Abstract
Background
Current measurement tools have difficulty identifying the automatic physiologic processes maintaining continence, and many questions still remain about pelvic floor muscle (PFM) function during automatic events.
Objective
To perform a feasibility study to characterise the displacement, velocity, and acceleration of the PFM and the urethra during a cough.
Design, setting, and participants
A volunteer convenience sample of 23 continent women and 9 women with stress urinary incontinence (SUI) from the general community of San Francisco Bay Area was studied.
Measurements
Methods included perineal ultrasound imaging, motion tracking of the urogenital structures, and digital vaginal examination. Statistical analysis used one-tailedunpaired student t tests, and Welch’s correction was applied when variances were unequal.
Results and limitations
The cough reflex activated the PFM of continent women to compress the urogenital structures towards the pubic symphysis, which was absent in women with SUI. The maximum accelerations that acted on the PFM during a cough were generally more similar than the velocities and displacements. The urethras of women with SUI were exposed to uncontrolled transverse acceleration and were displaced more than twice as far (p = 0.0002), with almost twice the velocity (p = 0.0015) of the urethras of continent women. Caution regarding the generalisability of this study is warranted due to the small number of women in the SUI group and the significant difference in parity between groups.
Conclusions
During a cough, normal PFM function produces timely compression of the pelvic floor and additional external support to the urethra, reducing displacement, velocity, and acceleration. In women with SUI, who have weaker urethral attachments, this shortening contraction does not occur; consequently, the urethras of women with SUI move further and faster for a longer duration.
Keywords: Acceleration, Cough, Image processing, Pelvic floor muscles, Ultrasound, Urethra, Velocity
1. Introduction
It has been suggested that pelvic floor muscles (PFMs) contribute to urinary continence by providing external support to the urethra [1,2]. PFM contraction increases with the intensity of a cough [3] and with abdominal pressure (Pabd) generated during stress [4]. The failure of the PFMs to resist increases in Pabd may contribute to the pathophysiology of stress urinary incontinence (SUI) and pelvic-organ prolapse [5,6]. A number of electromyography (EMG) studies have indicated that PFM activation patterns are altered in women with SUI compared with those of healthy volunteers, showing delayed activation, shorter activation periods, lack of response, or paradoxical inhibition [7[en]10]. Yet one study has indicated that women with incontinence had increased PFM EMG compared with continent women, both prior to and during stress associated with unexpected loading [11]. Another study concludes that the preprogrammed PFM activity may not be important in the maintenance of continence [12].
PFMs are thought to perform multiple functions. Yet if PFMs have to balance continence and pelvic-organ support [6], spinal stability [13], respiration [14], and containment of Pabd [15], then, given the multipurpose role of theses muscles, the motor-control challenge would be immense. The efficiency of the PFMs would not only rely on the anatomic integrity of the pelvic floor (PF) but would depend on the response of the central nervous system (CNS) to satisfy hierarchic demands of function, generating a coordinated response so that the muscle activities occur at the right time with the appropriate level of force.
The physiologic mechanisms by which the PFMs achieve these tasks are not clearly understood, due to the limitation of current measurement tools to evaluate automatic events, such as coughing. EMG activity does not indicate in which direction any force is generated [16], so without visual access to the PFM, it is impossible to understand in what way the muscle is producing EMG activity, that is, whether the muscle is actively shortening or lengthening.
This paper aims to characterise the automatic dynamic function of the PFM and the urethra during a cough using two-dimensional (2D), perineal, real-time ultrasound imaging with image-processing methodology. The primary hypothesis was that under normal conditions, the cough reflex would regulate the response of the PFM so that there was a limit to the magnitude of the displacement, velocity, and acceleration of the PF and the urethra when compared to women with SUI. Subsequently, the urethras of women with SUI would move further and faster in response to a cough compared to those of continent women.
2. Methods
2.1. Ethical approval
The Institutional Review Board (IRB) of Stanford University approved the experimental protocol used. Witnessed, written, informed consent was obtained from all volunteers prior to the commencement of the investigation.
2.2. Subjects
A convenience sample of 33 female volunteers was recruited from the general community of the San Francisco Bay Area. Women were recruited through networking and fliers posted at schools, hospitals, sports associations, and Stanford University. They completed a demographic questionnaire, a validated short-form Incontinence Impact Questionnaire (IIQ-7), and the Urogenital Distress Inventory (UDI-6).
The investigators were blinded to the continence status of the volunteers, who, after evaluation, were divided on the basis of history: self-reported symptoms, no reported incontinence, and incontinence severity defined by a 12-point scale [17]. The study excluded women according to the following criteria: previous genitourinary surgery, symptoms associated with overactive bladder (OAB) or currently using pharmacotherapy for OAB, currently pregnant, back or pelvic pain within the past 3 mo, neurologic or psychiatric disease, major medical condition, significant prolapse, urinary tract infection, or vaginal infection.
2.3. Equipment
Imaging was performed using a commercially available 2D ultrasound scanner (Hitachi EUB-52, Hitachi Medical Corporation, Tokyo, Japan) with a 3.5[en]5 MHz high-definition, curved linear-array transducer with a footprint 10 cm long. To keep the out-of-plane rotation of the ultrasound transducer in an acceptable range (less than ±5°), a measuring device with six degrees of freedom, the Flock of Birds (FOB) tracking system (Ascension Technology Corporation, Burlington, VT, USA), was used. The FOB was fixed on the handle of the ultrasound transducer, and the orientation (ie, azimuth, elevation, and roll angles) of the ultrasound transducer was visualised in real-time during scanning measurements. Ultrasound images 320 × 240 pixels in size with an 8-bit resolution were recorded at a rate of 30 frames per second. Analysis of the video data was performed using a dual central-processing unit (CPU) Hewlett Packard workstation (Hewlett Packard, Palo Alto, CA, USA) using MATLAB 7.1 software (MathWorks, Natick, MA, USA). The 8-bit greyscale ultrasound images were captured using a video-to-universal serial bus (USB) capture card and were stored in the uncompressed advanced video interface (AVI) format for image analysis on a personal computer (Hewlett Packard Compaq nx9010, Hewlett Packard, Palo Alto, CA, USA).
2.4. Testing protocol
Volunteers were asked to void 1 h before testing, and then to drink 450 ml of water and to refrain from voiding until after the test sequences.
2.4.1. Perineal ultrasound
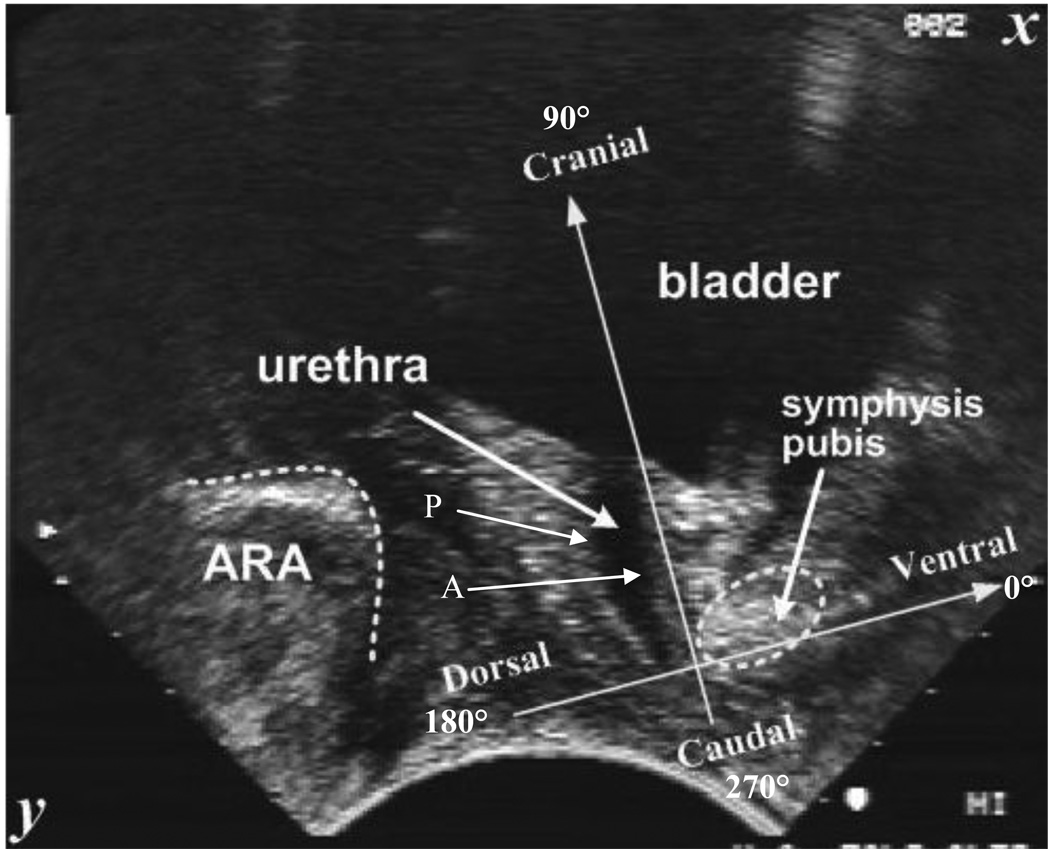
Using one operator, all volunteers were tested in the supine position, lying with their hips and knees bent and supported, with one pillow under their head. The transducer was covered in ultrasound gel covered by an unpowdered glove and followed by more ultrasound gel before being placed on the perineum in a midsagittal direction, orienting the transducer so that the clearest images of the urogenital structures were viewed (Fig. 1). The exact position of the transducer on the perineum was dependent on the ultrasound image observed and was applied with sufficient skin pressure to maintain the location and orientation without distortion of pelvic structures [18]. The volunteers were asked to do a perceived maximum cough three times with a 5-s rest between each cough.
Fig. 1.
[en] Typical transperineal view on an ultrasound scan with coordinate system placed on pubis symphysis.
2.4.2. Image processing methodology
The segmentation methodology for the urethra [19], motion tracking algorithms for the pubic symphysis (PS) and the anorectal angle (ARA) [20], and intertester reliability have been reported in detail elsewhere [21]. In summary, motion of the ARA and PS were measured by speckle tracking that matches the whole area of the structure being observed frame by frame, thereby eliminating error introduced by the operator having to accurately select one specific point on either the PS or the ARA [20]. The urethra has considerable deformation over a short period and is unsuitable for speckle tracking; therefore, the boundaries of the anterior and posterior edges of urethras studied were segmented in the binary images using an automatic segmentation algorithm [19].
Displacements of the urethra and ARAs were measured with respect to an orthogonal coordinate system fixed on the PS, parallel and vertical to the urethra at rest (Fig. 1). When the tissues moved, the coordinate system maintained its original position, and the subsequent trajectory of urogenital structures could then be measured relative to this fixed axis. To accurately map the trajectory of the ARA and the urethra, the apparent motion of the PS, created by movement of the transducer during the cough, was tracked and subtracted from the displacements of the ARA and the urethra.
The intraclass correlation coefficient for interobserver reliability analysis of image processing was good (0.73) for determining the ARA displacement in the SUI group, and it was excellent (0.93) in the continent group. Urethral segmentation was excellent for both groups at 0.86 for the SUI group and 0.88 for the continent group, respectively.
2.4.3. Statistical analysis
Mean and standard deviation (SD) of the displacement, velocity, and acceleration were calculated and presented graphically. Statistical comparisons using one-tailed unpaired t tests were performed to evaluate the mean values (plus SD) and levels of significant differences. Welch’s correction was applied where the variances were unequal, and a level of p < 0.05 was considered significant. GraphPad Prism v.5.01 for Windows (GraphPad Software, San Diego, CA, USA) was used for statistical analysis.
2.4.4. Graph alignment
Graphs were aligned so that the maximum caudal displacement occurred at a time of 2 s. The data values in tables and statistical tests were calculated on the average of the maximum values from individual tests regardless of time. In the first case, the maximum of the average was calculated, while in second case, the average of the maxima was calculated; consequently, the graphs do not correspond exactly with the tables. Angles were measured from the ventral axis so that the dorsal direction was 180° (−X); the ventral direction was 0° (+X); the cranial direction was 90° (−Y); and the caudal direction was 270° (+Y) (Fig. 1 and Fig. 2).
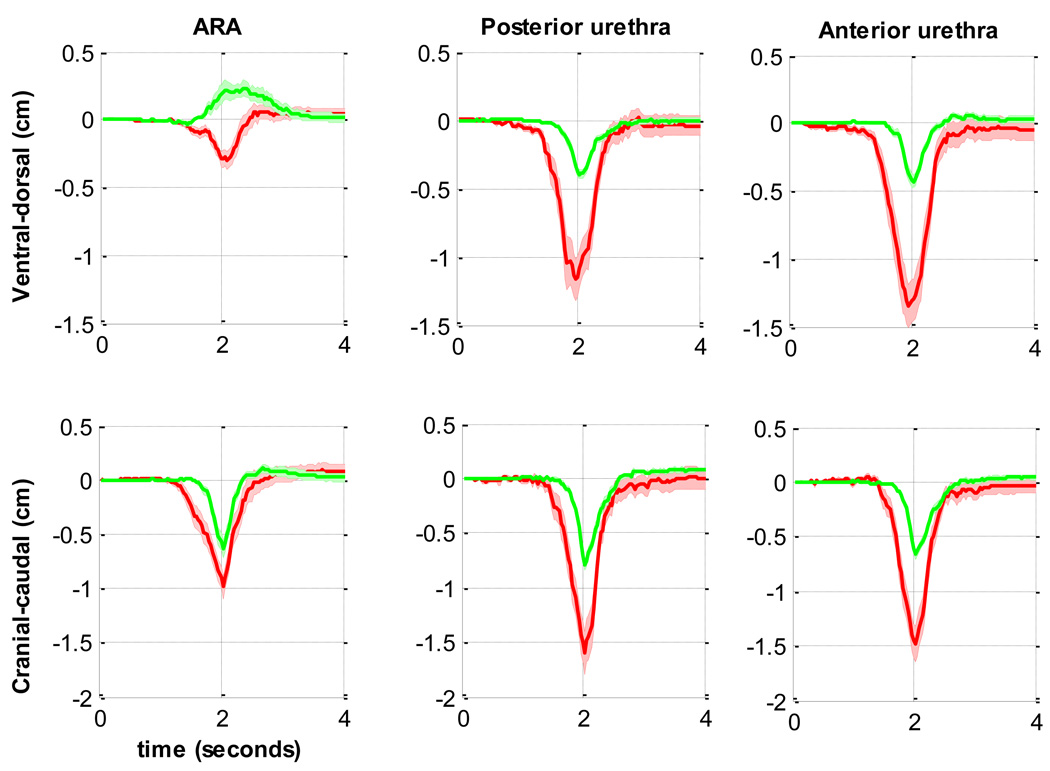
Fig. 2.
[en] Comparison of the mean displacements of the anorectal angle (ARA), and both edges of the urethra during a cough in supine continent women (n = 22; green line) and in women with stress urinary incontinence (SUI) (n = 9; red line).
3. Results
One volunteer was excluded due to symptoms of OAB. The general demographic of the two groups and standardised manual voluntary PFM muscle testing during a contraction are described below (Table 1). ARAs and urethral displacements, velocities, and accelerations were normally distributed.
Table 1.
[en] Mean and standard deviation (SD) of age, parity, body mass index (BMI), manual muscle testing (Oxford scale), and continence severity scale (CSS)
| Continent (n = 23) | SUI (n = 9) | p value | |
|---|---|---|---|
| Age (yr), mean ± SD | 41.1 ± 13.6 | 47.9 ± 13.2 | p = 0.21* |
| Parity, mean ± SD | 0.5 ± 0.9 | 1.6 ± 0.7 | p = 0.01 |
| BMI, mean ± SD | 22.0 ± 2.0 | 23.9 ± 2.6 | p = 0.09* |
| Oxford scale, mean ± SD | 3.6 ± 1.2 | 2.6 ± 0.88 | p = 0.03 |
| CSS | Continent | 5 slight SUI 4 moderate SUI | [en] |
SUI = stress urinary incontinence.
Not significant.
3.1. Displacement
When a woman in the continent group coughed, the ARA moved in a ventrocaudal direction towards the PS, whereas when a woman in the SUI group coughed, the ARA and urethra of the SUI group moved in a dorsocaudal direction away from the PS (Fig. 2 and Fig. 3). The urethras of women in the continent group moved barely a third of the distance of those of women in the SUI group along a linear path and returned almost along the same path (Table 2, Fig. 3). The urethras of women in the SUI group moved along a more convoluted path, contrasting with the collinear trajectory of urethras of women in the continent group. In the continent group, the anterior edge of the urethra moved significantly less than the posterior edge (p = 0.036), although there was no difference in the angle of displacement between the edges (p = 0.51). In the SUI group, there was a significant difference of 10° in the displacement angle between the anterior and posterior edges of the urethra (p = 0.029) (Table 2).
Fig. 3.
[en] Comparison of the average velocities of the anorectal angle (ARA), and both edges of the urethra during a cough in supine continent women (n = 22; green line) and in women with stress urinary incontinence (SUI) (n = 9; red line). The shaded area represents the standard error.
Table 2.
[en] Mean and standard deviation (SD) of the maximum displacements and angle of the anorectal angle (ARA) and urethral displacement direction during a cough in supine position‡
| Dorsal-ventral | Cranial-caudal | Resultant | Angle, ° | |
|---|---|---|---|---|
| ARA | ||||
| Continent (cm), mean ± SD | 0.18 ± 0.36 | −0.63 ± 0.37 | 0.77 ± 0.36 | 285 ± 40 |
| SUI (cm), mean ± SD | −0.20 ± 0.26 | −1.01 ± 0.42 | 1.1 ± 0.40 | 260 ± 20 |
| p value | p = 0.0038 | p = 0.0084 | p = 0.022 | p = 0.012* |
| Anterior urethra | ||||
| Continent (cm), mean ± SD | −0.43 ± 0.28 | −0.66 ± 0.25 | 0.84 ± 0.30 | 240 ± 15 |
| SUI (cm), mean ± SD | −1.22 ± 0.48 | −1.7 ± 0.51 | 2.1 ± 0.65 | 235 ± 10 |
| p value | p < 0.0001 | p = 0.0002* | p = 0.0002* | p = 0.16*† |
| Posterior urethra | ||||
| Continent (cm), mean ± SD | −0.41 ± 0.21 | −0.80 ± 0.27 | 0.92 ± 0.28 | 240 ± 10 |
| SUI (cm), mean ± SD | −1.1 ± 0.42 | −1.7 ± 0.61 | 2.0 ±0.71 | 240 ± 5 |
| p value | p = 0.0009* | p = 0.0014* | p = 0.0009* | p = 0.15† |
SUI = stress urinary incontinence.
Continent cohort: n = 23; SUI cohort: n = 9.
Welch’s correction applied for unequal variances.
Not significant.
3.2. Velocity
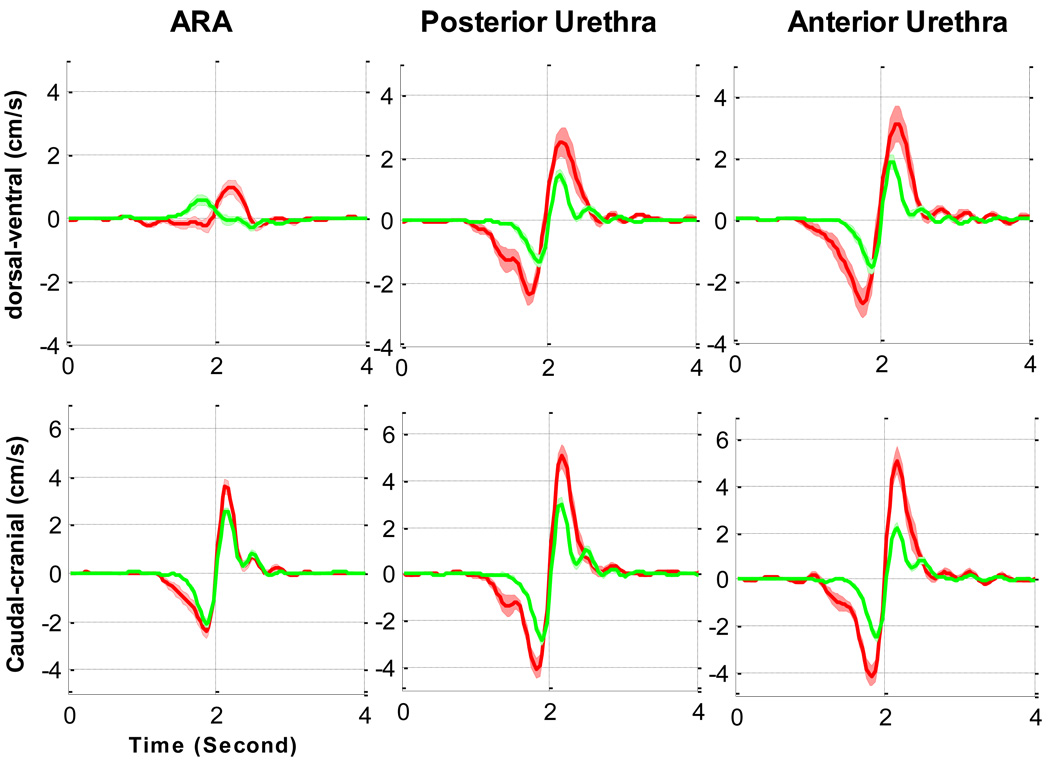
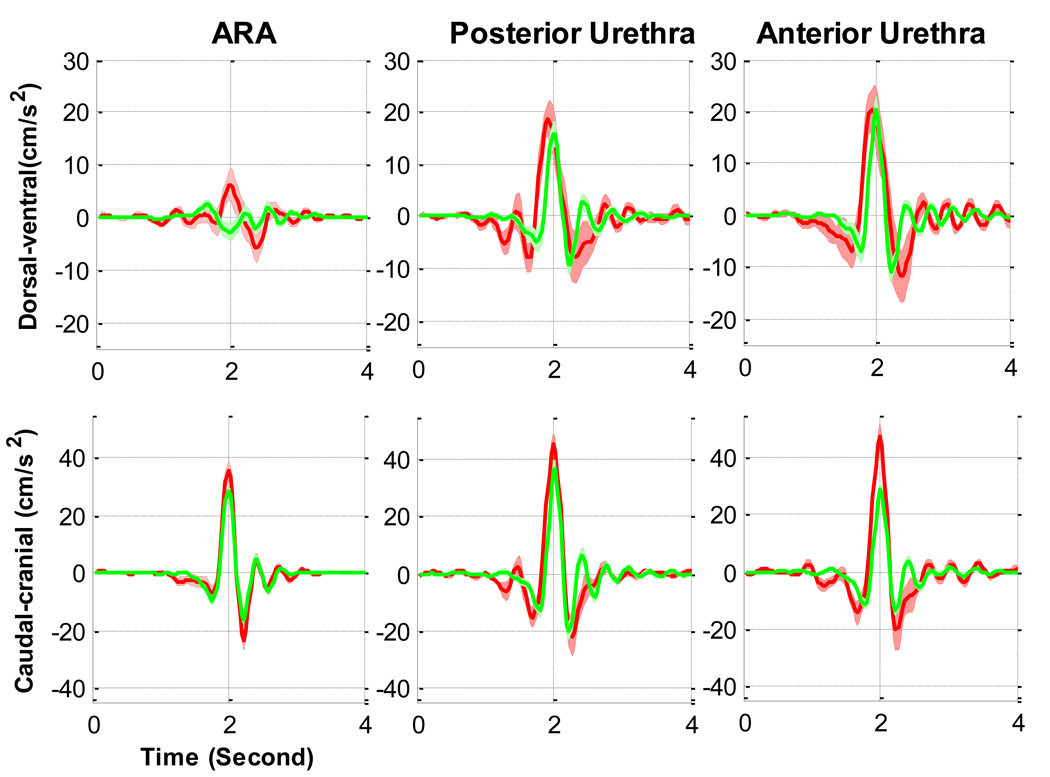
The duration of displacement of both the ARA and the urethra was longer for the women in SUI group than for the women in the continent group (Fig. 2 and Fig. 4). Before the synchronisation point (at a time of 2 s), the ARA in the continent group moved at its maximum ventral velocity towards the PS, whereas the ARA in the SUI group moved dorsally away. The ARAs of women in the SUI group moved ventrally only after the maximum caudal displacement. The caudocranial velocity of the ARAs of women in the incontinent group were higher, although not significantly different (Table 3). The urethras of women in both groups moved in a dorsocaudal direction, although the absolute angles and maximum velocities varied. Comparing the SUI group to the asymptomatic group, in the dorsal-ventral direction the urethras moved with at least one and a half times the velocity and up to twice the caudocranial velocity (Table 3). The “rebound” occurred after the time of maximum caudal (−Y) displacement aligned on the graphs at a time of 2 s. In the continent group, the maximum velocities of the anterior urethra of continent women were the same or lower after the rebound, whereas they were larger after the rebound for the SUI cases (Fig. 4).
Fig. 4.
[en] Comparison of the mean velocity of the anorectal angle (ARA), anterior and posturethra during a cough in supine continent women (n = 22; green line) and in women with stress urinary incontinence (SUI) (n = 9; red line).
Table 3.
[en] Mean and standard deviation (SD) of the maximum velocity of the anorectal angle (ARA) and the urethra in supine position during a cough before and after the synchronisation point‡
| Dorsal-ventral direction | Caudal-cranial direction | |||
|---|---|---|---|---|
| Before | After | Before | After | |
| ARA | ||||
| Continent (cm s−1), mean ± SD | 1.8 ± 0.95 | −1.3 ± 0.75 | −2.9 ± 1.5 | 3.3 ± 1.4 |
| SUI (cm s−1), mean ± SD | −1.5 ± | 2.2 ± 0.98 | −3.6 ± 1.0 | 4.1 ± 1.5 |
| p value | p < 0.0001 | p < 0.0001 | p = 0.10† | p = 0.085† |
| Anterior urethra | ||||
| Continent (cm s−1), mean ± SD | −2.8 ± 1.4 | 2.8 ± 1.3 | −3.4 ± 1.3 | 3.1 ± 1.0 |
| SUI (cm s−1), mean ± SD | −4.2 ± 1.3 | 4.5 ± 2.3 | −5.1 ± 1.3 | 6.3 ± 2.6 |
| p value | p = 0.011 | p = 0.0072 | p = 0.0015 | p = 0.0031* |
| Posterior urethra | ||||
| Continent (cm s−1), mean ± SD | −2.5 ± 1.4 | 2.5 ± 1.2 | −3.7 ± 1.4 | 4.2 ± 1.7 |
| SUI (cm s−1), mean ± SD | −3.9 ± 1.1 | 4.0 ± 2.1 | −5.4 ± 1.6 | 5.8 ± 2.4 |
| p value | p = 0.0021 | p = 0.034* | p = 0.0044 | p = 0.0041 |
SUI = stress urinary incontinence.
Continent cohort: n = 23; SUI cohort: n = 9.
Welch’s correction applied for unequal variances.
Not significant.
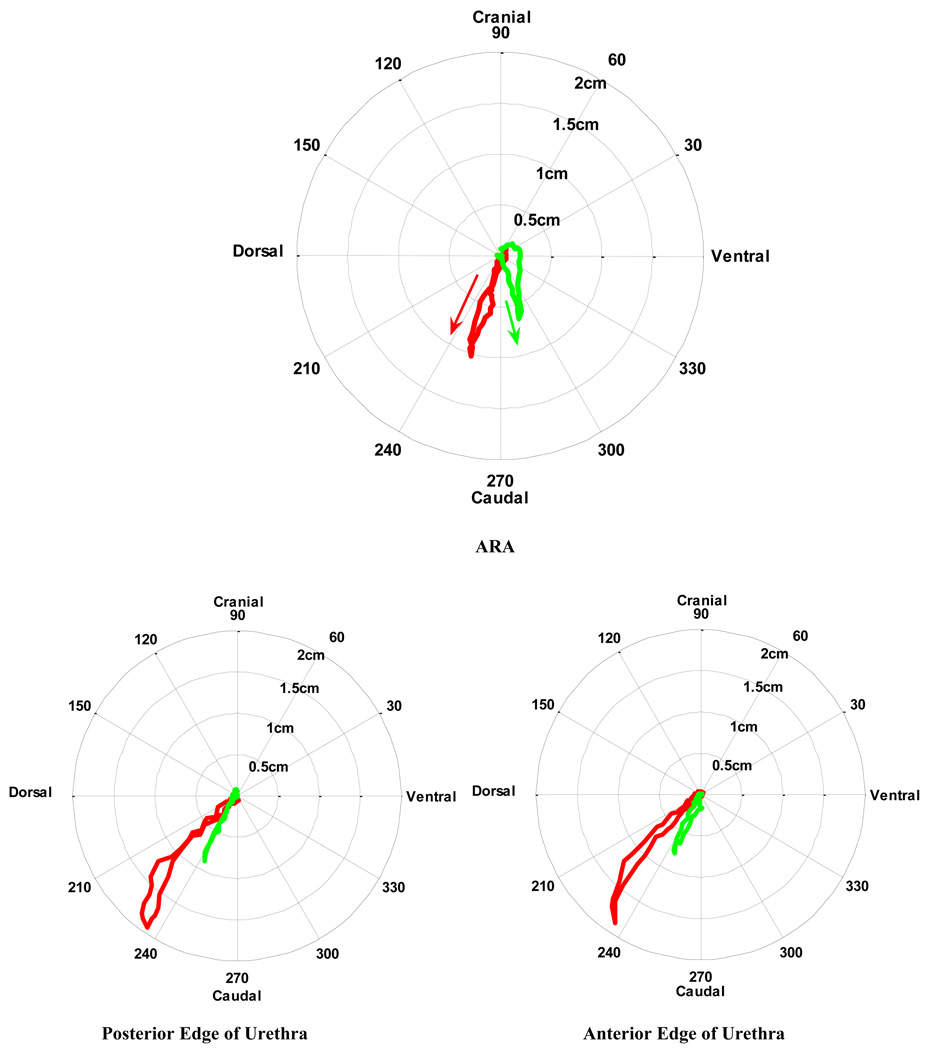
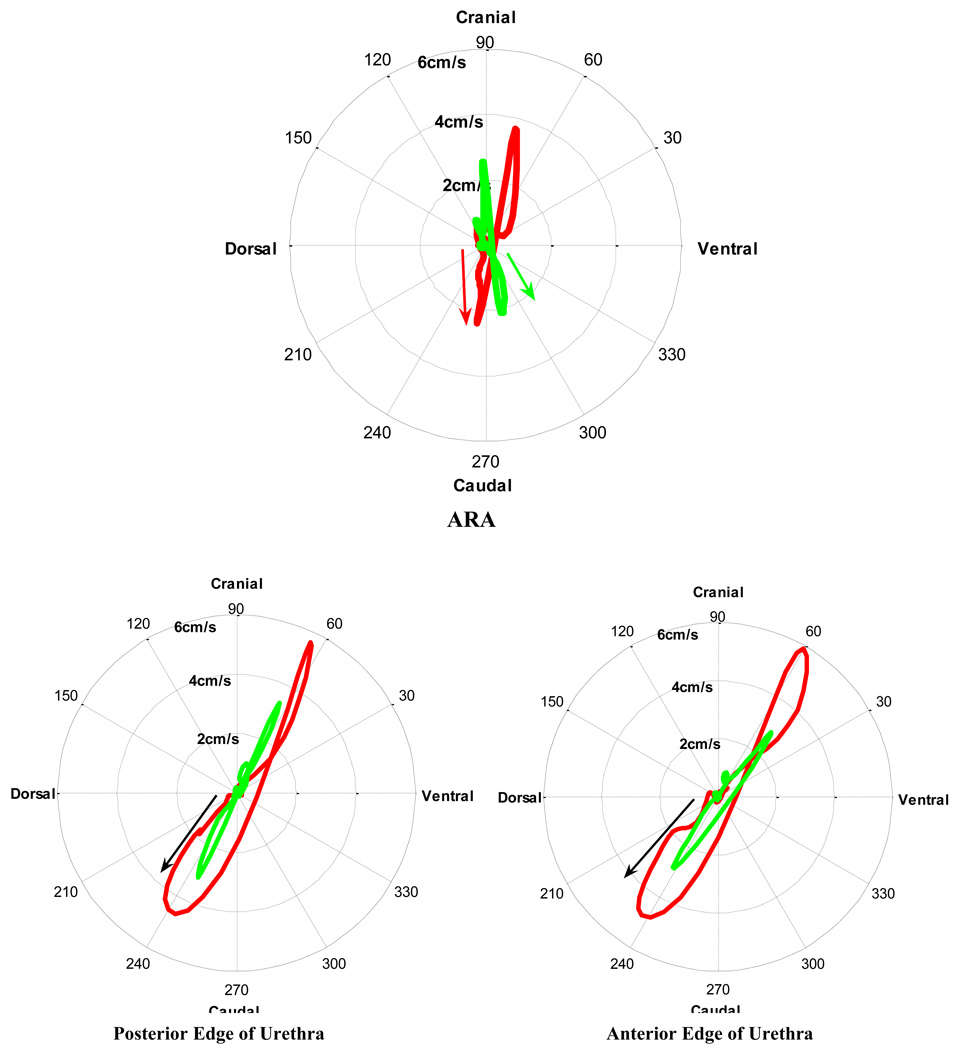
The disparity between groups can further be illustrated by plotting the amplitude and direction of the velocities of the ARAs and urethras in polar plots (Fig. 5); these show the initial ventrocaudal velocities of the ARAs in continent women compared with the dorsocaudal movement of the ARAs in women with SUI. Furthermore, they highlight the additional transverse velocities of the urethras of women in the SUI group, which were absent in the collinear path of the continent group.
Fig. 5.
[en] Comparison of the mean acceleration of the anorectal angle (ARA), and both edges of the urethra during a cough in supine continent women (n = 22; green line) and in women with stress urinary incontinence (SUI) (n = 9; red line).
3.3. Acceleration
A tendency for the accelerations to be larger in the SUI group was shown, although in general the magnitudes of the maximum accelerations between groups were more similar than the velocities and displacements (Table 4; Fig. 5 and Fig. 6). The timing of the ARA acceleration was different between groups, indicated by the initially low ventral acceleration of the ARA in the continent group compared with the negligible acceleration in the incontinent group (Fig. 6). Additionally, at the point when the caudal displacement was a maximum, both groups attained their maximum ventral-dorsal direction acceleration, but they were accelerating in opposite directions. In a cranial-caudal direction, there were no significant differences in the maximum accelerations.
Table 4.
[en] Mean and standard deviation (SD) of the maximum acceleration of the ano-rectal angle (ARA) and urethra in a supine position during a cough‡
| Ventral direction | Dorsal direction | Cranial direction | Caudal direction | |
|---|---|---|---|---|
| ARA | ||||
| Continent (cm s−2), mean ± SD | 15 ± 6.8 | −18 ± 8.9 | 35 ± 18 | −26 ± 12 |
| SUI (cm s−2), mean ± SD | 24 ± 9.9 | −21 ± 8.9 | 41 ± 13 | −33 ± 8.6 |
| p value | p = 0.015 | p = 0.16† | p = 0.20† | p = 0.092† |
| Anterior urethra | ||||
| Continent (cm s−2), mean ± SD | 35 ± 17 | −25 ± 9.9 | 38 ± 13 | −28 ± 9.4 |
| SUI (cm s−2), mean ± SD | 39 ± 17 | −34 ± 16 | 55 ± 19 | −48 ± 21 |
| p value | p = 0.27† | p = 0.030 | p = 0.0052 | p = 0.012* |
| Posterior urethra | ||||
| Continent (cm s−2), mean ± SD | 31 ± 13 | −24 ± 9.2 | 33 ± 24 | −39 ± 16 |
| SUI (cm s−2), mean ± SD | 37 ± 13 | −34 ± 16 | 55 ± 16 | −47 ± 16 |
| p value | p = 0.11† | p = 0.021 | p = 0.0060 | p = 0.12† |
SUI = stress urinary incontinence.
Continent cohort: n = 23; SUI cohort: n = 9.
Welch’s correction applied for unequal variances.
Not significant.
Fig. 6.
[en] Comparison of the average acceleration of the anorectal angle (ARA), and both edges of the urethra during a cough in supine continent women (n = 23; green line) and in women with stress urinary incontinence (SUI) (n = 9; red line) women. The shaded area represents the standard error.
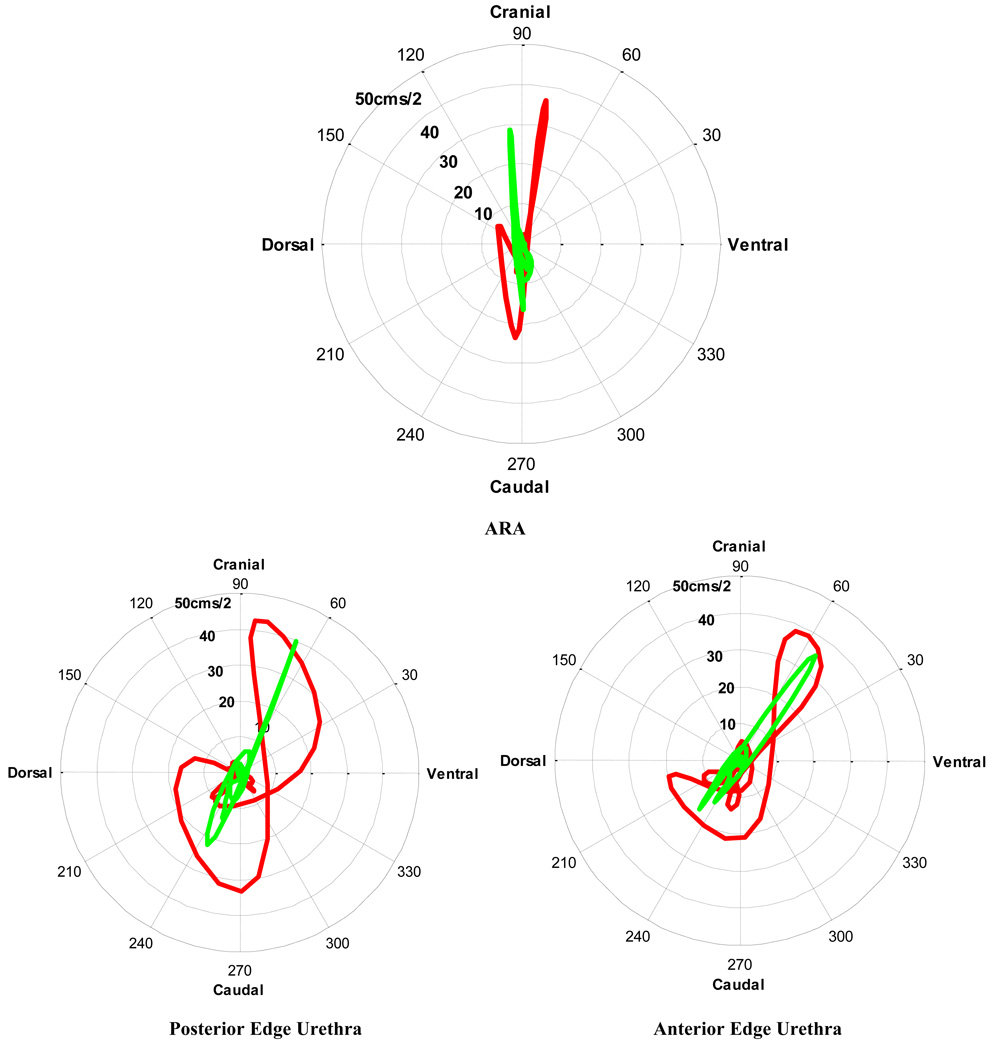
The maximum acceleration of the urethras in the continent group was less than that in the SUI group, particularly cranially and dorsally, and although there was a significant difference in maximum acceleration of the anterior edge, caudally there was significant variance in the SUI group. The uncontrolled transverse acceleration observed in the SUI group was particularly obvious in the ventral cranial direction after the maximum cranial displacement (Fig. 7), and it contrasted with the difference in collinear acceleration of the ARAs and edges of the urethra in the asymptomatic group.
Fig. 7.
[en] Comparison of the mean acceleration (cms-2) and direction (degrees) of the ARA, and both edges of the urethra during a cough in supine continent women (n = 23; green line) and in women with stress urinary incontinence (SUI) (n = 9; red line) women. In the SUI group, bigger transverse velocities exist, indicated by the loops/kinks in the waveform.
4. Discussion
4.1. Direction of pelvic floor muscle support during a cough
We hypothesised that the PFMs, in terms of their timing and direction of contraction, would play an important role in maintaining continence. During a cough the PFM of continent women actively shortened, as indicated by the reduction in distance between the ARA and the PS and the initial direction of displacement, velocity, and acceleration of the ARA. The implication of this observation is that in the continent group either there was an anticipatory PFM contraction or the restoring forces of the PFM responded quickly to prevent any dorsal displacement caused by the rise in Pabd during a cough. In women with SUI, the muscle was lengthening, indicated by the increase in distance between the ARA and the PS.
Active shortening of the PFM would also produce compression of the tissues and stiffening of the PF, providing external support to the urethra and acting like a brake to reduce the velocity and displacement of the urethra and PF during a cough. This current study therefore provides evidence for and supports the hammock hypothesis first proposed by DeLancey [6]. Additionally, as the PFM contracted simultaneously with the diaphragm and abdominal wall muscles to build Pabd [14], the PFM contraction in the asymptomatic group also may have helped to tense the suburethral fascial layer, thereby enhancing urethral compression.
Ashton-Miller and DeLancey [2] also theorised that if there were any interruption in the endopelvic fascia or if the PFM was damaged, the supportive layer under the urethra would be more compliant, so that closure of the urethral lumen would be delayed, and stress incontinence would be likely to occur. This current study has indicated that in the SUI group, the supportive layer under the urethra is elongated back and down during a cough and is much more compliant than that of the continent PF. Consequently, in the SUI group, the PFM stiffness was reduced, so the PF and urethra were displaced more before the restoring forces were sufficient to bring the tissue back to a state of equilibrium. Thus, in the SUI women, the PF was like a saggy passive trampoline; once the tissues have been exposed to this downward stretch, they rebound with a greater velocity.
4.2. Urethral displacement, velocity, and acceleration
During a cough, the urethra in both groups was displaced in a dorsocaudal direction, although in asymptomatic volunteers, the urethra was displaced less than half the distance with a smaller velocity. This implies that in the continent group, in addition to a stiffer PF to support the urethra, the fascial attachments of the urethra were stronger. Any tear or break in the continuity of the fascia will limit the external support of the urethra, therefore contributing to greater displacement. The increased likelihood of fascial disturbances in the SUI group is illustrated by the fact that the anterior edge of the urethra moved more than the posterior edge. In continent women, the anterior edge of the urethra moved less than the posterior edge, which supports some anatomic studies [22] that indicate that the anterior edge is fixed to the posterior edge of the pubic symphysis, whereas the posterior edge has less direct fascial attachment.
The combination of stronger urethral control due to intact fascia and the presence of a PFM contraction in the continent group would account for the limitation of both the dorsal and caudal motion on the urethra during the cough and would explain the linear path of displacement, velocity, and acceleration. Although forces were not measured, the velocity and acceleration figures (Fig. 5 and Fig. 6) imply that the net forces on the urethra in the continent group are principally along a single direction and that the restoring forces in the transverse direction are balanced. In contrast, the convoluted path of the urethra in the SUI group suggest that there are uncontrolled forces on the urethra in the transverse direction as indicated by the “loops/kinks” in the polar plots of velocity and acceleration.
4.3. Limitations
Caution regarding the generalisability of this feasibility study is warranted because the number of women in the SUI group is small, no power analysis was calculated beforehand, and there was a significant difference in parity status between groups. Future studies with larger numbers of parity-matched, asymptomatic, and SUI groups are necessary; however, this current study does provide further comprehension of dynamic changes occurring in the PF and urethra during events that typically trigger SUI.
Although the direction of the forces involved can be visualised using 2D ultrasound imaging, there is insufficient information to measure the amplitude of the PFM force vectors during PFM activity, and the use of simultaneous EMG and or pressure recordings would have clarified both the timing of contraction and indicated force amplitude. However, insertion of any device into the vagina would artificially distort the PF and affect the image processing.
In the data preprocessing, the velocity and acceleration were smoothed using an eighth-order, Butterworth, low-pass filter (cut-off frequency: 3Hz), image-processing methodology. The use of such a filter may produce excesses in the time domain if the waveform contains rapid changes with frequency content in the region of the cut-off frequency. This may explain some of the ripple seen in the time waveforms, especially for the continent group, where the response was faster.
5. Conclusions
This study characterises the automatic dynamic function of the PFM and accurately describes the trajectory of the urethra during a cough. Significant differences exist in the behaviour of the PFM and the urethras of women with SUI compared with continent controls. Normal PFM function during a cough produces timely compression of the PF and additional external support to the urethra, reducing the velocity and acceleration of the PF and the urethra. In response to a cough, the PF and the urethra of women with SUI move further and faster and for a longer duration than in asymptomatic women. The restraining forces do not increase as rapidly with displacement as those in continent women; this is evidence that the urethra and the PF of asymptomatic women are stiffer than those of continent women.
Acknowledgment statement
The authors acknowledge Vicky Wolf, Tina Alley, and Inder Perkash for their assistance in the experimental part of this project and the volunteers who participated in this intimate study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: none
Funding/Support and role of the sponsor: This work was funded in part by NIH grant 1R21 EB001654-1.
References
- 1.DeLancey JOL, Trowbridge ER, Miller JM, et al. Stress urinary incontinence: relative importance of urethral support and urethral closure pressure. Urology. 2008;179:2286–2290. doi: 10.1016/j.juro.2008.01.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashton-Miller J, Delancey JOL. Functional anatomy of the female pelvic floor. Ann NY Acad Sci. 2007;1101:266–296. doi: 10.1196/annals.1389.034. [DOI] [PubMed] [Google Scholar]
- 3.Amarenco G, Ismael SS, Lagauche D, et al. Cough anal reflex: strict relationship between intravesical pressure and pelvic floor muscle electromyographic activity during cough. Urodynamic and electrophysiological study. Urology. 2005;173:149–152. doi: 10.1097/01.ju.0000147305.00443.df. [DOI] [PubMed] [Google Scholar]
- 4.Shafik A, Doss S, Asaad S. Etiology of the resting myoelectric activity of the levator ani muscle: physioanatomic study with a new theory. World J Surg. 2003;27:309–314. doi: 10.1007/s00268-002-6584-1. [DOI] [PubMed] [Google Scholar]
- 5.Constantinou CE, Govan DE. Spatial distribution and timing of transmitted and reflexly generated urethral pressures in healthy women. Urology. 1982;127:964–969. doi: 10.1016/s0022-5347(17)54148-8. [DOI] [PubMed] [Google Scholar]
- 6.DeLancey JO. Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol. 1994;170:1713–1720. doi: 10.1016/s0002-9378(94)70346-9. [DOI] [PubMed] [Google Scholar]
- 7.Deindl FM, Vodusek DB, Hesse U, Schussler B. Pelvic floor activity patterns: comparison of nulliparous continent and parous urinary stress incontinent women. A kinesiological EMG study. Br J Urol. 1994;73:413–417. doi: 10.1111/j.1464-410x.1994.tb07606.x. [DOI] [PubMed] [Google Scholar]
- 8.Barbic M, Kralj B, Cor A. Compliance of the bladder neck supporting structures: importance of activity pattern of levator ani muscle and content of elastic fibers of endopelvic fascia. Neurourol Urodyn. 2003;22:269–276. doi: 10.1002/nau.10116. [DOI] [PubMed] [Google Scholar]
- 9.Deffieux X, Hubeaux K, Porcher R, Ismael SS, Raibaut P, Amarenco G. Pelvic floor muscle activity during coughing: altered pattern in women with stress urinary incontinence. Urology. 2007;70:443–447. doi: 10.1016/j.urology.2007.03.084. [DOI] [PubMed] [Google Scholar]
- 10.Smith MD, Coppieters MW, Hodges PW. Postural activity of the pelvic floor muscles is delayed during rapid arm movements in women with stress urinary incontinence. Int Urogynecol. 2007;18:901–911. doi: 10.1007/s00192-006-0259-7. [DOI] [PubMed] [Google Scholar]
- 11.Smith MD, Coppieters MW, Hodges PW. Postural response of the pelvic floor and abdominal muscles in women with and without incontinence. Neurourol Urodyn. 2007;26:377–385. doi: 10.1002/nau.20336. [DOI] [PubMed] [Google Scholar]
- 12.Verelst M, Leivseth G. Are fatigue and disturbances in pre-programmed activity of pelvic floor muscles associated with female stress urinary incontinence? Neurourol Urodyn. 2004;23:143–147. doi: 10.1002/nau.20004. [DOI] [PubMed] [Google Scholar]
- 13.Pool-Goudzwaard A, van Dijke GH, van Gurp M, Mulder P, Snijders C, Stoeckart R. Contribution of pelvic floor muscles to stiffness of the pelvic ring. Clin Biomech. 2004;6:564–571. doi: 10.1016/j.clinbiomech.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Hodges PW, Sapsford R, Pengel LH. Postural and respiratory functions of the pelvic floor muscles. Neurourol Urodyn. 2007;26:362–371. doi: 10.1002/nau.20232. [DOI] [PubMed] [Google Scholar]
- 15.Hemborg B, Moritz U, Lowing H. Intra-abdominal pressure and trunk muscle activity during lifting. IV. The causal factors of the intra-abdominal pressure rise. Scand J Rehab Med. 1985;17:25–38. [PubMed] [Google Scholar]
- 16.Turker KS. Electromyography: some methodological problems and issues. Phys Ther. 1993;73:698–710. doi: 10.1093/ptj/73.10.698. [DOI] [PubMed] [Google Scholar]
- 17.Sandvik H, Seim A, Vanvik A, Hunskaar S. A severity index for epidemiological surveys of female urinary incontinence: comparison with 48-hour pad-weighing tests. Neurourol Urodyn. 2000;19:137–145. doi: 10.1002/(sici)1520-6777(2000)19:2<137::aid-nau4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 18.Schaer GN, Koechli OR, Schuessler B, Haller U. Perineal ultrasound: determination of reliable examination procedures. Ultrasound Obstet Gynecol. 1996;7:347–352. doi: 10.1046/j.1469-0705.1996.07050347.x. [DOI] [PubMed] [Google Scholar]
- 19.Peng Q, Jones RC, Constantinou CE. 2D ultrasound image processing in identifying responses of urogenital structures to pelvic floor muscle activity. Ann Biomed Eng. 2006;34:477–493. doi: 10.1007/s10439-005-9059-3. [DOI] [PubMed] [Google Scholar]
- 20.Peng Q, Jones R, Shishido K, Constantinou CE. Ultrasound evaluation of dynamic responses of female pelvic floor muscles. Ultrasound Med Biol. 2007;3:342–352. doi: 10.1016/j.ultrasmedbio.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lovegrove Jones RC, Peng Q, Stokes M, Humphreys VF, Constantinou CE. The inter-tester reliability of 2D ultrasound and image processing methods to evaluate the pelvic floor muscles (PFM) and urethra during a cough in continent and stress urinary incontinent (SUI) women [abstract 247]. Abstract presented at: the 38th Annual Meeting of the International Continence Society; Cairo, Egypt. Oct 24, 2008. pp. 20–24. https://www.icsoffice.org/ASPNET_Membership/Membership/Abstracts/Publish/46/000247.pdf. [Google Scholar]
- 22.Mostwin JL. Current concepts of female pelvic anatomy and physiology. Urol Clin North Am. 1991;18:175–195. [PubMed] [Google Scholar]