chemopreventive and chemotherapeutic agents in hepatocarcinogenesis. Eur J Cancer Prev. 2009 Feb;18(1):13–25. doi: 10.1097/CEJ.0b013e3282f0c090. [DOI] [PubMed] [Google Scholar]
- 23.Shabtay A, Sharabani H, Barvish Z, Kafka M, Amichay D, Levy J, et al. Synergistic antileukemic activity of carnosic acid-rich rosemary extract and the 19-nor Gemini vitamin D analogue in a mouse model of systemic acute myeloid leukemia. Oncology. 2008;75(3-4):203–14. doi: 10.1159/000163849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharabani H, Izumchenko E, Wang Q, Kreinin R, Steiner M, Barvish Z, et al. Cooperative antitumor effects of vitamin D3 derivatives and rosemary preparations in a mouse model of myeloid leukemia. Int J Cancer. 2006 Jun 15;118(12):3012–21. doi: 10.1002/ijc.21736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh RP, Dhanalakshmi S, Tyagi AK, Chan DC, Agarwal C, Agarwal R. Dietary feeding of silibinin inhibits advance human prostate carcinoma growth in athymic nude mice and increases plasma insulin-like growth factor-binding protein-3 levels. Cancer Res. 2002 Jun 1;62(11):3063–9. [PubMed] [Google Scholar]
- 26.Bhatia N, Zhao J, Wolf DM, Agarwal R. Inhibition of human carcinoma cell growth and DNA synthesis by silibinin, an active constituent of milk thistle: comparison with silymarin. Cancer Lett. 1999 Dec 1;147(1-2):77–84. doi: 10.1016/s0304-3835(99)00276-1. [DOI] [PubMed] [Google Scholar]
- 27.Singh RP, Deep G, Chittezhath M, Kaur M, Dwyer-Nield LD, Malkinson AM, et al. Effect of silibinin on the growth and progression of primary lung tumors in mice. J Natl Cancer Inst. 2006 Jun 21;98(12):846–55. doi: 10.1093/jnci/djj231. [DOI] [PubMed] [Google Scholar]
- 28.Son YG, Kim EH, Kim JY, Kim SU, Kwon TK, Yoon AR, et al. Silibinin sensitizes human glioma cells to TRAIL-mediated apoptosis via DR5 up-regulation and down-regulation of c-FLIP and survivin. Cancer Res. 2007 Sep 1;67(17):8274–84. doi: 10.1158/0008-5472.CAN-07-0407. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Gao Y, Zhang L, Zeng J, He D, Sun Y. Silibinin inhibits cell growth and induces apoptosis by caspase activation, down-regulating survivin and blocking EGFR-ERK activation in renal cell carcinoma. Cancer Lett. 2008 Dec 8;272(1):61–9. doi: 10.1016/j.canlet.2008.06.033. [DOI] [PubMed] [Google Scholar]
- 30.Chittezhath M, Deep G, Singh RP, Agarwal C, Agarwal R. Silibinin inhibits cytokine-induced signaling cascades and down-regulates inducible nitric oxide synthase in human lung carcinoma A549 cells. Mol Cancer Ther. 2008 Jul;7(7):1817–26. doi: 10.1158/1535-7163.MCT-08-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim KW, Choi CH, Kim TH, Kwon CH, Woo JS, Kim YK. Silibinin inhibits glioma cell proliferation via Ca2+/ROS/MAPK-dependent mechanism in vitro and glioma tumor growth in vivo. Neurochem Res. 2009 Aug;34(8):1479–90. doi: 10.1007/s11064-009-9935-6. [DOI] [PubMed] [Google Scholar]
- 32.Singh RP, Raina K, Deep G, Chan D, Agarwal R. Silibinin suppresses growth of human prostate carcinoma PC-3 orthotopic xenograft via activation of extracellular signal-regulated kinase 1/2 and inhibition of signal transducers and activators of transcription signaling. Clin Cancer Res. 2009 Jan 15;15(2):613–21. doi: 10.1158/1078-0432.CCR-08-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Velmurugan B, Singh RP, Tyagi A, Agarwal R. Inhibition of azoxymethane-induced colonic aberrant crypt foci formation by silibinin in male Fisher 344 rats. Cancer Prev Res (Phila Pa) 2008 Oct;1(5):376–84. doi: 10.1158/1940-6207.CAPR-08-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin CJ, Sukarieh R, Pelletier J. Silibinin inhibits translation initiation: implications for anticancer therapy. Mol Cancer Ther. 2009 Jun;8(6):1606–12. doi: 10.1158/1535-7163.MCT-08-1152. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Maceira P, Mateo J. Silibinin inhibits hypoxia-inducible factor-1alpha and mTOR/p70S6K/4E-BP1 signalling pathway in human cervical and hepatoma cancer cells: implications for anticancer therapy. Oncogene. 2009 Jan 22;28(3):313–24. doi: 10.1038/onc.2008.398. [DOI] [PubMed] [Google Scholar]
- 36.Tyagi A, Singh RP, Ramasamy K, Raina K, Redente EF, Dwyer-Nield LD, et al. Growth inhibition and regression of lung tumors by silibinin: modulation of angiogenesis by macrophage-associated cytokines and nuclear factor-kappaB and signal transducers and activators of transcription 3. Cancer Prev Res (Phila Pa) 2009 Jan;2(1):74–83. doi: 10.1158/1940-6207.CAPR-08-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang SN, Lee MH, Kim KM, Cho D, Kim TS. Induction of human promyelocytic leukemia HL-60 cell differentiation into monocytes by silibinin: involvement of protein kinase. C Biochem Pharmacol. 2001;61(12):1487–95. doi: 10.1016/s0006-2952(01)00626-8. [DOI] [PubMed] [Google Scholar]
- 38.Danilenko M, Wang Q, Wang X, Levy J, Sharoni Y, Studzinski GP. Carnosic acid potentiates the antioxidant and prodifferentiation effects of 1α, 25-dihydroxyvitamin D3 in leukemia cells but does not promote elevation of basal levels of intracellular calcium. Cancer Res. 2003 Mar 15;63(6):1325–32. [PubMed] [Google Scholar]
- 39.Wang Q, Salman H, Danilenko M, Studzinski GP. Cooperation between antioxidants and 1,25-dihydroxyvitamin D3 in induction of leukemia HL60 cell differentiation through the JNK/AP-1/Egr-1 pathway. J Cell Physiol. 2005 Sep;204(3):964–74. doi: 10.1002/jcp.20355. [DOI] [PubMed] [Google Scholar]
- 40.Laverny G, Penna G, Uskokovic M, Marczak S, Maehr H, Jankowski P, et al. Synthesis and anti-inflammatory properties of 1alpha,25-dihydroxy-16-ene-20-cyclopropyl-24-oxo-vitamin D3, a hypocalcemic, stable metabolite of 1alpha,25-dihydroxy-16-ene-20-cyclopropyl-vitamin D3. J Med Chem. 2009 Apr 23;52(8):2204–13. doi: 10.1021/jm801365a. [DOI] [PubMed] [Google Scholar]
- 41.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976 Aug;33(4):451–8. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, Posner GH, Danilenko M, Studzinski GP. Differentiation-inducing potency of the seco-steroid JK-1624F2-2 can be increased by combination with an antioxidant and a p38MAPK inhibitor which upregulates the JNK pathway. J Steroid Biochem Mol Biol. 2007 Jun-Jul;105(1-5):140–9. doi: 10.1016/j.jsbmb.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 44.Kang SN, Lee MH, Kim KM, Cho D, Kim TS. Induction of human promyelocytic leukemia HL-60 cell differentiation into monocytes by silibinin: involvement of protein kinase C. Biochem Pharmacol. 2001 Jun 15;61(12):1487–95. doi: 10.1016/s0006-2952(01)00626-8. [DOI] [PubMed] [Google Scholar]
- 45.Danilenko M, Wang Q, Wang X, Levy J, Sharoni Y, Studzinski GP. Carnosic acid potentiates the antioxidant and prodifferentiation effects of 1alpha,25-dihydroxyvitamin D3 in leukemia cells but does not promote elevation of basal levels of intracellular calcium. Cancer Res. 2003 Mar 15;63(6):1325–32. [PubMed] [Google Scholar]
- 46.Kolla SS, Studzinski GP. Resolution of multiple AP-1 complexes in HL-60 cells induced to differentiate by 1,25-dihydroxyvitamin D3. J Cell Physiol. 1993 Jul;156(1):63–71. doi: 10.1002/jcp.1041560110. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Studzinski GP. The requirement for and changing composition of the activating protein-1 transcription factor during differentiation of human leukemia HL60 cells induced by 1,25-dihydroxyvitamin D3. Cancer Res. 2006 Apr 15;66(8):4402–9. doi: 10.1158/0008-5472.CAN-05-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ji Y, Studzinski GP. Retinoblastoma protein and CCAAT/enhancer-binding protein beta are required for 1,25-dihydroxyvitamin D3-induced monocytic differentiation of HL60 cells. Cancer Res. 2004 Jan 1;64(1):370–7. doi: 10.1158/0008-5472.can-03-3029. [DOI] [PubMed] [Google Scholar]
- 49.Marcinkowska E, Garay E, Gocek E, Chrobak A, Wang X, Studzinski GP. Regulation of C/EBPbeta isoforms by MAPK pathways in HL60 cells induced to differentiate by 1,25-dihydroxyvitamin D3. Exp Cell Res. 2006 Jul 1;312(11):2054–65. doi: 10.1016/j.yexcr.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koschmieder S, Agrawal S, Radomska HS, Huettner CS, Tenen DG, Ottmann OG, et al. Decitabine and vitamin D3 differentially affect hematopoietic transcription factors to induce monocytic differentiation. Int J Oncol. 2007 Feb;30(2):349–55. [PubMed] [Google Scholar]
- 51.Roy S, Kaur M, Agarwal C, Tecklenburg M, Sclafani RA, Agarwal R. p21 and p27 induction by silibinin is essential for its cell cycle arrest effect in prostate carcinoma cells. Mol Cancer Ther. 2007 Oct;6(10):2696–707. doi: 10.1158/1535-7163.MCT-07-0104. [DOI] [PubMed] [Google Scholar]
- 52.Bang CI, Paik SY, Sun DI, Joo YH, Kim MS. Cell growth inhibition and down-regulation of survivin by silibinin in a laryngeal squamous cell carcinoma cell line. Ann Otol Rhinol Laryngol. 2008 Oct;117(10):781–5. doi: 10.1177/000348940811701014. [DOI] [PubMed] [Google Scholar]