Abstract
Dystrophin and the α-dystrobrevins bind directly to the adaptor protein syntrophin to form membrane-associated scaffolds. At the blood-brain barrier, α-syntrophin co-localizes with dystrophin and the α-dystrobrevins in perivascular glial endfeet and is required for localization of the water channel aquaporin-4. We have previously shown that localization of the scaffolding proteins γ2-syntrophin, α-dystrobrevin-2, and dystrophin to glial endfeet is also dependent upon the presence of α-syntrophin. In the present study, we show that the expression levels of α-syntrophin, γ2-syntrophin, and dystrophin at the blood-brain barrier are reduced in α-dystrobrevin-null mice. This is the first demonstration that assembly of an astroglial protein scaffold containing syntrophin and dystrophin in perivascular astrocytes is dependent upon the presence of α-dystrobrevin.
Keywords: glia, dystrophin, syntrophin, perivascular astrocyte
INTRODUCTION
Since the discovery of dystrophin as the product of the Duchenne muscular dystrophy gene, numerous proteins have been identified which associate with dystrophin to form membrane-based scaffolds [reviewed in refs. 1 and 2]. These dystrophin-associated scaffolds provide structural integrity to the cell by connecting the extracellular matrix with the cytoskeleton and create intracellular docking points for regulatory proteins. The dystrobrevins are a family of proteins that interact directly with dystrophin and syntrophin adaptor proteins providing a link to a network of signaling proteins that regulate key cellular processes such as water transport and nitric-oxide-mediated events [3–5] Two separate dystrobrevin genes named α- and β-dystrobrevin have been identified, both of which yield multiple transcripts [6–10]. α- and β-dystrobrevin are both expressed in brain but have distinct locations [8].
The α-dystrobrevin isoforms (currently six have been identified) have been characterized most thoroughly in muscle where several are required for proper formation of the neuromuscular synapse [11]. However, the dystrobrevins are also present in the brain where they are present in both neurons and glia[10–16]. Immunofluorescence studies show αDb at glial endfeet and endothelial cells at the blood-brain barrier [13,15], whereas αDb-1 and -2 localize specifically to glial endfeet [12,16]. We have previously shown that the localization of αDb-2 to glial endfeet at the blood brain barrier is specifically dependent upon the presence of α-syntrophin [16]. Using the α-dystrobrevin-null mouse, we now show that the expression levels of α-syntrophin, γ2-syntrophin, and dystrophin in the vasculature are dependent on α-dystrobrevin. These data indicate that α-syntrophin and α-dystrobrevin are mutually dependent on each other and that α-dystrobrevin plays a critical role in assembly of the DAPC at the blood-brain barrier.
METHODS
The syntrophin antibodies used in this study were mAb1351 (Affinity Bioreagents; MA1-745), which recognizes α-, β1-, and β2-syntrophin , and syntrophin-isoform specific affinity-purified rabbit polyclonal antibodies to α-syntrophin (Syn17; [17]) and γ2-syntrophin (Ab 212; [16,18,19]). The dystrophin (MANDRA-mouse monoclonal) was purchased from Sigma (St. Louis, MO) and the α-dystrobrevin antibodies (Ab 638, Ab αDB2-rabbit polyclonals) have been characterized previously [20,21].
For immunofluorescence, animals were euthanized in accordance with the NIH guide for the care of laboratory animals. After light sedation with CO2 gas, 6-week and 3-month-old male C57Bl6 and α-dystrobrevin-null mice [22] and corresponding wild type littermates were quickly euthanized and brains were dissected. Cerebella were removed, bisected and placed in PBS containing 4% paraformaldehyde/10% sucrose for 12 min on ice. Cerebellar tissue was then transferred to PBS containing 25% sucrose and placed at 4°C overnight. Tissue was embedded in OCT and frozen in isopentane cooled with liquid nitrogen. Twenty µm thick cryosections were adhered to superfrost plus slides (VWR) and stored at −80°C. Immunoflurescence and confocal images were collected under identical conditions, as described previously [15].
RESULTS
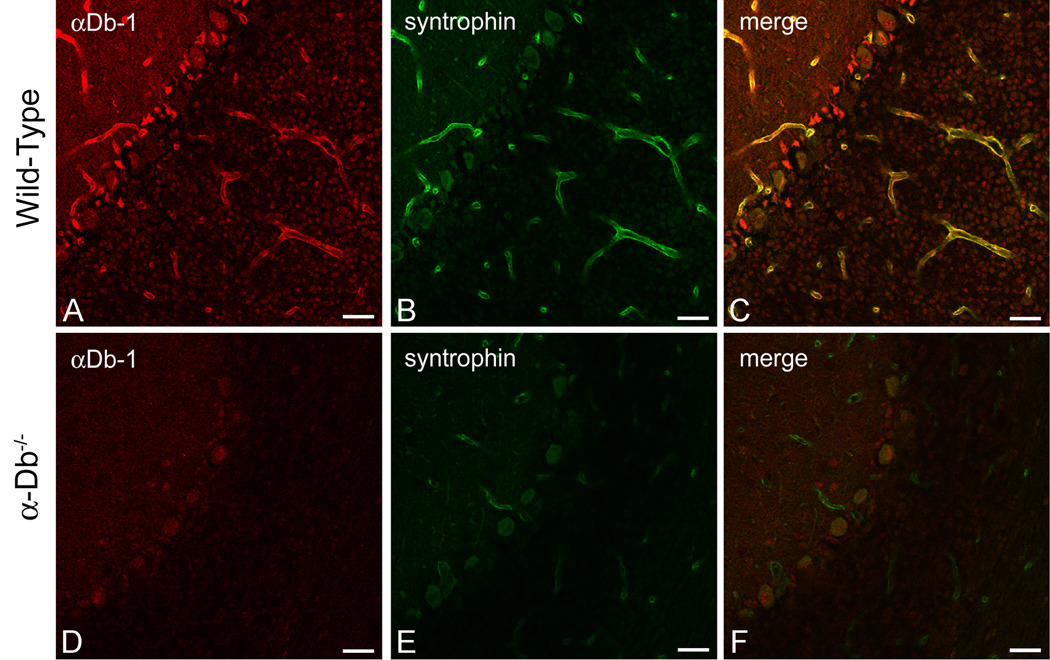
The assembly of the dystrophin-associated protein complex at the blood-brain barrier is dependent upon the presence of α-syntrophin [16]. Specifically, α-syntrophin is required for the localization of γ2-syntrophin, α-dystrobrevin-2, and dystrophin to the perivascular astrocyte endfoot membrane. Since localization of αDB-2, but not αDB-1, requires α-syntrophin, we questioned how the DAPC would be affected by the loss of α-dystrobrevin isoforms. We performed immunolocalization for α-syntrophin on brains from α-dystrobrevin null animals [Figure 1D–F; 22] and wild-type littermates (Figure 1A–C). The merged image in Figure 1C indicates that, in wild type mice, α-syntrophin co-localizes with α-dystrobrevin 1 in perivascular astrocyte endfeet, as reported previously. Furthermore, we observed a striking decrease in the level of α-syntrophin immunofluorescence in the α-dystrobrevin-null mice (compare 1E to 1B).
Figure 1. α-Syntrophin is Reduced at the Blood-Brain Barrier in the Absence of α-Dystrobrevin.
We performed double-immunolabeling for α-dystrobrevin-1 (red, A, D) and syntrophin (green, B, E) in wild type (A–C) and α-dystrobrevin-null (D–F) adult mouse cerebellum. α-Syntrophin and α-dystrobrevin-1 co-localize in perivascular astrocyte endfeet (see merged image, 1C). We observed a reduction in fluorescence intensity for α-syntrophin in the brains of α-dystrobrevin-null mice (E), indicating that localization of α-syntrophin to perivascular astrocyte endfeet is dependent on α-dystrobrevin. The pan-syntrophin antibody (mAb 1351; B, E) or an α-syntrophin-specific rabbit polyclonal antibody (syn17, data not shown) was used to recognize α-syntrophin, and an αDb-1 specific rabbit polyclonal [26] was used to localize αDb-1. As expected, αDb-1 is absent in the α-dystrobrevin-null cerebellum (D). C and F are merged images of [A, B] and [D, E], respectively. Scale bar, 25 µm.
We have previously shown that the localization of γ2-syntrophin to perivascular endfeet is dependent on α-syntrophin [16]. Since we find that expression of α-syntrophin at glial endfeet is dependent on the presence of α-dystrobrevin, we determined whether the localization of γ2-syntrophin was disrupted by the loss of α-dystrobrevin. We compared γ2-syntrophin immunolocalization (green, Fig 2B, E) in wild-type (Figure 2A–C) and α-dystrobrevin-null (Figure 2D–F) cerebellum. γ2-Syntrophin immunofluorescence was reduced in the surrounding cerebellar vasculature in the absence of α-dystrobrevin (compare 2E to 2B). As in the α-syntrophin null animals [16], γ2-syntrophin expression is unaffected in neurons (see arrows, Figure 2). Taken together, these data indicate that expression of γ2-syntrophin in non-neuronal cells is dependent on both α-syntrophin and α-dystrobrevin.
Figure 2. γ2-Syntrophin is Reduced at the Blood-Brain Barrier in the Absence of α-Dystrobrevin.
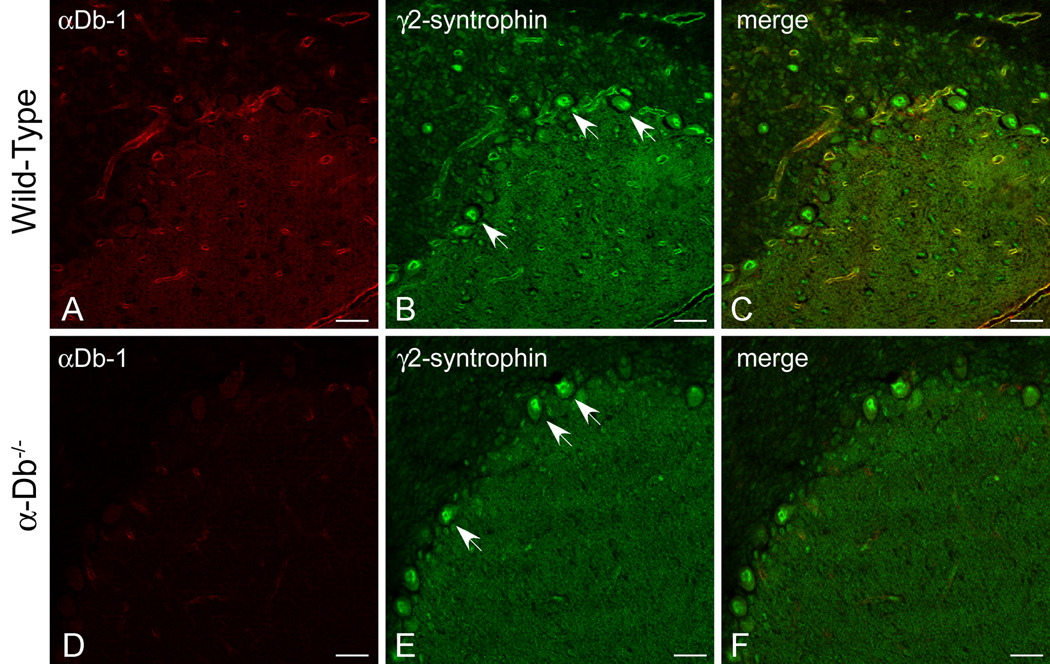
We performed double-immunolabeling for α-dystrobrevin-1 (red, A, D) and γ2-syntrophin (green, B, E) in wild type (A–C) and α-dystrobrevin-null (D–F) adult mouse cerebellum. As shown previously [16], γ2-syntrophin localizes to Purkinje neurons and to astrocyte endfeet in the adult cerebellum (B). In the absence of α-dystrobrevin (E), γ2-syntrophin is reduced in astrocyte endfeet but unaffected in Purkinje neurons. As expected, αDb-1 labeling is lost in the α-dystrobrevin-null animals (D). C and F are merged images of [A, B] and [D, E], respectively. Scale bar, 25 µm.
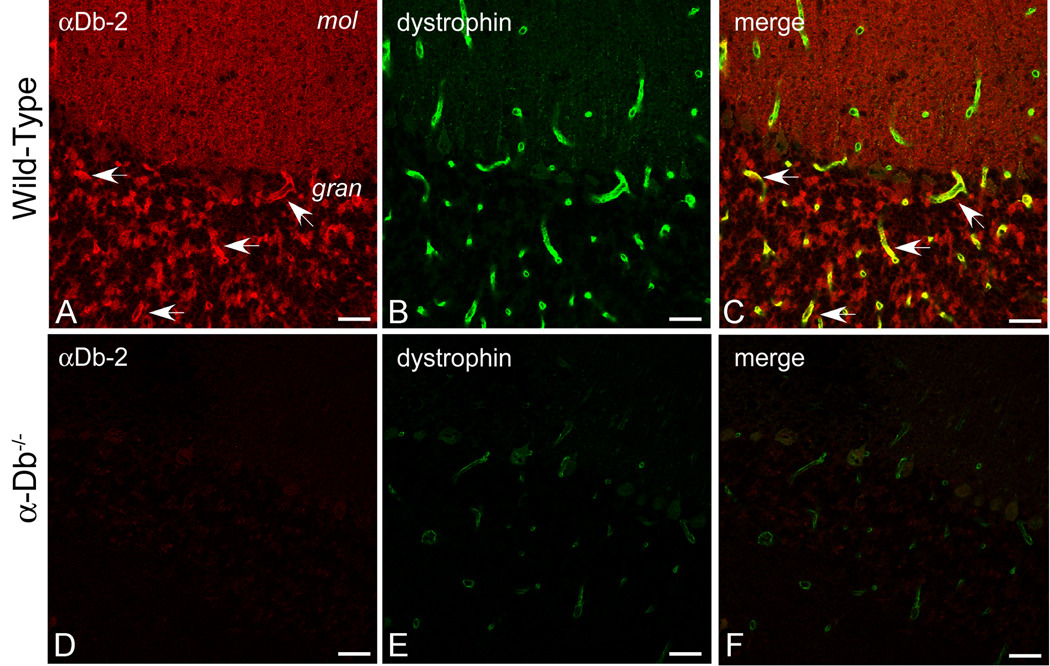
Dystrophin colocalizes with α-syntrophin in both the granular layer and the molecular layer of the cerebellum. The α-Db-1 and -2 isoforms exhibit discrete distributions in the vasculature of these layers but both colocalize with α-sybtrophin. Thus, dystrophin and a form of α-dystrobrevin are colocalized in the vasculature throughout the cerebellum. Dystrophin localization at the blood-brain barrier is contingent on the presence of α-syntrophin [16]. Since α-syntrophin localization is disrupted in the α-dystrobrevin-null mice, the localization of dystrophin might also be affected. We assessed immunofluorescence for dystrophin (green, Fig 3B, E) and αDb-2 (red, Fig 3A, D) in cerebellum from wild type (Fig 3A–C) and α-dystrobrevin-null mice (Fig 3D–F). As shown previously, αDb-2 is present in the granular layer of cerebellar cortex (Fig 3A, see arrows). As expected, the α-dystrobrevin-null cerebellum is negative for α-Db2 (Fig 3D). Dystrophin is associated with the blood-brain barrier throughout the cerebellar cortex (Fig 3B), and its expression levels are substantially reduced in the α-dystrobrevin-null mouse (compare 3E to 3B).
Figure 3. Dystrophin is Reduced at the Blood-Brain Barrier in the Absence of α-Dystrobrevin.
We performed double-immunolabeling for α-dystrobrevin-2 (red, A, D) and dystrophin (green, B, E) in wild type (A–C) and α-dystrobrevin-null (D–F) adult mouse cerebellum. αDB-2 is restricted to the blood-brain barrier in the granular layer of cerebellum (see arrows, A), whereas dystrophin localizes to the blood-brain barrier throughout the cerebellar cortex (B). In α-dystrobrevin null cerebellum (D–F), labeling for αDB-2 is absent (D), and immunolocalization of dystrophin is reduced (E). Arrows in C denote co-localization of αDB-2 and dystrophin in the granular layer of cerebellum. C and F are merged images of [A, B] and [D, E], respectively. mol, molecular layer; gran, granular layer. Scale bar, 25 µm.
DISCUSSION
Taken together with our previous published results [16], the main finding of this work is that α-syntrophin and α-dystrobrevin are mutually dependent upon one another for their expression and/or localization in the cerebrovasculature. Our earlier conclusion [16] that α-syntrophin is the central regulator of the DAPC at endfeet must now be modified to include the α-dystrobrevin/α-syntrophin subcomplex. Whether other syntrophin isoforms are required remains to be determined. Interestingly, co-dependence of α-syntrophin and α-dystrobrevin occurs also in skeletal muscle fibers. In mice lacking α-syntrophin, sarcolemmal staining intensity for αDB-1 and αDB-2 is also decreased [23]. Likewise, in α-dystrobrevin-null mice, levels of α-, β1- and β2-syntrophin were also decreased [11,22].
How might these protein members of the dystrophin complex be mutually dependent on each other for proper localization? One possibility is that they are assembled into a vesicular complex [24,25] prior to transport to glial endfeet. In this capacity, loss of either protein may have effects downstream in the trafficking pathway. Alternatively, α-syntrophin and α-dystrobrevin may act in concert to retain components of the DAPC at the cellular plasma membrane. Future experiments using endothelial-astrocyte co-cultures from normal and α-syntrophin null mice may be informative in revealing the dynamics of DAPC complex trafficking, assembly, and stability at the blood-brain interface.
Loss of either α-syntrophin or α-dystrobrevin results in a reduction of dystrophin from the vasculature. In skeletal muscle, dystrophin is considered the central component of the dystrophin-associated protein complex whose presence is critical for sarcolemmal localization of α-dystrobrevins and certain syntrophin isoforms [11,22,23]. The abence of α-dystrobrevins and syntrophins do not affect sarcolemmal dystrophin levels. Thus, despite some similarities discussed above, assembly and/or maintenance of this dystrophin-associated subcomplex may be regulated differentially in brain and skeletal muscle.
CONCLUSION
We show for the first time that the loss of α-dystrobrevin results in the decreased expression of α-syntrophin, γ2-syntrophin, and dystrophin in astrocytes. Taken together with our previous study [16], these data indicate that α-syntrophin and α-dystrobrevin are both essential for the proper assembly and maintenance of the dystrophin-based scaffold in the cerebrovasculature. Therefore, altered expression of either of these proteins could compromise both the structural and signaling functions of this scaffold.
ACKNOWLEDGEMENTS
We thank Drs. Stephan Kroger and Mahmood Amiry-Moghaddam for advice regarding brain immunofluorescence protocols, Drs Marv Adams and Ruth Westenbroek for assistance with mouse perfusions, Greg Martin for assistance with confocal imaging, Yan Tesch and Kendra Anderson for their help with mouse husbandry and genotyping, and Charles Mahan for preparation of cryosections. We also thank Drs. Mark Grady and Joshua Sanes of Washington University, St Louis, MO, and Harvard University, Cambridge, MA, respectively, for generously providing α-dystrobrevin-null animals.
Sources of Support: NIH grant RO1 NS33145 to SCF, NIH training grant T32 NS007332 to ADB and SSD and NIH training grant T32 EB0001650 to SSD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Banks GB, Fuhrer C, Adams ME, Froehner SC. The postsynaptic submembrane machinery at the neuromuscular junction: requirement for rapsyn and the utrophin/dystrophin-associated complex. J Neurocytol. 2003;32:709–726. doi: 10.1023/B:NEUR.0000020619.24681.2b. [DOI] [PubMed] [Google Scholar]
- 2.Dalkilic I, Kunkel LM. Muscular dystrophies: genes to pathogenesis. Curr Opin Genet Dev. 2003;13:231–238. doi: 10.1016/s0959-437x(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 3.Albrecht DE, Froehner SC. Syntrophins and dystrobrevins: defining the dystrophin scaffold at synapses. Neurosignals. 2002;11:123–129. doi: 10.1159/000065053. [DOI] [PubMed] [Google Scholar]
- 4.Percival JM, Adams ME, Froehner SC. Syntrophin: A Molecular Adapter Conferring a Signaling Role to the Dystrophin-Associated Protein Complex. In: Winder SJ, editor. Molecular Mechanisms of Muscular Dystrophies. Landes Bioscience; 2004. pp. 46–92. [Google Scholar]
- 5.Blake DJ. Dystrobrevin dynamics in muscle-cell signalling: a possible target for therapeutic intervention in Duchenne muscular dystrophy? Neuromuscul Disord. 2002;12 Suppl 1:S110–S117. doi: 10.1016/s0960-8966(02)00091-3. [DOI] [PubMed] [Google Scholar]
- 6.Enigk RE, Maimone MM. Differential expression and developmental regulation of a novel alpha-dystrobrevin isoform in muscle. Gene. 1999;238:479–488. doi: 10.1016/s0378-1119(99)00358-3. [DOI] [PubMed] [Google Scholar]
- 7.Puca AA, Nigro V, Piluso G, Belsito A, Sampaolo S, Quaderi N, et al. Identification and characterization of a novel member of the dystrobrevin gene family. FEBS Lett. 1998;425:7–13. doi: 10.1016/s0014-5793(98)00097-0. [DOI] [PubMed] [Google Scholar]
- 8.Blake DJ, Nawrotzki R, Loh NY, Gorecki DC, Davies KE. beta-dystrobrevin, a member of the dystrophin-related protein family. Proc Natl Acad Sci U S A. 1998;95:241–246. doi: 10.1073/pnas.95.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadoulet-Puccio HM, Khurana TS, Cohen JB, Kunkel LM. Cloning and characterization of the human homologue of a dystrophin related phosphoprotein found at the Torpedo electric organ post-synaptic membrane. Hum Mol Genet. 1996;5:489–496. doi: 10.1093/hmg/5.4.489. [DOI] [PubMed] [Google Scholar]
- 10.Blake DJ, Nawrotzki R, Peters MF, Froehner SC, Davies KE. Isoform diversity of dystrobrevin, the murine 87-kDa postsynaptic protein. J Biol Chem. 1996;271:7802–7810. doi: 10.1074/jbc.271.13.7802. [DOI] [PubMed] [Google Scholar]
- 11.Grady RM, Zhou H, Cunningham JM, Henry MD, Campbell KP, Sanes JR. Maturation and maintenance of the neuromuscular synapse: genetic evidence for roles of the dystrophin--glycoprotein complex. Neuron. 2000;25:279–293. doi: 10.1016/s0896-6273(00)80894-6. [DOI] [PubMed] [Google Scholar]
- 12.Blake DJ, Hawkes R, Benson MA, Beesley PW. Different dystrophin-like complexes are expressed in neurons and glia. J Cell Biol. 1999;147:645–658. doi: 10.1083/jcb.147.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueda H, Baba T, Terada N, Kato Y, Fujii Y, Takayama I, et al. Immunolocalization of dystrobrevin in the astrocytic endfeet and endothelial cells in the rat cerebellum. Neurosci Lett. 2000;283:121–124. doi: 10.1016/s0304-3940(00)00925-3. [DOI] [PubMed] [Google Scholar]
- 14.Moukhles H, Carbonetto S. Dystroglycan contributes to the formation of multiple dystrophin-like complexes in brain. J Neurochem. 2001;78:824–834. doi: 10.1046/j.1471-4159.2001.00466.x. [DOI] [PubMed] [Google Scholar]
- 15.Haenggi T, Soontornmalai A, Schaub MC, Fritschy JM. The role of utrophin and Dp71 for assembly of different dystrophin-associated protein complexes (DPCs) in the choroid plexus and microvasculature of the brain. Neuroscience. 2004;129:403–413. doi: 10.1016/j.neuroscience.2004.06.079. [DOI] [PubMed] [Google Scholar]
- 16.Bragg AD, Amiry-Moghaddam M, Ottersen OP, Adams ME, Froehner SC. Assembly of a perivascular astrocyte protein scaffold at the mammalian blood-brain barrier is dependent on alpha-syntrophin. Glia. 2006;53:879–890. doi: 10.1002/glia.20347. [DOI] [PubMed] [Google Scholar]
- 17.Peters MF, Adams ME, Froehner SC. Differential association of syntrophin pairs with the dystrophin complex. J Cell Biol. 1997;138:81–93. doi: 10.1083/jcb.138.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puwarawuttipanit W, Bragg AD, Frydenlund DS, Mylonakou MN, Nagelhus EA, Peters MF, et al. Differential effect of alpha-syntrophin knockout on aquaporin-4 and Kir4.1 expression in retinal macroglial cells in mice. Neuroscience. 2006;137:165–175. doi: 10.1016/j.neuroscience.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 19.Alessi A, Bragg AD, Percival JM, Yoo J, Albrecht DE, Froehner SC, et al. gamma-Syntrophin scaffolding is spatially and functionally distinct from that of the alpha/beta syntrophins. Exp Cell Res. 2006;312:3084–3095. doi: 10.1016/j.yexcr.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 20.Kramarcy NR, Vidal A, Froehner SC, Sealock R. Association of utrophin and multiple dystrophin short forms with the mammalian M(r) 58,000 dystrophin-associated protein (syntrophin) J Biol Chem. 1994;269:2870–2876. [PubMed] [Google Scholar]
- 21.Peters MF, Sadoulet-Puccio HM, Grady MR, Kramarcy NR, Kunkel LM, Sanes JR, et al. Differential membrane localization and intermolecular associations of alpha-dystrobrevin isoforms in skeletal muscle. J Cell Biol. 1998;142:1269–1278. doi: 10.1083/jcb.142.5.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grady RM, Grange RW, Lau KS, Maimone MM, Nichol MC, Stull JT, et al. Role for alpha-dystrobrevin in the pathogenesis of dystrophin-dependent muscular dystrophies. Nat Cell Biol. 1999;1:215–220. doi: 10.1038/12034. [DOI] [PubMed] [Google Scholar]
- 23.Adams ME, Kramarcy N, Krall SP, Rossi SG, Rotundo RL, Sealock R, et al. Absence of alpha-syntrophin leads to structurally aberrant neuromuscular synapses deficient in utrophin. J Cell Biol. 2000;150:1385–1398. doi: 10.1083/jcb.150.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marchand S, Stetzkowski-Marden F, Cartaud J. Differential targeting of components of the dystrophin complex to the postsynaptic membrane. Eur J Neurosci. 2001;13:221–229. [PubMed] [Google Scholar]
- 25.Ort T, Voronov S, Guo J, Zawalich K, Froehner SC, Zawalich W, et al. Dephosphorylation of beta2-syntrophin and Ca2+/mu-calpain-mediated cleavage of ICA512 upon stimulation of insulin secretion. EMBO J. 2001;20:4013–4023. doi: 10.1093/emboj/20.15.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters MF, O'Brien KF, Sadoulet-Puccio HM, Kunkel LM, Adams ME, Froehner SC. beta-dystrobrevin, a new member of the dystrophin family. Identification, cloning, and protein associations. J Biol Chem. 1997;272:31561–31569. doi: 10.1074/jbc.272.50.31561. [DOI] [PubMed] [Google Scholar]