Abstract
Novel therapeutics to overcome the toxic effects of organophosphorus (OP) chemical agents are needed due to the documented use of OPs in warfare (e.g. 1980–1988 Iran/Iraq war) and terrorism (e.g. 1995 Tokyo subway attacks). Standard OP exposure therapy in the United States consists of atropine sulfate (to block muscarinic receptors), the acetylcholinesterase (AChE) reactivator (oxime) pralidoxime chloride (2-PAM), and a benzodiazepine anticonvulsant to ameliorate seizures. A major disadvantage is that quaternary nitrogen charged oximes, including 2-PAM, do not cross the blood brain barrier (BBB) to treat brain AChE. Therefore, we have synthesized and evaluated pro-2-PAM (a lipid permeable 2-PAM derivative) that can enter the brain and reactivate CNS AChE, preventing seizures in guinea pigs after exposure to OPs. The protective effects of the pro-2-PAM after OP exposure were shown using a) surgically-implanted radiotelemetry probes for electroencephalogram (EEG) b) neurohistopathology of brain, c) cholinesterase activities in the PNS and CNS, and d) survivability. The PNS oxime 2-PAM was ineffective at reducing seizures/status epilepticus (SE) in diisopropyl-fluorophosphate (DFP)-exposed animals. In contrast, pro-2-PAM significantly suppressed and then eliminated seizure activity. In OP-exposed guinea pigs, there was a significant reduction in neurological damage with pro-2-PAM, but not 2-PAM. Distinct regional areas of the brains showed significantly higher AChE activity 1.5 h after OP exposure in pro-2-PAM treated animals compared to the 2-PAM treated ones. However, blood and diaphragm showed similar AChE activities in animals treated with either oxime, as both 2-PAM and pro 2-PAM are PNS active oximes. In conclusion, pro-2-PAM can cross the BBB, is rapidly metabolized inside the brain to 2-PAM, and protects against OP-induced SE through restoration of brain AChE activity. Pro-2-PAM represents the first non-invasive means of administering a CNS therapeutic for the deleterious effects of OP poisoning by reactivating CNS AChE.
Keywords: Acetylcholinesterase, Diisopropyl-fluorophosphate, Electroencephalogram (EEG), Guinea pigs, Organophosphate agent, Pro-2-Pralidoxime, Soman (GD), status epilepticus
1. Introduction
The chemical warfare nerve agent organophosphates (OP) such as sarin (GB) are intended to disrupt neurotransmission in humans by rapidly inhibiting acetylcholinesterase (AChE), which prolongs the excitatory action of the neurotransmitter acetylcholine (ACh) by preventing its hydrolysis and removal from cholinergic receptors [1]. The accumulation of excess ACh causes miosis (iris muscle constriction) and rhinorrhea (nasal discharge) that proceed to bronchoconstriction, fasciculation, and seizures due to inhibition of central nervous system AChE. Severe and untreated AChE inhibition from acute OP exposure ultimately results in a cholinergic crisis and death, usually by respiratory failure.
OPs, by virtue of their organic-like features, readily penetrate the skin unless the agent is thickened [2] and also the usually highly exclusionary blood brain barrier (BBB). In the CNS, OPs exhibit short and long-term detrimental effects [3]; in mild OP exposures, there is depressed mental status and respiratory drive. In OP survivors with prolonged and repeated seizures (status epilepticus) resulting from CNS AChE inhibition and the cascade of overstimulation of excitatory amino acid receptors, severe neuropathology and neurobehavioral abnormalities were observed [4]. The long term CNS effects of sarin exposure to humans were documented in victims of the 1995 Tokyo subway incident. A victim exhibited tonic-clonic convulsions 7 minutes after exposure, was treated with the antimuscarinic atropine sulfate and oxime AChE reactivator 2-PAM, but exhibited long-term personality changes characterized by passivity and shallowness [5]. However, 2-PAM, as well as other positively charged, hydrophilic small molecule oximes such as obidoxime, HI-6, and MMB-4, do not penetrate the BBB and therefore cannot reactivate OP-inhibited brain AChE [6] (Figure 1). More recently, matched control and sarin exposed Tokyo victims were analyzed for brain morphological changes using diffusion tensor magnetic resonance imaging [7]. Compared to controls, the imaging from the brains of victims exposed to sarin exhibited significant signs of sarin intoxication and subsequent damage; smaller than normal insular cortex and hippocampus areas in exposed individuals correlated (P < 0.05) with decreased serum cholinesterase levels and chronic somatic complaints related to anxiety.
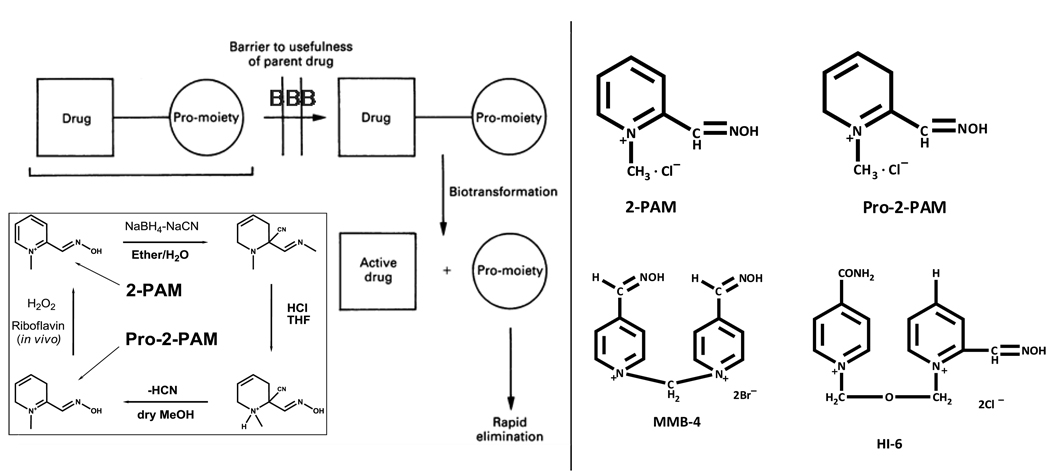
Figure 1.
Pro-2-PAM synthesis and in vivo distribution in comparison to 2-PAM. On the lower left, synthesis of pro-2-PAM from 2-PAM [11]. Pro-2-PAM, a pro-drug form of 2-PAM, exhibits lipid solubility due to its reduced charge, readily penetrates the blood brain barrier to the CNS as well as the PNS. Inside the brain, Pro-2-PAM is rapidly converted to 2-PAM [13] (modified from [12]). 2-PAM, due to its quaternary charged pyridine ring, cannot cross the BBB. Right, structures of 2-PAM, Pro-2-PAM, MMB-4, and HI-6.
Currently, there is no direct treatment for OP poisoning of CNS inhibited AChE [8]. Therapy for OP poisoning in the United States consists of a combined administration of an AChE reactivator (the peripheral nervous system oxime 2-PAM), a muscarinic receptor antagonist (CNS active atropine sulfate), and an anticonvulsant (CNS active diazepam) [9]. While the efficacy of this drug combination significantly increases survival in animals and humans, it does not treat the origin of seizure in the brain, which is the inhibition of CNS AChE. Thus, seizure/status epilepticus is an outcome of OP exposure in the CNS where 2-PAM is excluded by the BBB [10].
Since there is no direct countermeasure to OP exposure in the CNS, there is a critical need for new therapeutics to maintain survival while limiting the long-term sequelae of CNS effects after OP exposure. To overcome the lack of BBB penetration by oximes such as 2-PAM or HI-6, we investigated pro-2-PAM, the pro-drug of 2-PAM. The highly charged pyridyl ring of 2-PAM is replaced with a markedly less charged dihydropyridyl moiety (Figure 1), changing the cLogP values from −3.7 to +0.99, respectively. Positive cLogP values, indicating lipophilicity, are associated with drugs that penetrate the BBB [11, 12]. Once in the CNS, pro-2-PAM is rapidly biotransformed by oxidation to its parent compound and therapeutic oxime 2-PAM to reactivate CNS (as well as PNS) inhibited AChE [13]. Pro-2-PAM was reported to modestly improve the protective ratio over 2-PAM against DFP and sarin in mice and against soman in guinea pigs, and treatment with pro-2-PAM produced higher brain AChE activity than 2-PAM therapy [14].
We now describe that pro-2-PAM is a CNS therapeutic for OP poisoning. First, it reactivated peripheral AChE equivalent to 2-PAM. Second, pro-2-PAM additionally reactivated CNS AChE (frontal cortex). Third, we monitored the effect of reactivation of CNS AChE for reduction in the sequelae of a CNS cholinergic crisis. Thus, we performed 24 hour continuous electroencephalography recordings (EEG) of guinea pigs with radiotelemetry instrumentation to quantify status epilepticus convulsions. Pro-2-PAM was observed to abrogate seizures. Fourth, we processed the guinea pig brains for histopathology (H&E and fluoro-jade staining), which provided strong evidence for protection by pro-2-PAM against CNS pathological damage induced by CNS penetrating OP, which was not observed for 2-PAM.
2. Methods
2.1. Synthesis of pro-2-PAM (Fig. 1)
We synthesized the pro-drug, pro-2-PAM, as previously described [11], but with the slight modification of allowing the final step (E1 elimination of hydrogen cyanide) to proceed overnight at 4°C to increase the product yield. Synthesized pro-2-PAM was fully characterized by 1H and 13C NMR, elemental analysis, reverse phase HPLC, mass spectrometry (MS), and its bioactivity per unit weight by AChE reactivation assay after complete oxidation to 2-PAM in vitro utilizing hydrogen peroxide. Pro-2-PAM is rapidly oxidized to 2-PAM [13]. Pro-2-PAM is stored long-term at −30°C as a dry tan-colored powder and dissolved in 0.9% saline (~pH 5.0) just prior to drug administration, which is similar to the storage and use of the oxime HI-6.
2.2 Animals
All studies used a guinea pig model of organophosphate exposure [15, 16], which were conducted in compliance with the Animal Welfare Act and other Federal statutes and regulations relating to animals and experiments involving animals and adhered to principles stated in the Guide for the Care and Use of Laboratory Animals, NRC publication, 1996 edition, and approved by the WRAIR (Walter Reed Army Institute of Research) and USAMRICD (United States Army Medical Research Institute of Chemical Defense) IACUCs. Adult, 2 month-old, male Hartley guinea pigs (~ 300 g body wt.) were purchased from Charles River Laboratories (Portage, MI) and housed in the WRAIR animal facility, with regulated temperature, humidity, and a 12/12 h light/dark cycle. Rodent chow (Prolab® Laboratory Animal Diet; PMI Nutrition, LLC; Brentwood, MO) enhanced with vitamin C and water was provided ad libitum, and fresh fruit and vegetables were provided as additional supplementary enrichment. A one week stabilization period preceded surgery and also post-surgery (see 2.3 Animal surgery).
2.3 Animal surgery. (Figure 2)
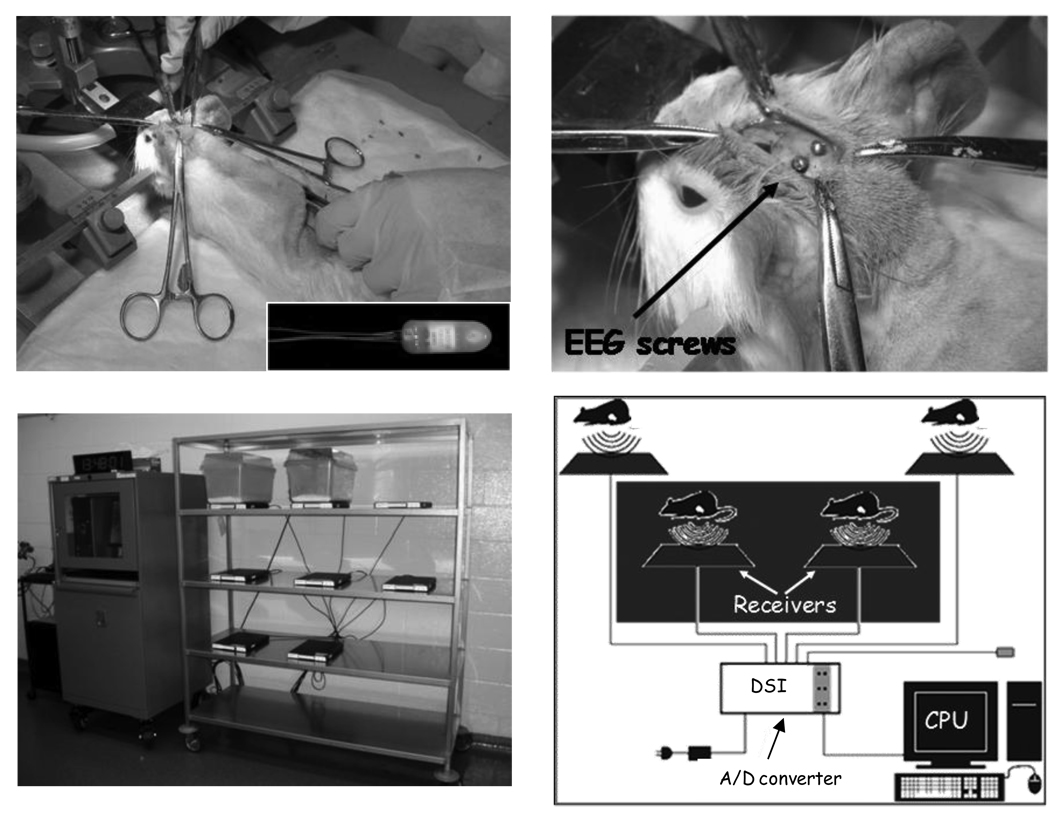
Fig. 2.
Radiotelemetry probe implantation and recorder instrumentation. Top panels: Left, surgical implantation of a radiotelemetry probe (inset). The guinea pig’s nose is held in an isoflurane delivery cone. On the right, EEG leads are tunneled to screws in the skull. Probe body is sutured underneath the skin of the back. Bottom panels: Left, radiotelemetry recorder set up on animal rack with 2 holding cages in place. Right, schematic drawing of the radiotelemetry recorder. The pads receive the probe’s radio signal, permitting untethered animal movement, which is processed, stored, and visualized on the computer.
Guinea pigs were fasted for several hours, anesthetized (2–5% isoflurane, oxygen 1.5 L/min), shaved on the head and back, and their heads placed in a stereotaxic instrument (David Kopf Instruments, Tujunga, CA). The radiotelemetry system consisted of 8 receivers and TL11M2-F40-EET bipotential radiotelemetry probes (Figure 2, top left panel, inset; Data Sciences International, St. Paul, MN) turned on and off by hand with a magnet. Probes were reused by sterilization with 4% glutaraldehyde and handled as instructed by the manufacturer. Briefly, the surgery proceeded as follows: radiotelemetry probes were surgically implanted under the back skin, with wire leads fixed to the skull to record brain activity [17] (Fig. 2, top panels). Cyano-acrylate glue was used to keep the skull electrodes in place. Incisions were sutured and then covered with TissueMend glue (Webster’s Veterinary Supply, Sterling, MA). Guinea pigs were then housed individually in microisolator cages with a 12 hour light/dark cycle.
2.4. DFP exposure and Radiotelemetry
In our studies, we exposed guinea pigs to the organophospate diisopropyl-fluorophosphate (DFP) as a surrogate for sarin (GB), for which it has a similar structure and ChE reactivity. On the day of the experiment, the standard military exposure paradigm was used and only the oxime delivery time was modified [9] as follows: guinea pigs were pretreated (i.p.) with the FDA approved prophylactic drug pyridostigmine bromide (PB) at 0.026 mg/kg. PB is a carbamate [1,23] that temporarily inhibits and sequesters AChE activity, but since it does not cross the BBB, PB does not protect CNS ChEs. In the periphery, PB spontaneously decarbamylates when OP is gone, restoring the active site of ChEs. After 20 minutes, the animals were injected (s.c.) with DFP (8 mg/kg) followed 1 min later by atropine methyl bromide (i.m., 2 mg/kg), which poorly penetrates the CNS [18]. At various times post-OP exposure, equivalent doses of 2-PAM or pro-2-PAM were injected i.m. (1.5 auto-injectors, 13 mg/kg) to approximate use of the Mark I nerve agent antidote kit provided to military personnel. Controls were given saline injections alone or PB and atropine injections, but in both cases did not receive oximes and DFP. Likewise, some animals received PB and atropine followed by DFP exposure, without oxime treatment. The EEG of the animals were continuously monitored and recorded by radiotelemetry for 24 h (Fig. 2, bottom panels). Guinea pigs were euthanized after 24 h by injection with 75 mg/kg pentobarbital followed by terminal cardiac puncture exsanguination. Brain (frontal cortex) and whole blood were collected and frozen at −80°C for ChE assays. Some animals were euthanized with pentobarbital at 1.5 h after treatment, blood taken by cardiac puncture, and then whole body perfusion with heparinized saline to remove blood from the brain. Whole blood and brain samples from these animals were collected and frozen for ChE assays as described above.
2.5 WRAIR cholinesterase assay
The WRAIR Assay [19] was performed in 96-well microtiter plates; the final concentrations of substrates were 1 mM each of acetylthiocholine, propionylthiocholine, butyrylthiocholine iodides, and 0.2 mM 4,4’-dithiodipyridine, the indicator for the hydrolyzed thiocholine, and UV absorbance measured at 324 nm, which avoids the hemoglobin interference observed with Ellman’s reagent [20]. Guinea pig blood was collected with EDTA and heparin and tissues were frozen at − 80°C. To perform the ChE assays, a small aliquot of blood, typically 10 µL, was diluted 20-fold in distilled water. Brain samples were homogenized (on ice) using a ground glass homogenizer until completely emulsified. Homogenates were centrifuged for 10 min at 15,000 × g at 4°C and the supernatants assayed for ChE activity in triplicate (final volume of 300 µL using 50 mM sodium phosphate buffer, pH 8.0). A four-minute kinetic assay was performed at 25°C using a Molecular Devices SpectraMax Plus384 microtiter spectrophotometer (Sunnyvale, CA). Data were subjected to linear least squares analysis from which the activitiy of AChE (U/mL) was calculated using SoftMax v5.2 and an Excel spreadsheet. A 2-tail t-test was used with a p-value of ≤ 0.05 to indicate significance.
2.6 Histopathology
Twenty four hours post-exposure to DFP, guinea pigs were euthanized as above, the brain removed, and forebrain taken for ChE activity assay. The remainder of the brain was subjected to immersion fixation, for at least two weeks, in 4% formaldehyde (stabilized with 0.5% methanol). Next, the formaldehyde-preserved guinea pig brains were transverse sectioned using a rodent brain matrix (model: RMB-5000C; ASI Instruments, Inc., Warren, MI). A 2 mm thick section was cut from each brain, using microtome blades hand dropped into the matrix, at the middle of the hippocampus, adjacent to the midbrain. Brain slices were processed into microscope slides containing paraffin embedded 6 µm transverse sections (microtome cut) stained with hematoxylin and eosin (H&E) or fluoro-jade in duplicate (FD Neurotechnologies, Inc; Ellicott City, MD). H&E stain is reactive towards membrane lipids and proteins, and highlights the general structural morphology of all cells. In contrast, fluoro-jade stain penetrates only leaky membranes and thus highlights dead cells. Prepared slides were examined at 40× magnification under an Olympus axial light microscope equipped with an image capturing camera (Olympus Provis AX80/DP70; Olympus, Center Valley, PA). Standard bright field and fluorescence (FITC filter) illuminations were used on the H&E and fluoro-jade stained slides, respectively. The lower-outside pyramidal layer of the hippocampus (CA1-CA2 region), a region known to be hypersensitive to OP-agent damage, was chosen for examination. Photographic images were captured of neurons and granular cells comprising this regional zone.
2.7 Assessment of protection by pro-2-PAM against percutaneous GD (soman) exposure
Experiments using GD (soman) were performed at the U.S. Army Medical Research Institute of Chemical Defense (USAMRICD; Aberdeen Proving Grounds, MD). Two month old adult male guinea pigs were used but without radiotelemetry probes. Each animal had a 12-cm2 area of bare skin exposed on the right lateral side by shaving, using an electric clipper with #40 blades, and a central 3 × 4 cm block was marked off using a black permanent marker as the site for GD application. This area of application represents ~10% of the surface area of the animal for assessing skin toxicity of chemical warfare agents [21]. At 30 min prior to OP-agent exposure, animals were pre-treated with an i.p. injection of pyridostigmine bromide (0.026 mg/kg). Animals were then fully sedated with an i.m. injection of 44 mg/kg ketamine and 7 mg/kg xylazine, at 5 min prior to OP-agent exposure. In a chemical fume hood, neat GD was percutaneously (p.c.) placed as a droplet in the center of the marked area of shaved skin using a glass micro-syringe, mimicking an exposure in the field. The amount of GD applied was 17, 23, 45, 68, 91, 113, 136, 170, 215, 339, or 537 mg/kg, which represents 1.5, 2, 4, 6, 8, 10, 12, 15, 19, 30, and 47-times, respectively, the LD50 for GD applied to unprotected animals (1 LD50 = 11 mg/kg GD) [22]. One minute after GD exposure, the animals were then treated with i.m. injections of 16 mg/kg atropine sulfate (which passes the BBB) plus two AI (auto-injectors) of pro-2-PAM. Control, animals were given 45, 68, and 79 mg/kg of GD and 1 min later 3 AI of 2-PAM, which does not enter the brain. The LD50 for guinea pigs protected by 2-PAM is 66 mg/kg GD (5.8 × LD50 of unprotected controls) [22]. Animals that survived 24 h were euthanized and blood and brain collected for AChE assay and histopathology, as previously described.
3. Results
3.1. Radiotelemetry monitoring of brain EEG activity: Pro-2-PAM, but not 2-PAM, abrogates DFP-induced status epilepticus seizures
We compared 2-PAM and pro-2-PAM in guinea pigs, the model for OP poisoning because its repertoire of OP detoxifying enzymes more closely matches the human enzyme complement than rats and mice. Control, DFP alone, or DFP followed by the oximes 2-PAM or pro-2-PAM treated animals were continuously monitored for 24 h for brain electrical activity (EEG, Fig. 3). EEG measurement and seizure allowed us to evaluate the protective effects of pro-2-PAM in comparison to 2-PAM after exposure to the OP-agent DFP. The amount of oxime (2-PAM or pro-2-PAM) administered as a single dose was equivalent to a 1.5 human auto-injector dose by body weight (13 mg/kg), which was found to be most efficacious, although we also evaluated doses of 1, 2, and 3 auto-injector equivalents (not shown). Control animals received PB, atropine, and saline instead of DFP and/or oximes.
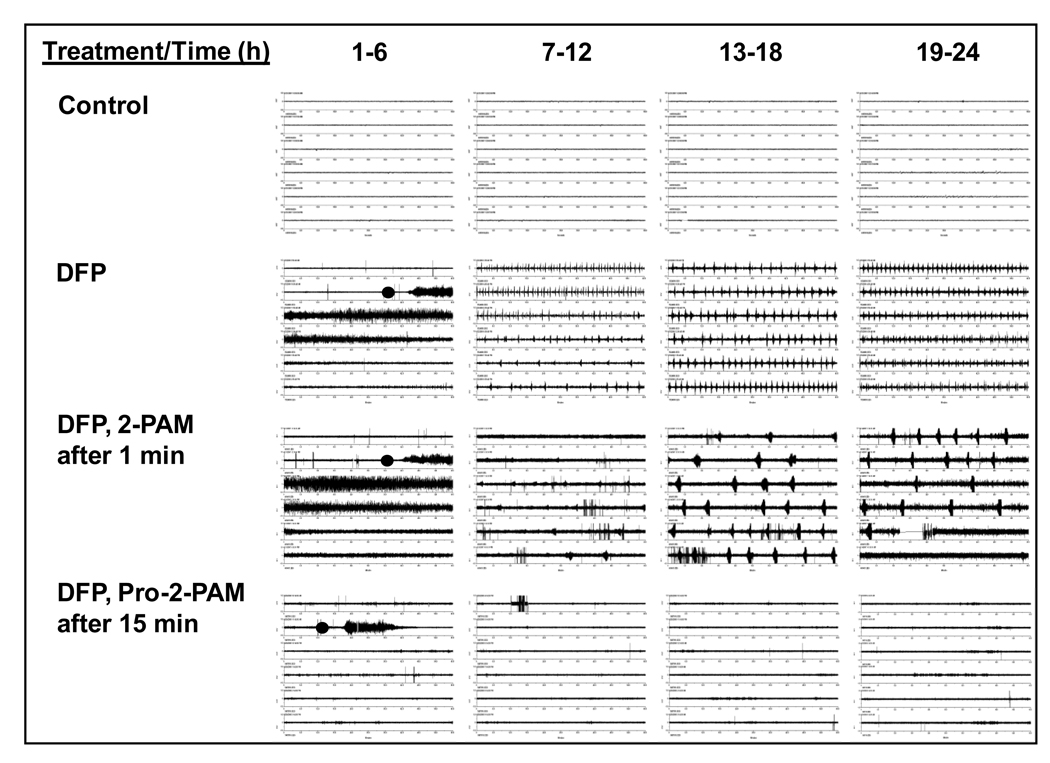
Fig. 3.
Representative EEG traces for Control, DFP, DFP then 2-PAM, and DFP then Pro-2-PAM treated guinea pigs. Each block shown is 6 h of EEG recording, with a continuous trace to 24 h for each treatment. Animals received pyridostigmine bromide (0.026 mg/kg, i.p.) then 20 min later DFP (8 mg/kg, s.c; black circle) followed at 1 min by atropine methylbromide (2 mg/kg) and 1.5 human auto-injector equivalents of each oxime (13 mg/kg, i.m.). 2-PAM was injected at 1 min post-DFP exposure, whereas pro-2-PAM treatment was delayed in this example by 15 min. The 19–24 h EEG panels show the efficacy of pro-2-PAM, but not 2-PAM, to alleviate SE.
Control guinea pigs (n = 19) displayed stable EEG tracing (Fig. 3, Control, with only random single spikes due to noise picked up by the telemetry instrumentation readily differentiated from repetitive spikes observed with SE, Fig. 3, DFP). All control animals were normal and survived the 24 h recording period. In contrast, 100 % of the guinea pigs exposed to DFP (n = 6) produced intense SE seizures that continued for the full 24 h period, with a survival rate of 33% (Fig. 3, DFP). The oxime 2-PAM was ineffective at reducing SE seizures in DFP-exposed animals (n = 24) (Fig. 3, 2-PAM), since the guinea pigs exhibited seizure activity for the full 24 h recording period (63% survival rate). Note that 2-PAM was injected at 1 min post-DFP exposure. These data support reports that 2-PAM cannot pass the BBB at therapeutically relevant doses [6, 23]. In notable contrast, pro-2-PAM (n = 27 treated animals) blocked seizure activity and increased the 24 h survival rate to 85%, when using the standard military regimen of dosage and time, 1 min after OP-agent exposure (not shown). Remarkably, pro-2-PAM abrogated seizure activity even when the injection was delayed for 15 min post-exposure (n = 9) (Fig. 3, pro-2-PAM), which produced a similar 24 h survival rate of 78%. These results suggest that pro-2-PAM - but not 2-PAM - may extend the time to treat OP-agent exposed soldiers for SE seizures.
3.2. Cholinesterase assays of peripheral (blood) and CNS (frontal cortex) tissues: Pro-2-PAM, but not 2-PAM, restores activity of DFP-inhibited brain AChE
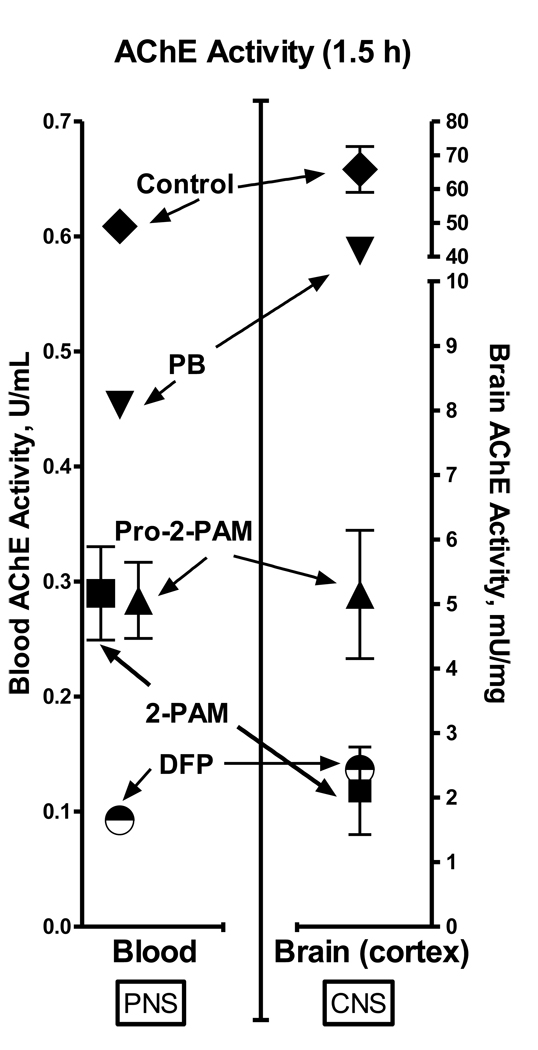
Consistent with the observations of protection of the CNS from SE by pro-2-PAM, but not 2-PAM, the frontal cortex of brain showed significantly higher (p ≤ 0.05) AChE activity, at least 2-fold, at 1.5 h after DFP exposure in pro-2-PAM treated animals (n = 4) (in this case, administered 1 min after DFP) compared to those given 2-PAM (n = 4) (Fig. 4, pro-2-PAM and 2-PAM, triangle and square, respectively). The data in Fig. 4 clearly shows that 2-PAM did not pass the BBB nor reactivate frontal cortex AChE, since DFP alone (n = 1) (half-filled circle) and 2-PAM treated animals showed equivalent inhibited AChE activity. Restoration of brain AChE activity was not complete, however, since saline and PB plus methyl atropine only treated controls (n = 2 and 1, respectively) exhibited activities about 10-fold higher than DFP then pro-2-PAM treated animals. As previous suggested [4,6], only a critical fraction of cholinesterase is required for restoration of function. As described in section 3.1 (Fig 3; EEG trace), pro-2-PAM was efficacious in stopping seizures even when delayed in injection at 15 min after DFP exposure. The AChE activity of frontal cortex for the 15 min delayed pro-2-PAM animals (n = 9), at 24 h post-DFP exposure, was 3-fold greater (p ≤ 0.05) than for those treated with DFP alone (data not shown). Thus, even with a delay in administration of pro-2-PAM, it was able to restore significant CNS AChE activity.
Fig. 4.
AChE activity in guinea pig blood (left panel; U/ml) and brain frontal cortex (right panel; mU/mg) at 1.5 h post-treatment with saline (control; diamond), PB and atropine (inverted-triangle), DFP then pro-2-PAM (triangle), DFP then 2-PAM (square), and DFP without oxime (half-filled circle); number of animals = 2, 1, 4, 4, and 1, respectively.
In the periphery, red blood cell bound AChE has been used as a marker for OP-agent inhibition and oxime reactivation [24]. Similar reactivation of blood AChE after the 2-PAM or pro-2-PAM treatments confirms that both oximes are equivalent in the PNS (Fig. 3). There was about a 2-fold increase in the blood AChE activity restored by a 1.5 auto-injector dose of 2-PAM or pro-2-PAM at 1.5 h after DFP exposure (injected 1 min after DFP).
3.3. Histopathology of brain sections, stained with fluoro-jade: Pro-2-PAM, but not 2-PAM, prevents DFP-induced apoptosis in hippocampus neurons
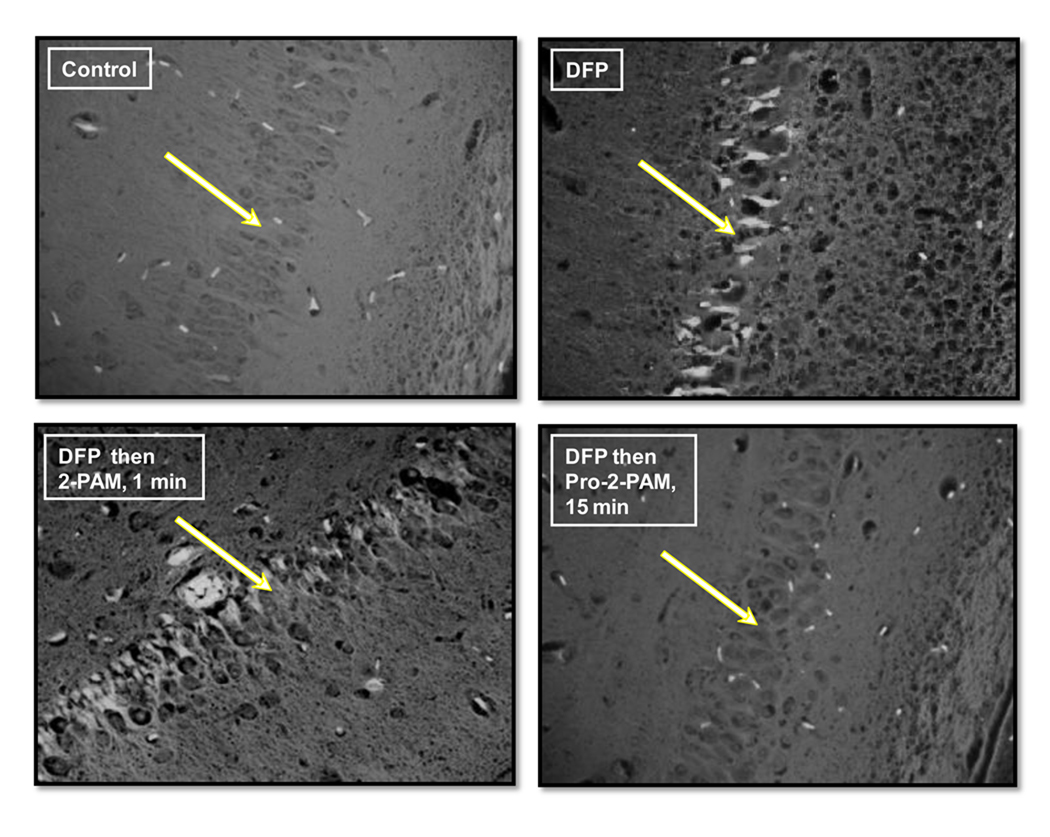
Using histopathology staining and microscopy, distinct differences between the 2-PAM treatment (at 1 min time delay) and pro-2-PAM treatment (15 min time delay) were quantified in the transverse sections cut from guinea pig brains collected 24 h after DFP exposure (section 2.5). Under fluoro-jade staining, which penetrates and highlights apoptotic neurons as bright fluorescence, the lower-outside pyramidal layer of the hippocampus exhibited missing, heavily distorted, and apoptotic granular cells and neurons in the DFP then 2-PAM treated animals (Fig. 5, lower left panel). The dead cells were mostly found to be interspersed and disrupting the order of the pyramidal layer. The pattern of cellular destruction was nearly identical in appearance to that seen for the hippocampus of animals treated with DFP alone (Fig. 5, upper right panel). In contrast, for control (PB + atropine only; Fig. 5, upper left panel) animals, the hippocampus pyramidal neuron layer was well defined with no apoptotic cells visible. Similar in appearance to controls, the DFP then pro-2-PAM (given 15 min post-exposure) treated animals completely lacked dead cells in this region and displayed minimally swollen cells. These results suggest that pro-2-PAM shielded brain neurons from most adverse effects of DFP exposure. Consistent with this observation, scoring of hippocampus neuronal cell death by fluoro-jade visualization showed that pro-2-PAM treated animals had significantly less damage compared to those treated with 2-PAM (data not shown).
Fig. 5.
Fluoro-jade staining of representative guinea pig brains (40× magnification) for the hippocampus pyramidal neuron layer (lower CA1–CA2 region) at 24 h. White arrows point to the hippocampus neuron layer. Apoptotic cells, highlighted by fluorescence above background, are distinctly seen in the DFP and DFP then 2-PAM treatment 1 min after OP exposure.
H&E staining, which highlights general cell morphology, was also carried out on identical brain sections for the control, DFP alone, DFP then 2-PAM (1 min delay), and DFP then pro-2-PAM (15 min delay) treated animals (data not shown). Under 40× magnification microscopy (bright field light), the same hippocampus region of the DFP alone and DFP then 2-PAM treated animals showed grossly swollen granular cells and pyramidal neurons with deformed nuclei, and the presence of numerous vacant holes that indicate early tissue necrosis. In contrast, the control or DFP then pro-2-PAM treated cells exhibited relatively normal morphological appearance. These H&E staining results are consistent with the fluoro-jade stain, but not as informative for identification of cells that have undergone apoptosis.
3.4. Survival outcomes after skin exposure to increasing amounts of GD (soman): Pro-2-PAM is more effective than 2-PAM at promoting 24 h survivability against GD exposure
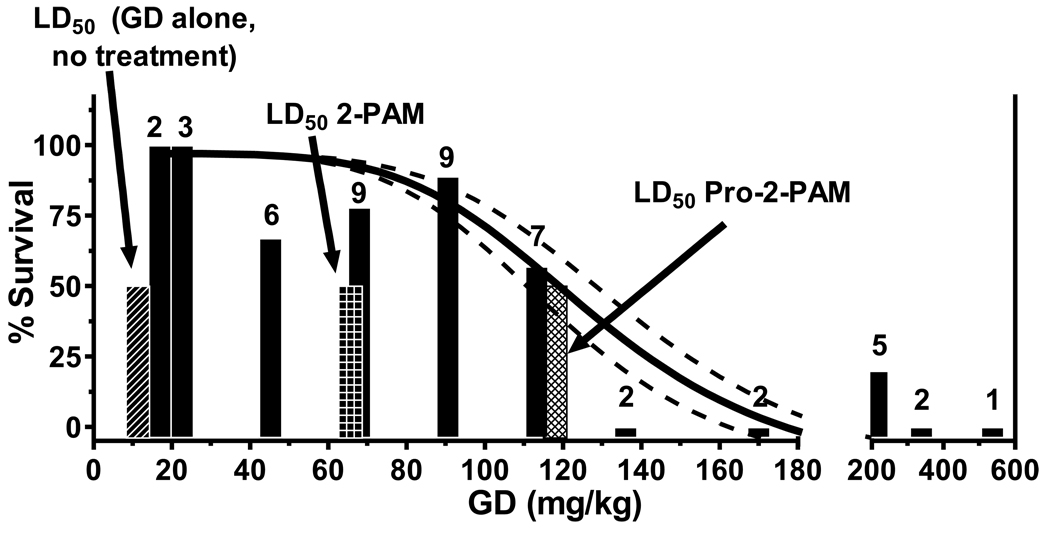
Guinea pigs, pre-treated with pyridostigmine bromide, were percutaneously exposed to increasing amounts of GD (soman), and then 1 min afterward given 2 auto-injectors of pro-2-PAM along with atropine sulfate, that also enters the brain. Survival to 24 h post-exposure was used as the experimental end point. Percentage of animals surviving 24 h after exposure to 17, 23, 45, 68, 91, 113, 136, 170, 215, 339, or 537 mg/kg of GD, then treated with pro-2-PAM, were 100, 100, 67, 78, 89, 57, 0, 0, 20, 0, and 0%, respectively; with group sizes of 2, 3, 6, 9, 9, 7, 2, 2, 5, 2, and 1, respectively (Fig. 6). The 24 h survival curve yielded a 50% lethal dose (LD50) of 118 mg/kg GD (Fig. 6, cross-hatched bar). This GD exposure level, with pro-2-PAM treatment, is 2-fold greater than the LD50 observed for guinea pigs similarly protected by 3 auto-injectors of 2-PAM (66 mg/kg GD; Fig. 6, checkered bar), and 10-fold higher than the LD50 for unprotected animals (11 mg/kg GD; Fig 6, diagonal striped bar) [22]. As judged by H&E staining of representative brain sections, animals treated with pro-2-PAM showed an absence of hippocampus neuronal cell damage at exposure levels of 17 – 113 mg/kg GD (data not shown). In contrast, animals treated with 2-PAM after GD exposure (45, 68, and 79 mg/kg) showed marked disruption of the cellular integrity for the neurons comprising the hippocampus. These results clearly demonstrate that pro-2-PAM increased survivability and markedly reduced neuropathy compared to 2-PAM, even when pro-2-PAM is administered at a 33% lower dose.
Fig. 6.
Twenty-four h survival outcome for animals exposed to GD and 2 auto-injectors of pro-2-PAM 1 min later. Animals used for each treatment are shown above the corresponding bars. The diagonal striped bar shows the LD50 for untreated control animals (11 mg/kg GD). The checkered bar represents the LD50 of GD (66 mg/kg) observed for treatment with 3 auto-injectors of 2-PAM 1 min after exposure. In contrast, the cross-hatched bar shows the LD50 of GD (118 mg/kg) that was obtained for 2 auto-injectors of pro-2-PAM treatment 1 min after exposure. Dotted lines are the 95% confidence intervals for the survival curve.
4. Discussion
OP-based pesticides and chemical warfare agents prevents AChE’s critical function to remove ACh from neuronal synapses [1]. Severe cases of OP exposure can lead to cholinergic overload and over-stimulation of downstream excitatory amino acid pathways in the CNS, yielding status epilepticus and neuronal metabolic exhaustion and apoptosis. Current standard treatment for reactivating OP inhibited AChE is to include an oxime therapeutic, such as 2-PAM, but all clinically approved oximes cannot penetrate the BBB. Oximes are therefore limited to restoration of PNS AChE to maintain diaphragm muscle contractions, and breathing. There is a crucial need to develop oximes that can readily enter the CNS, restore AChE activity, and maintain higher neurological functions. To this end, our goal was to assess the ability of pro-2- PAM, a pro-drug derivative of 2-PAM, to restore brain AChE activity in guinea pigs exposed to a lethal amount of DFP, abolish formation of SE seizures, reduce neuronal cell death, and increase survival.
We found that an s.c. injection of DFP in guinea pigs readily produced SE seizures, as judged by their EEG recordings, with death frequently occurring before 24 h. Consistent with this, their brain and blood AChE levels were severely depressed. Animals that did survive to 24 h exhibited severe neurological damage and unresponsiveness. Histopathology analysis of brains from survivors showed extensive apoptotic neurons in the hippocampus. Likewise, an i.m. injection of 2-PAM 1 min after DFP-exposure did not abrogate the SE seizures and brain AChE activity remained unchanged. While some 2-PAM treated animals survived for 24 h post-exposure due to support of diaphragm function by the oxime, they displayed obvious behavioral disturbances. This deficit was confirmed by histopathology, which showed the presence of apoptotic neurons throughout the hippocampus. These results support the observation that 2-PAM, a highly charged molecule, is incapable of entering the brain and only affords protection against OP’s by restoring peripheral AChE activity [6]. In contrast, an equivalent dose of pro-2-PAM, even after a delay of 15 min, completely abrogated DFP-induced seizures over 24 h, without obvious signs of behavioral impairment subsequently. Pro-2-PAM’s mechanism of action was by significant regeneration of brain AChE activity. Increases in blood AChE activity (PNS action), however, were identical to that found for 2-PAM. Consistent with rescue of brain AChE activity and stoppage of seizures, there was an absence of dead neurons in the hippocampus at 24 h as documented by histopathology. Consequently, pro-2-PAM appears to be equivalent to 2-PAM in the PNS and substantially more effective than 2-PAM in the CNS.
Chemical warfare OP-agents are among the most toxic compounds known to man, and due to low-cost, ease of manufacturing, rapid onset of symptoms, and resulting panic, OPs have high probability for use by terrorists. Indeed, GB (sarin) victims of the Tokyo attack, although aggressively treated with 2-PAM, showed signs of permanent brain damage more than a decade later [25, 7]. The poor long-term prognosis for these patients was likely a result of 2-PAM being incapable of crossing the BBB, precluding CNS AChE reactivation, and inability to modulate ongoing SE, which caused neuropathy.
In light of the apparent inadequacy of 2-PAM in treating human cases of OP-agent exposure, our studies were extended to guinea pigs subjected to percutaneous exposure with GD (soman). When administered immediately (~1 min) after GD application to preclude aging, Pro-2-PAM increased the LD50 compared to those given 2-PAM (Fig. 6). The increase in survival protection even occurred when pro-2-PAM was administered at a one-third lower dose than 2-PAM. Histopathology analysis of brains from the pro-2-PAM treated animals indicated that the hippocampus neurons remained relatively undamaged over the entire range of GD exposures up to the LD50 for pro-2-PAM, while 2-PAM treated guinea pigs showed extensive cellular perturbations far below this value.
5. Conclusions
In summary, this study showed in a guinea pig model that pro-2-PAM readily circumvented the deficiencies of 2-PAM as a therapeutic measure against OP poisoning. Pro-2-PAM, unlike 2-PAM, readily crossed the BBB, was rapidly converted inside the brain to bioactive 2-PAM, restored OP inhibited CNS AChE, suppressed seizure formation, and ultimately protected neurons in the hippocampus from apoptosis. We also demonstrated that pro-2-PAM yielded an improved 24 h survival after exposure to OP. Pro-2-PAM thus holds great promise as a readily available, easily administered, fast acting, PNS and CNS reactivating oxime that can benefit the warfighter and civilian personnel at the international, Federal, State, and local levels.
Acknowledgements
This work was supported by the Defense Threat Reduction Agency project 1.E0033_07_WR_C and NIH CounterAct U01 NS058166-01. “The studies were in compliance with the Animal Welfare Act and implementing Animal Welfare Regulations and adherence to the principles in “The Guide for the Care and Use of Laboratory Animals.” The opinions or assertions contained herein are private views of the authors, and are not to be construed as official or as reflecting views of the Department of the Army or the Department of Defense. We thank Ms Ruth Cho for editorial assistance.
Abbreviations
- 2-PAM
2-Pralidoxime
- ACh
Acetylcholine
- AChE
Acetylcholinesterase
- AI
Auto-injector
- BBB
Blood brain barrier
- CA1
Cornu Ammonis 1
- CA2
Cornu Ammonis 2
- ChE
Cholinesterase
- CNS
Central nervous system
- DFP
Diisopropyl-fluorophosphate
- EDTA
Ethylenediaminetetraacetic Acid
- EEG
Electroencephalogram
- FITC
Fluorescein isothiocyanate
- GB
Sarin
- GD
Soman
- H&E
Hematoxylin and eosin
- HI-6
Pyridinium, 1-[[[4-(aminocarbonyl)pyridinio]methoxy]methyl]-2-[(hydroxyimino)methyl] dichloride
- HPLC
High performance liquid chromatography
- IACUC
Institutional Animal Care and Use Committee
- I.M.
Intramuscular
- I.P.
Intraperitoneal
- LC
Liquid chromatography
- LD50
Lethal dose that kills 50% of animals tested
- MMB-4
1,1'-Methylenebis(4-[(hydroxyimino)methyl]pyridinium) dichloride
- MS
Mass spectrometry
- NMR
Nuclear magnetic resonance
- OP
Organophosphate
- P.C.
Percutaneous
- PB
Pyridostigmine bromide
- PNS
Peripheral nervous system
- Pro-2-PAM
Pro-2-Pralidoxime
- S.C.
Subcutaneous
- SE
Status epilepticus
- USAMRICD
United States Army Medical Research Institute of Chemical Defense
- VX
V-agent
- WRAIR
Walter Reed Army Institute of Research
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Taylor P. In: Goodman & Gilman's The Pharmacological Basis of Therapeutics. 8th edition. Gilman AG, Rall TW, Neis AS, Taylor P, editors. 1990. pp. 131–149. [Google Scholar]
- 2.Gordon RK, Clarkson ED Rapid Decontamination of Chemical Warfare Agents. In: Handbook of Toxicology of Chemical Warfare Agents. Gupta RC, editor. Vol. 71. 2009. pp. 1069–1081. [Google Scholar]
- 3.Grob D, Harvey AM. The effects and treatment of nerve gas poisoning. Am J Med. 1953;14:52–63. doi: 10.1016/0002-9343(53)90358-1. [DOI] [PubMed] [Google Scholar]
- 4.Carpentier P, Foquin A, Rondouin G, Lerner-Natoli M, De Groot D, Lallement G. Effects of atropine sulphate on seizure activity and brain damage produced by soman in guinea-pigs: EcoG correlates of neuropathology. Neurotoxicol. 2000;21(4):521–540. [PubMed] [Google Scholar]
- 5.Hatta K, Miura Y, Asukai N, Hamabe Y. Amnesia from sarin poisoning. Lancet. 1996;347:1343. doi: 10.1016/s0140-6736(96)90998-8. [DOI] [PubMed] [Google Scholar]
- 6.Bajgar J, Fusek J, Kuca K, Bartosova L, Jun D. Treatment of organophosphate intoxication using cholinesterase reactivators: facts and fiction. Mini Rev Med Chem. 2007;7(5):461–466. doi: 10.2174/138955707780619581. [DOI] [PubMed] [Google Scholar]
- 7.Yamasue H, Osamu A, Kasai K, Motomu S, Iwanami A, Haruyasu Y, Tochigi M, Ohtani T, Rogers M, Sasaki T, Aoki S, Kato T, Kato N. Human brain structural change related to acute single exposure to sarin. Ann Neurol. 2007;61:37–46. doi: 10.1002/ana.21024. [DOI] [PubMed] [Google Scholar]
- 8.Marrs TC, Rice P, Vale AJ. The role of oximes in the treatment of nerve agent poisoning in civilian casualties. Toxicol Rev. 2006;25(4):297–323. doi: 10.2165/00139709-200625040-00009. [DOI] [PubMed] [Google Scholar]
- 9.Newmark J. Therapy for nerve agent poisoning. Arch Neurol. 2004;61(5):649–652. doi: 10.1001/archneur.61.5.649. [DOI] [PubMed] [Google Scholar]
- 10.Lowenstein DH. Status epilepticus: an overview of the clinical problem. Epilepsia. 1999;40 Suppl 1:S3–S8. doi: 10.1111/j.1528-1157.1999.tb00872.x. [DOI] [PubMed] [Google Scholar]
- 11.Bodor N, Shek E, Higuchi T. Improved delivery through biological membranes. 1. Synthesis and Properties of 1-Methyl-1,6-dihydropyridine-2-carbaldoxime, a Pro-Drug of N-Methylpyridinium-2-carbaldoxime Chloride. J Med Chem. 1976;19:102–107. doi: 10.1021/jm00223a017. [DOI] [PubMed] [Google Scholar]
- 12.Bodor N, Buchwald P. Recent advances in the brain targeting of neuropharmaceuticals by chemical delivery systems. Advanced Drug Delivery Reviews. 1999;36:229–254. doi: 10.1016/s0169-409x(98)00090-8. [DOI] [PubMed] [Google Scholar]
- 13.Khan F, Campbell A, Hoyt B, Herdman C, Thangavelu S, Gordon R. Development of an in vitro assay for the conversion of Pro-2-PAM to 2-PAM; CBDS&T Conference; Dallas, TX. 2009. p. 282. #T066. [Google Scholar]
- 14.Clement JG. HI-6: reactivation of central and peripheral acetylcholinesterase following inhibition by soman, sarin and tabun in vivo in the rat. Biochem Pharmacol. 1982;31(7):1283–1287. doi: 10.1016/0006-2952(82)90017-x. [DOI] [PubMed] [Google Scholar]
- 15.de Jong LP, van Dijk C, Berhitoe D, Benschop HP. Hydrolysis and binding of a toxic stereoisomer of soman in plasma and tissue homogenates from rat, guinea pig and marmoset, and in human plasma. Biochem Pharmacol. 1993;46(8):1413–1419. doi: 10.1016/0006-2952(93)90106-7. [DOI] [PubMed] [Google Scholar]
- 16.Maxwell DM, Brecht KM, Lenz DE, O'Neill BL. Effect of carboxylesterase inhibition on carbamate protection against soman toxicity. J Pharmacol Exp Ther. 1988;246(3):986–991. [PubMed] [Google Scholar]
- 17.Tetz LM, Rezk PE, Ratcliffe RH, Gordon RK, Steele KE, Nambiar MP. Development of a rat pilocarpine model of seizure/status epilepticus that mimics chemical warfare nerve agent exposure. Toxicol Ind Health. 2006;33(6):255–266. doi: 10.1191/0748233706th268oa. [DOI] [PubMed] [Google Scholar]
- 18.Wolthuis OL, Philippens IH, Vanwersch RA. Side effects of therapeutic drugs against organophosphate poisoning. Neurotoxicol Teratol. 1989;11(3):221–225. doi: 10.1016/0892-0362(89)90062-7. [DOI] [PubMed] [Google Scholar]
- 19.Feaster SR, Gordon RK, Doctor BP. Assay for detecting, measuring and monitoring the activities and concentrations of proteins and methods of use thereof. U.S. Patent 6,746,850. 2004
- 20.Haigh JR, Lefkowitz LJ, Capacio BR, Doctor BP, Gordon RK. Advantages of the WRAIR whole blood cholinesterase assay: Comparative anaylsis to the micro-Ellman, Test-mate ChE, and Michel (Delta pH) assays. Chem Biol Interact. 2008;175(1–3):417–420. doi: 10.1016/j.cbi.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 21.Ecobichon DJ, editor. The Basis of Toxicity Testing. first edition. Ann Arbor: CRC Press; 1992. [Google Scholar]
- 22.Clarkson ED, Schulz SM, Railer RF, Sigler MA, Logan TP, Baskin SI. Cutaneous exposure to GD and VX: Timing of antidotes; The Toxicologist: 45th Annual Meeting and ToxExpo; 2006. p. 17. Abstract #81. [Google Scholar]
- 23.Kant GJ, Bauman RA, Feaster SR, Anderson SM, Saviolakis GA, Garcia GE. The combined effects of pyridostigmine and chronic stress on brain cortical and blood acetylcholinesterase, corticosterone, prolactin and alternation performance in rats. Pharmacol Biochem Behav. 2001;70(2–3):209–218. doi: 10.1016/s0091-3057(01)00596-2. [DOI] [PubMed] [Google Scholar]
- 24.Thiermann H, Kehe K, Steinritz D, Mikler J, Hill I, Zilker T, Eyer P, Worek F. Red blood cell acetylcholinesterase and plasma butyrylcholinesterase status: important indicators for the treatment of patients poisoned by organophosphorous compounds. Arh Hig Rada Toksikol. 2007;58(3):359–366. doi: 10.2478/v10004-007-0030-6. [DOI] [PubMed] [Google Scholar]
- 25.Miyaki K, Nishiwaki Y, Maekawa K, Ogawa Y, Asukai N, Yoshimura K, Etoh N, Matsumoto, Kikuchi Y, Kumagai N, Omae K. Effects of sarin on the nervous system of subway workers seven years after the Tokyo subway sarin attack. J Occup Health. 2005;47(2005):299–304. doi: 10.1539/joh.47.299. [DOI] [PubMed] [Google Scholar]