Abstract
There is an expanding body of work characterizing dopaminergic modulation of synaptic plasticity in the hippocampus CA1 region, an area known to be involved in learning and memory. However, in vitro studies to date have focused almost exclusively on the proximal and distal apical dendritic layers (strata radiatum and lacunosum moleculare, respectively). In this report we establish that dopaminergic activity can enhance long-term potentiation (LTP) in the basal dendritic layer (stratum oriens) of CA1 in the rat hippocampal slice preparation. Application of the D1/5 agonist SKF38393 (20μM) significantly increased the magnitude of basal LTP of the fEPSP response following high-frequency stimulation of the Schaffer collateral/commissural inputs in the stratum oriens layer. In addition, endogenous dopamine activity facilitated by the presence of cocaine (6μM) was also capable of enhancing the magnitude of basal LTP. Prior application of the D1/5 antagonist SKF83566 (2μM) prevented this effect of cocaine, indicating that endogenously released dopamine was exerting its LTP-enhancing effect in stratum oriens via activation of D1/5 receptors. This final result stands in contrast with the previously characterized effects of cocaine on apical LTP in the stratum radiatum, which instead have been shown to require D3 receptor activation. These observations demonstrate that dopaminergic mechanisms resulting in the enhancement of hippocampal LTP are lamina specific at Schaffer collateral/commissural synapses in the CA1 region.
Keywords: synaptic plasticity, stratum oriens, D1 receptor, SKF38393
INTRODUCTION
The hippocampus has been identified as a brain region involved with reinstatement of drug-seeking behavior in rats previously allowed to self-administer cocaine (Vorel et al., 2001). As a preclinical model, rodent self-administration is argued to have relevance for investigating the relapse of drug-taking in human addiction to drugs of abuse (Epstein et al., 2006). More specifically, the dorsal hippocampus is required for context-induced reinstatement of cocaine-seeking (Fuchs et al., 2005) and the ventral hippocampus is known to mediate both cue- and drug-primed reinstatement behavior (Rogers and See, 2007; Sun and Rebec, 2003). These findings emphasize the relevance of investigating the actions of cocaine in the hippocampus, which, as a brain structure known to be involved with learning and memory, may therefore be a key site of interest for investigating the “memories of addiction” as they may relate to the relapse of drug-taking behavior.
Prior studies have demonstrated that physiologically relevant concentrations of cocaine can increase the magnitude of long-term potentiation (LTP) in the CA1 region of rat hippocampal slices (Thompson et al., 2005). As LTP is thought to be one potential mechanism for information processing and storage in the brain that contributes to experience-dependent modification of neural circuitry (Malenka and Bear, 2004), cocaine-mediated enhancement of LTP may be an important component of the neuroadaptations that occur following drug exposure (Thompson et al., 2004), in addition to direct cocaine-induced potentiation itself (Ungless et al., 2001). Underlying its enhancement of LTP is cocaine’s action as an inhibitor of the dopamine transporter (DAT) and a subsequent increase in the activity of endogenous dopamine (Thompson et al., 2005). This effect of cocaine is mediated by the D3 subtype of dopamine receptor (Swant and Wagner, 2006). Interestingly, D3 receptor activation decreased evoked IPSCs and enhanced LTP specifically at apical synapses in the stratum radiatum layer, and no such effects were observed at basal synapses in the stratum oriens layer of CA1 (Swant et al., 2008).
This last observation, along with the known ability of D1/5 agonists (Otmakhova and Lisman, 1996) and dopamine transporter inhibitors (Thompson et al., 2005) to enhance LTP in the stratum radiatum, prompted us to further investigate the actions of cocaine and dopaminergic modulation of LTP in the basal dendritic compartment of CA1. Additionally, a recent report has described the presence of dopaminergic fibers in the stratum oriens (Kwon et al., 2008), further suggesting that LTP at basal dendrites may likewise be influenced by endogenously released dopamine.
METHODS
Hippocampal slices were prepared from male Sprague-Dawley rats (40–90 days old) as previously described (Stramiello and Wagner, 2008). A bipolar stimulating electrode (Kopf Instruments) was placed on the CA3-side of the CA1 region in the stratum oriens, and a 1.0 MΩ tungsten recording microelectrode (World Precision Instruments) was then positioned in the same layer in CA1. Data were digitized at 10 kHz, low-pass filtered at 1 kHz, and analyzed with pCLAMP 9.2 software (Axon Instruments). The initial slope of the population fEPSP was measured by fitting a straight line to a 1 msec window immediately following the fiber volley. A stimulus-response curve was obtained at the beginning of each experiment, with stimulus pulses consisting of a single square wave of 270 μs duration delivered at 40–160 μA. The stimulation intensity was adjusted to obtain a field EPSP of approximately 1mV in amplitude to begin baseline recording, and fEPSPs were elicited by stimulation of the Schaffer collateral-commissural pathway in stratum oriens once every 60 s (.0167 Hz) for the duration of the experiment. Synaptic responses were normalized by dividing all slopes by the average of the 5 fEPSP slopes obtained from the 5 min prior to tetanization. The tetanization protocol used to induce LTP in all experiments was a standard HFS protocol consisting of 3 trains of 100 Hz/1s administered at 20 s intertrain intervals. LTP measurements were calculated by averaging fEPSP slope values from 26–30 min following HFS administration. Slices exhibiting LTP values greater or less than 2 times the standard deviation of the mean of the respective test groups were discarded from the analysis as outliers (2 of 56 slices). A one-way ANOVA followed by Dunnet’s post hoc test was used to evaluate the group data reported in Fig. 2B. In reporting our results, n-values indicate first the number of slices, and then the number of animals. Drugs were bath-applied for at least 30 min prior to tetanus, and remained present for the duration of recording. None of the applied compounds had significant effects on the baseline fEPSP response during the wash-in period (range 0–7% change/30 min). (±)-1-Phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol hydrochloride (SKF38393) and 8-Bromo-2,3,4,5-tetrahydro-3-methyl-5-phenyl-1H-3-benzazepin-7-ol hydrobromide (SKF83566) were obtained from Tocris, and cocaine hydrochloride was obtained from NIDA (RTI).
Figure 2.
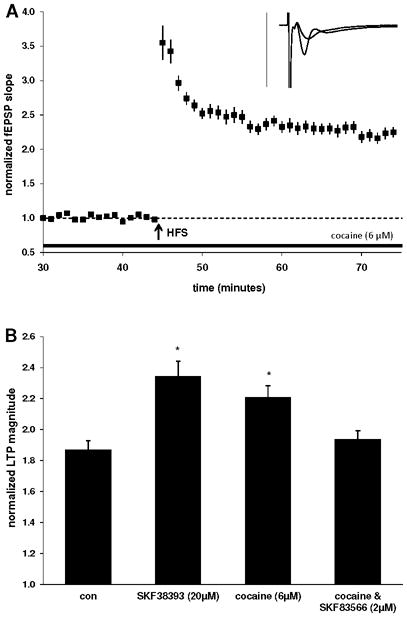
Cocaine enhances basal LTP via D1/5 receptor activation. A) Cocaine (6 μM) is also capable of significantly enhancing LTP in the stratum oriens. Insets are 50 ms sweeps averaged from all experiments illustrating the mean fEPSP 1–5 min prior to and 26–30 min post-tetanus (vertical scale bar is 3 mV). B) LTP magnitude in slices treated with either SKF38393 or cocaine was significantly enhanced when compared to the control group (*, p<0.05, one-way ANOVA/Dunnett’s), while coapplication of D1/5 antagonist SKF83566 (2 μM) was able to prevent cocaine-mediated LTP enhancement, resulting in LTP which was similar in magnitude to the control level. Bars represent LTP measured at 26–30 minutes post-HFS.
RESULTS/DISCUSSION
Afferent input to the hippocampus CA1 region arrives via two major pathways. One, the temporoammonic pathway, arises in layer III of the entorhinal cortex and synapses directly on distal apical dendrites of stratum lacunosum moleculare. The other pathway, consisting of Schaffer collateral/commissural fibers from the CA3 region, synapses on both apical dendrites in stratum radiatum and basal dendrites in stratum oriens. Several reports from the last twenty years have demonstrated that exogenously applied dopaminergic agonists and antagonists can modulate the magnitude and/or persistence of LTP in the stratum radiatum layer of the CA1 region of the hippocampus (Frey et al., 1990; Frey et al., 1991; Huang and Kandel, 1995; Otmakhova and Lisman, 1996; Swanson-Park et al., 1999; Swant and Wagner, 2006), and endogenously released dopamine is thought to serve an important role for novelty detection and long-term memory formation (Lisman and Grace, 2005). However, in contrast to this apical LTP, the study of dopaminergic modulation of basal LTP within stratum oriens synapses of CA1 has received minimal attention.
Compared to apical LTP in stratum radiatum, basal LTP evoked in stratum oriens has previously been characterized in vivo as exhibiting a lower threshold and higher amplitude (Kaibara and Leung, 1993), suggesting that there may be differences in how neuromodulators influence synaptic plasticity processes in these lamina. Indeed, recent evidence indicates that cholinergic tone from the basal forebrain hippocampal input can selectively enhance LTP in the stratum oriens (Doralp and Leung, 2008; Ovsepian, 2008). The physiological relevance of the differential distribution of LTP across basal and apical dendritic compartments is not known, but the behavioral state of the animal can alter the propensity for LTP recorded in the stratum oriens (Leung et al., 2003), thus the ratio of basal/apical potentiation may be a salient factor in the processing of information through the CA1 region of the hippocampus.
An exception to the relative lack of information concerning dopaminergic modulation of LTP at basal synapses in the CA1 is our recent report that D3 activation has no effect on LTP in the stratum oriens, despite its efficacy for enhancing LTP at apical dendritic synapses of the stratum radiatum (Swant et al., 2008). As evidence indicates that dopamine is present at a higher concentration in stratum oriens relative to the stratum radiatum (Gasbarri et al., 1994; Kwon et al., 2008), it was expected that basal LTP would be subject to dopaminergic modulation. In the current report we have added to the characterization of dopamine actions in the CA1 to now also include effects of D1/5 receptor activation and monoamine transport blockade on basal LTP in the stratum oriens. LTP measured 25–30 minutes following high frequency stimulation (100Hz/1 sec *3) in the control group measured 87 ± 6% (n = 12, 5; Fig. 1A). We found that both the D1/5 agonist SKF38393 (20μM; 134 ± 10%; n = 16, 7; p < 0.01; Fig. 1B) and the indirect dopamine agonist cocaine (6μM; 121 ± 8%; n = 16, 8; p < 0.05; Fig. 2A) were each capable of enhancing basal LTP. Additionally, the effects of cocaine were blocked by prior application of the D1/5 antagonist SKF83566 (2μM; 94 ± 6%; n = 10, 5; Fig. 2B), indicating that cocaine exerted its LTP-enhancing effect via D1/5 receptors in stratum oriens.
Figure 1.
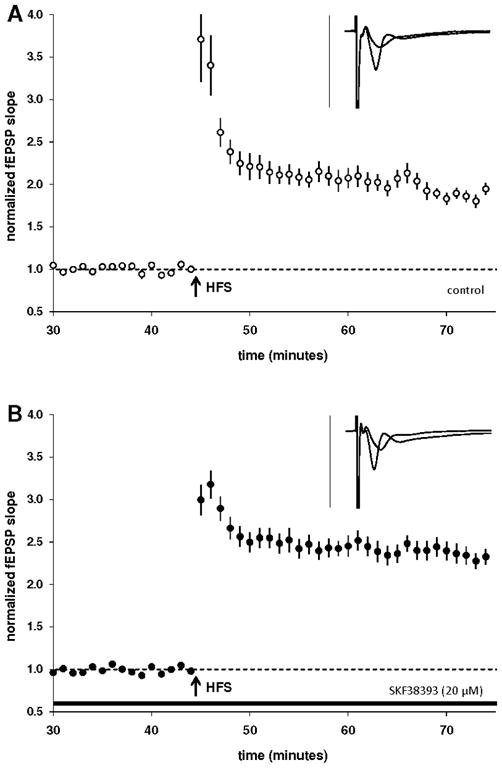
Comparison of CA1 basal LTP in controls with a group of slices treated with SKF38393 (20 μM). Insets are 50 ms sweeps averaged from all experiments illustrating the mean fEPSP 1–5 min prior to and 26–30 min post-HFS (vertical scale bar is 3 mV). A) Summary plot of normalized fEPSP slope measurements evoked and recorded in the stratum radiatum layer of the CA1 region. B) SKF38393 (20 μM) is capable of significantly enhancing LTP in the stratum oriens.
Of the five known subtypes of dopamine receptors, D2 receptors appear to be expressed at very low levels in the CA1 (Khan et al., 1998) and D4 receptors have been shown to inhibit NMDA receptor activity in the CA1 (Kotecha et al., 2002). Consequently, our work to date concerning the dopaminergic enhancement of LTP has focused on receptors of the D1/5 and D3 subtypes. These studies have demonstrated that activation of either dopamine receptor subtype (as well as DAT blockade) can enhance apical LTP (Table 1). As these receptors are also present in the stratum oriens, we sought to determine whether DA receptor activation would also be effective in enhancing LTP at the Schaffer collateral synapses of the basal dendrites. The data presented in Fig. 1 illustrates the capacity of an exogenously applied D1/5 agonist to enhance basal LTP, much as we have observed for apical LTP in the stratum radiatum (Stramiello and Wagner, 2008).
TABLE I.
Dopaminergic enhancement of LTP at Schaffer collateral/commissural inputs to CA1
| D1/5 | D3 | cocaine | references | |
|---|---|---|---|---|
| s. oriens | * | — a | * | a) Swant et al. (2008) |
| s. radiatum | *b | *c | *d |
b) Otmakhova & Lisman (1996) c) Swant & Wagner (2006) d) Thompson et al. (2005) |
significance relative to control LTP measured in the same layer.
With respect to the role of endogenously released dopamine, we have previously shown that cocaine (5–10 μM) is capable of enhancing apical LTP in the stratum radiatum, an effect that was blocked by coapplication of the D2-like antagonist eticlopride (Thompson et al., 2005). Further investigation with the DAT-specific compound GBR12935 (1 μM) showed that this effect in the stratum radiatum is dependent upon activation of the D3 receptors (Swant and Wagner, 2006), which likely enhances apical LTP via an increase in GABAA receptor endocytosis (Swant et al., 2008). In contrast, D1/5 receptor activation enhances apical LTP following enhancement of NR2B-containing NMDA receptor activity (Stramiello and Wagner, 2008). In the former scenario, a D3-mediated decrease in protein kinase A activity occurs whereas in the latter, a D1/5-mediated increase in protein kinase A activity occurs-the net effect of either resulting in a facilitation of LTP. As it is known that dopamine has a relatively high affinity for D3 receptors in comparison to D1/5 receptors (Missale et al., 1998; Sokoloff et al., 1992), and that endogenous DA transmitter is scarce in the stratum radiatum, it is reasonable that DAT blockers such as cocaine and GBR12935 exert their effects via D3 receptors in this layer where action at a distance is required. The relative abundance and proximity of DA in stratum oriens (Gasbarri et al., 1994; Kwon et al., 2008) would be one explanation for why cocaine can exert its neuromodulatory effect on basal LTP via D1/5 receptor activation in this layer (Fig. 2), despite the lower affinity of dopamine for these receptors.
In this study, we have demonstrated that either direct D1/5 receptor activation or increased activity of endogenously released dopamine is capable of enhancing basal LTP in the stratum oriens of CA1 pyramidal neurons. A summary of findings to date (Table 1) illustrates that the enhancement of LTP at Schaffer collateral synapses via endogenous dopamine release in the hippocampus can exhibit laminar specificity in the CA1 region with respect to the dopamine receptor subtype being activated. Alterations in the balance of synaptic plasticity between apical and basal dendritic compartments of the CA1 region by dopaminergic systems may underlie both physiological (learning & memory) activity and pathophysiological (e.g. schizophrenia, addiction) disease states related to hippocampal function.
Acknowledgments
J.J.W. was funded by National Institute on Drug Abuse (DA 16302).
References
- Doralp S, Leung LS. Cholinergic modulation of hippocampal CA1 basal-dendritic long-term potentiation. Neurobiol Learn Mem. 2008;90(2):382–388. doi: 10.1016/j.nlm.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Epstein D, Preston K, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189(1):1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Matthies H, Reymann K, Matthies H. The effect of dopaminergic D1 receptor blockade during tetanization on the expression of long-term potentiation in the rat CA1 region in vitro. Neurosci Lett. 1991;129(1):111–114. doi: 10.1016/0304-3940(91)90732-9. [DOI] [PubMed] [Google Scholar]
- Frey U, Schroeder H, Matthies H. Dopaminergic antagonists prevent long-term maintenance of posttetanic LTP in the CA1 region of rat hippocampal slices. Brain Res. 1990;522(1):69–75. doi: 10.1016/0006-8993(90)91578-5. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The Role of the Dorsomedial Prefrontal Cortex, Basolateral Amygdala, and Dorsal Hippocampus in Contextual Reinstatement of Cocaine Seeking in Rats. Neuropsychopharmacology. 2005;30(2):296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Verney C, Innocenzi R, Campana E, Pacitti C. Mesolimbic dopaminergic neurons innervating the hippocampal formation in the rat: a combined retrograde tracing and immunohistochemical study. Brain Res. 1994;668(1–2):71–79. doi: 10.1016/0006-8993(94)90512-6. [DOI] [PubMed] [Google Scholar]
- Huang Y, Kandel E. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proc Natl Acad Sci U S A. 1995;92(7):2446–2450. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibara T, Leung LS. Basal versus apical dendritic long-term potentiation of commissural afferents to hippocampal CA1: a current-source density study. J Neurosci. 1993;13(6):2391–2404. doi: 10.1523/JNEUROSCI.13-06-02391.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan ZU, Gutierrez A, Martin R, Penafiel A, Rivera A, Calle ADL. Differential regional and cellular distribution of dopamine D2-like receptors: An immunocytochemical study of subtype-specific antibodies in rat and human brain. J Comp Neurol. 1998;402(3):353–371. doi: 10.1002/(sici)1096-9861(19981221)402:3<353::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Kotecha S, Oak J, Jackson M, Perez Y, Orser B, Van_Tol H, MacDonald J. A D2 class dopamine receptor transactivates a receptor tyrosine kinase to inhibit NMDA receptor transmission. Neuron. 2002;35(6):1111–1122. doi: 10.1016/s0896-6273(02)00859-0. [DOI] [PubMed] [Google Scholar]
- Kwon OB, Paredes D, Gonzalez CM, Neddens, Hernandez L, Vullhorst D, Buonanno A. Neuregulin-1 regulates LTP at CA1 hippocampal synapses through activation of dopamine D4 receptors. Proceedings of the National Academy of Sciences. 2008;105(40):15587–15592. doi: 10.1073/pnas.0805722105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung LS, Shen B, Rajakumar N, Ma J. Cholinergic Activity Enhances Hippocampal Long-Term Potentiation in CA1 during Walking in Rats. J Neurosci. 2003;23(28):9297–9304. doi: 10.1523/JNEUROSCI.23-28-09297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Grace A. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46(5):703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: An Embarrassment of Riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine Receptors: From Structure to Function. Physiol Rev. 1998;78(1):189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Otmakhova N, Lisman J. D1/D5 Dopamine Receptor Activation Increases the Magnitude of Early Long-Term Potentiation at CA1 Hippocampal Synapses. J Neurosci. 1996;16(23):7478–7486. doi: 10.1523/JNEUROSCI.16-23-07478.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsepian SV. Differential cholinergic modulation of synaptic encoding and gain control mechanisms in rat hippocampus. Neurosci Res. 2008;61(1):92–98. doi: 10.1016/j.neures.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Rogers JL, See RE. Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats. Neurobiol Learn Mem. 2007;87(4):688–692. doi: 10.1016/j.nlm.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P, Martres M-P, Giros B, Bouthenet M-L, Schwartz J-C. The third dopamine receptor (D3) as a novel target for antipsychotics. Biochem Pharmacol. 1992;43(4):659–666. doi: 10.1016/0006-2952(92)90227-a. [DOI] [PubMed] [Google Scholar]
- Stramiello M, Wagner JJ. D1/5 receptor-mediated enhancement of LTP requires PKA, Src family kinases, and NR2B-containing NMDARs. Neuropharmacology. 2008;55(5):871–877. doi: 10.1016/j.neuropharm.2008.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Lidocaine Inactivation of Ventral Subiculum Attenuates Cocaine-Seeking Behavior in Rats. J Neurosci. 2003;23(32):10258–10264. doi: 10.1523/JNEUROSCI.23-32-10258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson-Park J, Coussens C, Mason-Parker S, Raymond C, Hargreaves E, Draqunow M, Cohen A, Abraham W. A double dissociation within the hippocampus of dopamine D1/D5 receptor and beta-adrenergic receptor contributions to the persistence of long-term potentiation. Neuroscience. 1999;92(2):485–497. doi: 10.1016/s0306-4522(99)00010-x. [DOI] [PubMed] [Google Scholar]
- Swant J, Stramiello M, Wagner JJ. Postsynaptic dopamine D3 receptor modulation of evoked IPSCs via GABAA receptor endocytosis in rat hippocampus. Hippocampus. 2008;18(5):492–502. doi: 10.1002/hipo.20408. [DOI] [PubMed] [Google Scholar]
- Swant J, Wagner J. Dopamine transporter blockade increases LTP in the CA1 region of the rat hippocampus via activation of the D3 dopamine receptor. Learn Mem. 2006;13(2):161–167. doi: 10.1101/lm.63806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AM, Swant J, Gosnell BA, Wagner JJ. Modulation of long-term potentiation in the rat hippocampus following cocaine self-administration. Neuroscience. 2004;127(1):177–185. doi: 10.1016/j.neuroscience.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Swant J, Wagner JJ. Cocaine-induced modulation of long-term potentiation in the CA1 region of rat hippocampus. Neuropharmacology. 2005;49(2):185–194. doi: 10.1016/j.neuropharm.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411(6837):583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to Cocaine-Seeking After Hippocampal Theta Burst Stimulation. Science. 2001;292(5519):1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]


