Abstract
Introduction
The objective of this study is to determine which cognitive processes underlying spelling are most affected in the three variants of Primary Progressive Aphasia (PPA): Logopenic variant primary progressive aphasia (lvPPA), Semantic variant primary progressive aphasia (svPPA), and Nonfluent variant primary progressive aphasia (nfvPPA).
Methods
23 PPA patients were administered The Johns Hopkins Dysgraphia Battery to assess spelling. Subtests evaluate for effects of word frequency, concreteness, word length, grammatical word class, lexicality (words vs. pseudowords), and “regularity” by controlling for the other variables. Significant effects of each variable were identified with chi square tests. Responses on all spelling to dictation tests were scored by error type. 16 of the 23 subjects also had a high resolution MRI brain scan to identify areas of atrophy.
Results
We identified 4 patterns of spelling that could be explained by damage to one or more cognitive processes underlying spelling. Nine patients (3 unclassifiable, 4 with lvPPA, 2 with svPPA) had dysgraphia explicable by impaired access to lexical representations, with reliance on sublexical phonology-to-orthography conversion (POC). Two patients (with nfvPPA) showed dysgraphia explicable by impaired access to lexical representations and complete disruption of sublexical POC. Seven patients (4 with lvPPA, 1 with svPPA, 2 unclassifiable) showed dysgraphia explicable by impaired access to lexical-semantic representations and/or lexical representations with partially spared sublexical POC mechanisms. Five patients (1 with nfvPPA, 2 with svPPA, 1 with lvPPA, and 1 unclassifiable) showed dysgraphia explicable by impairment of the graphemic buffer.
Conclusions
Any cognitive process underlying spelling can be affected in PPA. Predominance of phonologically plausible errors, more accurate spelling of regular words than irregular words, and more accurate spelling of pseudowords than words (indicating spared POC mechanisms) may indicate a low probability of progression to nfvPPA.
Keywords: Primary Progressive Aphasia, dysgraphia, spelling errors, neuroimaging, aphasia
1. Introduction
Primary Progressive Aphasia (PPA) is a neurodegenerative syndrome characterized by progressive impairment of language with sparing of other cognitive domains (except praxis) for at least two years (Mesulam, 2003). PPA has recently been divided into three clinical subtypes based on spoken language comprehension and production that appear to be associated with different regions of cortical atrophy and loosely associated with different pathologies (Gorno-Tempini et al., 2004; Mesulam et al., 2008; Posteraro et al., 1988; Hillis, 2008; Josephs et al., 2008; Gorno-Tempini, et al. submitted).
Nonfluent variant primary progressive aphasia (nfvPPA) is classically associated with atrophy in left posterior frontal cortex and insula and is characterized by difficulty with grammaticality of sentence production and apraxia of speech (Gorno-Tempini et al., 2004 and submitted; Rabinovici et al., 2008; Mesulam et al., 2008; Josephs et al., 2008). Many show disproportionately impaired naming of action verbs compared to concrete nouns (Hillis et al., 2004). Writing is sometimes relatively intact compared to speech, and many patients use writing as a primary means of communication (Holland et al., 1985; Hillis et al., 2002). Some patients show a progressive loss of verbs only in speech, while naming of nouns remains normal in speech and naming of verbs and nouns remains normal in writing (Hillis et al., 2002). These patients eventually also develop impaired written naming of verbs (and nouns), but it is often long after they have become mute.
In contrast, Semantic variant primary progressive aphasia (svPPA) is associated with atrophy in the left (more than the right) anterior and inferior temporal lobe and is characterized by deterioration of word and object meanings (Hodges et al., 1992; Mummery et al., 2000). There is often disproportionately impaired naming of nouns (objects) compared to verbs (actions) - opposite to the pattern seen in Nonfluent variant primary progressive aphasia (Hillis et al., 2004). Comprehension of object meanings is often worse than comprehension of action meanings as well (Rhee et al., 2001). These patients tend to show deterioration in written naming prior to deterioration of spoken naming. Writing and reading often show a reliance on sublexical phonological-orthographic conversion (or the opposite), resulting in phonologically plausible errors (e.g., creature spelled kreechure) (Graham et al., 1995).
Logopenic variant primary progressive aphasia (lvPPA), associated with atrophy in the left posterior, superior temporal, and inferior parietal cortex, is characterized by poor sentence repetition and poor naming of both nouns and verbs (Gorno-Tempini et al., 2004, 2008, submitted; Rabinovici et al., 2008; Hillis, 2008; Josephs et al., 2008). Unlike individuals with Semantic variant primary progressive aphasia, those with Logopenic variant primary progressive aphasia have relatively spared word and object meanings. Unlike patients with nfvPPA, they have relatively spared motor speech. Most or all have impaired phonological working memory. In part because this group was recently identified, the spelling performance of these patients has not been studied extensively.
These distinct behavioral patterns also provide clues as to the most likely etiology of the neurological condition. There are three main degenerative neurological pathologies, which can only be diagnosed at autopsy or by brain biopsy that can cause PPA. The three pathologies tend to affect different parts of the brain, so the subtype of PPA (which reflects what part of the brain is affected) allows one to predict the most likely pathology. Most autopsy studies have found that nfvPPA is most commonly associated with Corticobasal Degeneration or Frontotemporal Lobar Degeneration-tau (“tau-opathies”), while svPPA is most commonly associated with a different abnormal protein inclusion, ubiquitin (Kertesz et al., 2005; Davies et al., 2005; Snowden et al., 2007; Josephs et al., 2008). Logopenic variant primary progressive aphasia is most commonly associated with the pathology found in Alzheimer’s Disease (Gorno-Tempini et al., 2008; Rabinovici et al., 2008). These underlying conditions may respond to different medical interventions; therefore, identifying the pattern of language impairment may be useful for deciding on medical treatment. Because writing can be one of the earliest symptoms of language breakdown (particularly in svPPA), the distinct pattern of spelling impairment might predict the subsequent course of language deterioration. However, few studies have examined the breakdown of spelling in various types of PPA.
In this paper, we sought to identify which cognitive processes underlying spelling were impaired in 23 patients with PPA. Previous detailed analyses of spelling performance by patients with focal brain damage such as stroke, along with consideration of the computational demands of the task, have provided evidence for proposing that spelling requires a number of cognitive processes that can be independently disrupted by brain lesions. (Caramazza et al., 1987; Rapp, 2002; Beeson and Hillis, 2001). In brief, spelling can be accomplished either by recognizing the spoken word (phonological representation), accessing its meaning (lexical-semantic representation), and accessing the stored spelling of the word (orthographic representation) or through sublexical (phonology to orthography conversion) mechanisms. Accurate spelling may be accomplished through an interaction between these mechanisms, even if one or both are partially impaired (Hillis and Caramazza, 1991; Hillis and Caramazza, 1995; see also Patterson et al., 1994). Spelling of pseudowords (e.g., frunk) requires sublexical mechanisms, while spelling of irregular words may depend more on lexical and lexical-semantic representations (Rapp, 2002). Whether the spelling is accessed via lexical and semantic representations or assembled through sublexical mechanisms, the sequence of graphemes or abstract letter identities must be stored in working memory while the letters are written or spoken aloud (a mechanism known as the “graphemic buffer”(Caramazza et al., 1987; Posteraro et al., 1988; Hillis and Caramazza, 1989) (see Figure 1). Written naming requires the same cognitive processes as spelling to dictation of irregular words, except that recognition of the picture, rather than recognition of the spoken word, is required.
Figure 1. Schematic representation of the cognitive processes underlying spelling, with proposed disruptions that can account for each pattern of errors.
To identify the status of each of these cognitive processes underlying spelling in each patient and to determine whether or not there were differences across subtypes of PPA, we administered a battery of spelling tasks to 23 patients with PPA and analyzed their patterns of errors across tasks and stimuli.
2. Methods
2.1 Participants
Patients were diagnosed as having PPA, and the subtype was identified when possible on the basis of history, comprehensive neurological examination, brain MRI, brain SPECT or PET scan, and a battery of language tests. Language tests included: The Western Aphasia Battery (WAB, Kertesz, 1982); The Boston Naming Test (Goodglass et al., 1983); a test of oral and writing naming of nouns and verbs (Berndt et al., 1997); The Apraxia Battery for Adults (Dabul, 1979); telling of the Cinderella Story; and two tests of comprehension (sentence/picture matching and enactment of syntactically complex and syntactically simple, reversible and nonreversible sentences). Other cognitive tests included: forward and backward digit span; The Trail Making Test (Partington and Leiter, 1949); The Stroop Test (Trenerry et al., 1989); The Rey Auditory Verbal Learning Test (adapted from Rey, 1941); The Rey Complex Figure Test (Osterrieth, 1944); and The Mini Mental State Examination (Folstein et al., 1975). Patients were classified using criteria for each clinical subtype of PPA (nfvPPA,svPPAor lvPPA) developed by consensus of a large international group of PPA investigators (Gorno-Tempini et al., submitted; see addendum). Patients who did not meet criteria for any of these subtypes were unclassifiable; these patients had PPA with prominent word finding deficits and progressive spelling impairment but spared word comprehension, sentence repetition, and motor speech. All patients, or their spouses (in cases of impaired comprehension), provided informed consent for the study using forms and procedures approved by the Johns Hopkins Institutional Review Board.
2.2 Spelling Tests
The Johns Hopkins Dysgraphia Battery (Beeson and Hillis, 2001) was administered to all patients. This test includes written spelling to dictation of 326 words and 34 pseudowords, oral spelling to dictation of 42 words and 20 pseudowords, and delayed copy transcoding of 42 words and 20 pseudowords. Subtests evaluate for effects of word frequency, concreteness, word length, grammatical word class, lexicality (words vs. pseudowords), and regularity by controlling for the other variables. Significant effects of each variable were identified with chi square tests. Responses on all spelling to dictation tests were scored by error type: phonological plausible error (PPE; e.g., leopard-> lepperd), phonologically implausible nonword (PIN; e.g., leopard-> leoprand), semantically related word (e.g., leopard-> tiger), phonologically similar word (PSW; e.g., leopard-> shepherd), partial response (e.g., leopard-> leop), unrelated (e.g., leopard-> show), or mixed (e.g., leopard->leppand; leopard-> lyun). Patients were also administered a test of written naming of 30 actions (verbs) and 30 objects (nouns) matched for frequency, word length, and familiarity (Zingeser and Berndt, 1988, 1990). Lastly, they were asked to write a description of what was happening in the “cookie theft” picture of the Boston Diagnostic Aphasia Examination (Goodglass and Kaplan, 1972).
We used the spelling data to identify patterns of errors across tasks and stimuli, along with distribution of error types in spelling, that could be explained by proposing damage at the level of one or more components of the spelling process schematically depicted in Figure 1. The expected patterns that result from damage to particular components of the model are described in greater detail elsewhere (Rapp, 2002; Beeson & Hillis, 2001). The patients in this study often did not meet all expectations from damage to a particular component of the spelling system, particularly in terms of statistical significance of the effects of word length, lexicality, orthographic regularity, and so on (often because of insufficient power due to relatively low error rates). Each patient’s pattern of performance was examined to determine which component(s) seemed to be most impaired. That is, if a patient showed consistent trends in the expected directions for various expected effects from damage to a given component (e.g. graphemic buffer), we classified them as showing the pattern resulting from impairment at that level of the spelling process. There are limitations to this approach, because some of the trends could be due to chance, but it is much less likely that consistent trends in an expected direction are due to chance alone. Furthermore, we considered the distribution of error types along with the pattern of performance across stimuli, when identifying the most likely locus of impairment in the cognitive architecture of spelling.
2.3 MR data acquisition
A subset of 16 of the 23 participants also had a T1 volumetric MRI brain scan within 6 weeks of their behavioural testing. The remaining participants (n=6) either did not consent to have a MRI scan or were ineligible (for example, because they had a pacemaker). Focal gray and white matter density was estimated on the basis of T1-weighted anatomical whole brain images acquired with a Philips 3.0 Tesla Achieva magnetic resonance imaging (MRI) scanner with an 8 channel SENSE head coil. A T1-weighted three-dimensional (3D) MPRAGE (magnetization prepared rapid acquisition gradient echo) sequence was used to acquire 120 sagittal partitions with an image matrix of 256 × 256 yielding a final resolution of 1 mm3 [repetition time (TR)/echo time (TE)/flip angle (FA), 10/6/8 ms]. The same scanner parameters and scanner hardware were used for the acquisition of all anatomical volumes.
2.4 MR data processing and data analysis
The brain atrophy of each patient was automatically identified using a modified unified segmentation and an outlier detection algorithm using default parameters (for comprehensive details see procedure in Seghier et al., 2008). Firstly, the structural images were pre-processed with Statistical Parametric Mapping software (SPM5: Wellcome Trust Centre for Neuroimaging: http://www.fil.ion.ucl.ac.uk/spm) running under MATLAB 7.7 (MathWorks, Natick, MA, USA). Then the images were spatially normalized into standard Montreal Neurological Institute (MNI) space using a unified segmentation algorithm optimized for use in patients with focal brain lesions. The unified segmentation algorithm is a generative model that combines tissue segmentation, bias correction and spatial normalization in the inversion of a single unified model (Ashburner and Friston, 2005). This algorithm was developed to deal with normal subjects’ brains, but with patients (stroke, tumour and dementia diagnoses) it outperforms the previous ‘gold-standard’ of cost-function masking (Crinion et al., 2007). More recently, a modified version of the tissue segmentation component has been developed to further improve identification and spatial normalization of ‘brain’ as opposed to ‘non-brain’ components by adding in an extra tissue class, ‘lesion’, into which outlier voxels can be classified (Seghier et al., 2008). This method has been validated in the same heterogeneous group of patients as used by Crinion et al., 2007.
An outlier image was thus generated that coded the degree of abnormality of each voxel (i.e. how far the value at a given voxel is from the normal range of the 64 healthy controls used by Seghier et al. 2008). These images were then smoothed with an isotropic kernel of 8 mm at full-width half maximum to increase the chance that regional effects are expressed at a spatial scale in which homologies in structural anatomy are shared over subjects. Each image was then thresholded into a binary image and then all the binary images were overlapped (i.e. summed across subjects) to generate the maps illustrating the common patterns of brain atrophy. Therefore, the brain atrophy overlap map indicates the number of patients who have atrophy at any given voxel. Note that this procedure (overlapping binary outlier images) is used here only for illustrating the spatial distribution of brain atrophy over our patients. No statistical analyses were carried out on the images. We chose to employ the automated method (as opposed to manual segmentation of the images) for two major reasons. First, identifying our patients’ atrophy by hand/eye is difficult, subject to human error and dependent upon the subjective opinion of the classifier. Second, it is possible that there will be brain regions that suffer from volume loss of both gray and white matter. As opposed to VBM analyses that look for correlations between continuous gray or white matter values and a behavior this method assigns binary values to the combination of both gray and white matter outlier voxels, generating a single brain damage image for each patient and the overlap maps will be able to illustrate this. (see Figure 2).
Figure 2. Distribution of brain damage in our PPA patients.
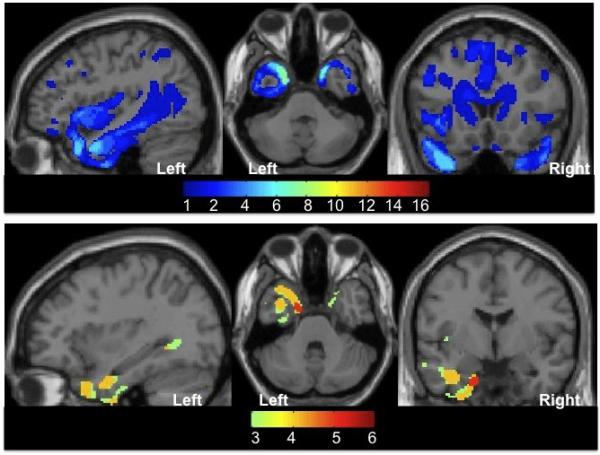
Images showing the spatial extent of damage at the group level. Sagittal, axial and coronal projections are displayed on a SPM5 single-subject brain T1 template. The range of the colour scale relates to the absolute number of patients with damage to any one brain voxel, where a minimum of one patient (panel A) or three patients (panel B) had damage according to the method described in Seghier et al. 2008. Panel A: Pattern of brain damage for our 16 PPA patients. The most common areas damaged were the left and right anterior temporal and left inferior frontal regions. This meant we had a good sampling of patients with damage across both hemispheres. Panel B: illustrates the pattern of brain damage for our six PPA patients with Pattern 1 spelling errors. All six patients had left temporal damage. In this group of patients the most common area damaged was the left anterior temporal with 5 out of 6 patients having damage to this region.
3. Results
We enrolled 3 patients with nfvPPA, 5 with svPPA, 9 with lvPPA, and 6 with unclassifiable PPA. There were no significant differences by ANOVA between subtypes of PPA in age or education. The mean age (and SD) in years was 67.0 (6.1) for svPPA, 74.3 (16.5) for nfvPPA, 64.3 (5.9) for lvPPA, and 69.2 (9.3) for unclassifiable PPA. The mean education in years was 16.8 (1.5) for svPPA, 16.3 (3.3) for nfvPPA, 16.4 (1.0) for lvPPA, and 15.8 (3.1) for unclassifiable PPA.
We identified four predominant patterns of spelling that could be explained by damage to one or more components of the spelling system as outlined above. All patients, like many neurologically unimpaired individuals, were more accurate in spelling high frequency than low frequency words. This effect was significant for all but one patient (GAR), who showed pattern 4 (impairment at the level of the graphemic buffer), as described below. Accuracy rates and distributions of error types for each patient are given in Tables 1 and 2.
Table 1.
Accuracy Rates as a Function of Lexical Variables
| Pattern 1: Impaired access to lexical representations with reliance on intact sublexical phonology-to-orthography conversion (POC) | ||||
|---|---|---|---|---|
| Patient (PPA Variant) | Words vs Pseudowords | High Prob vs Low Proba | Concrete vs. Abstract | 4-letter vs. 8-letter |
| BNR (LPA) | 56.0 vs. 61.8%; ns | 83.3 vs. 51.3%; p<0.005 | 66.7 vs. 42.9%; ns | 57.1 vs. 42.9%; ns |
| JRH (LPA) | 63.1 vs 91.2%; p<0.005 | 93.3 vs. 63.8%; p<0.005 | 52.4 vs. 57.1%; ns | 64.3 vs. 42.9%; ns |
| LLD (LPA) | 76.2 vs 88.2%; ns | 83.3 vs. 71.3%; ns | 71.4 vs. 81.0%; ns | 64.3 vs. 85.7%; ns |
| RPN (LPA) | 56.0 vs 91.2%; p<0.001 | 83.3 vs. 70%; ns | 47.6 vs. 38.1%; ns | DNCb |
| RLP (SD) | 9.5 vs 38.2%; p<0.001 | 10 vs. 10%; ns | 0 vs. 14%; ns | 0 vs. 0%; ns |
| RMR (SD) | 73.8 vs. 58.8%; ns | 100 vs. 82.5%; p<0.015 | 76.2 vs. 52.4%; ns | 78.6 vs. 42.9%; p<0.04 |
| JAN (UN)c | 70.2 vs. DNC | DNC | 95.2 vs. 61.9%; p<0.01 | DNC |
| MJE (UN) | 72.6 vs. 82.4%; ns | 83.3 vs. 67.5%; ns | 66.7 vs. 71.4%; ns | 64.3 vs. 64.3%; ns |
| Pattern 2: Impaired access to lexical representations and complete disruption of sublexical POC mechanisms | ||||
|---|---|---|---|---|
| Patient (PPA Variant) | Words vs. Pseudowords | High Prob vs. Low Prob | Concrete vs. Abstract | 4-letter vs. 8-letter |
| VBN (PNFA) | 51.2 vs. 0%; p<0.05 | 43.3 vs. 47.5%; ns | 76.2 vs. 47.6%; ns | 50.0 vs. 57.1%; ns |
| Pattern 3: Impaired access to lexical-semantic representations and/or lexical representations with partially spared sublexical POC mechanisms | ||||
|---|---|---|---|---|
| Patient (PPA Variant) | Words vs Pseudowords | High Prob vs. Low Prob | Concrete vs. Abstract | 4-letter vs. 8-letter |
| FHY (LPA) | 90 vs 61.8%; p<0.001 | 93.3 vs. 88.8%; ns | 95.2 vs. 81.0%; ns | 100 vs. 78.6; ns |
| FSE (LPA) | 65.5 vs 61.8%; ns | 80.0 vs. 83.8%; ns | 90.5 vs. 52.4%; p<0.01 | 85.7 vs. 92.9%; ns |
| JBH (LPA) | 94.0 vs DNC | 86.7 vs. 96.3%; ns | 100 vs. 81.0%; ns | 100 vs. 100%; ns |
| SRR (LPA) | 66.7 vs 26.5%; p<0.001 | 60 vs. 70%; ns | 100 vs. 76.2%; ns | 85.6 vs. 92.9%; ns |
| TEY (SD) | 78.6 vs 73.5%; ns | 73.3 vs. 80%; ns | 90.5 vs. 90.5%; ns | 64.3 vs. 78.6%; ns |
| EMY (UN) | 90.5 vs DNC | DNC | 100 vs. 95%; ns | 100 vs. 92.9%; ns |
| MRN (UN) | 96.4 vs 85.3%; p<0.05 | 93.3 vs. 95%; ns | 100 vs. 95.2%; ns | 100 vs. 100%; ns |
| Pattern 4: Impairment at the level of the graphemic buffer | ||||
|---|---|---|---|---|
| Patient (PPA Variant) | Words vs Pseudowords | High Prob vs. Low Prob | Concrete vs. Abstract | 4-letter vs. 8-letter |
| BKK (SD) | 34.5 vs 47.1%; ns | 63.3 vs. 51.3%; ns | 33.3 vs. 19%; ns | 50 vs. 21.4%; ns |
| GAR (LPA) | 67.9 vs 70.6%; ns | DNC | 52.4 vs. 57.1%; ns | DNC |
| ENN (SD) | 75.0 vs DNC | DNC | 76.2 vs. 76.2%; ns | 85.6 vs. 71.4%; ns |
| DNN (PNFA) | 77.4 vs DNC | 93.3 vs. 81.3%; ns | 76.2 vs. 47.6%; ns | 85.7 vs. 35.7%; p<0.01 |
| LBY (UN) | 28.1 vs 44%; ns | DNC | DNC | DNC |
high prob vs low prob= high probability of correct spelling by applying the most frequent phonology-orthography correspondences
DNC= did not complete testing
UN= unclassifiable
Table 2.
Distribution of Error Types
| Pattern 1: Impaired access to lexical representations, with reliance on intact sublexical phonology-to-orthography conversion (POC) | |||
|---|---|---|---|
| Patient (PPA Variant) | % PPE | % PIN | % PSW |
| BNR (LPA) | 60.7% | 29.0% | 7.1% |
| JRH (LPA) | 92.3% | 4.9% | 0.7% |
| LLD (LPA) | 77.6% | 16.3% | 1.0% |
| RPN (LPA) | 73.3% | 22.5% | 3.3% |
| RLP (SD) | 38.0% | 55.7% | 2.8% |
| RMR (SD) | 41.3% | 47.7% | 5.5% |
| JAN (UN) | 63.8% | 12.5% | 4.3% |
| MJE (UN) | 79.3% | 18.2% | 0.0% |
| Pattern 2: Impaired access to lexical representations and complete disruption of sublexical POC mechanisms | |||
|---|---|---|---|
| Patient (PPA Variant) | % PPE | % PIN | % PSW |
| VBN (PNFA) | 1.6% | 10.3% | 21.1% |
| Pattern 3: Impaired access to lexical-semantic representations and/or lexical representations with partially spared sublexical POC mechanisms | |||
|---|---|---|---|
| Patient (PPA Variant) | % PPE | % PIN | % PSW |
| FHY (LPA) | 46.2% | 23.1% | 23.1% |
| FSE (LPA) | 15.2% | 44.3% | 13.9% |
| JBH (LPA) | 11.8% | 29.4% | 47.1% |
| SRR (LPA) | 20.2% | 54.0% | 17.8% |
| TEY (SD) | 21.3% | 24.7% | 39.3% |
| EMY (UN) | 30.0% | 20.0% | 20.0% |
| MRN (UN) | 8.0% | 32.0% | 36.0% |
| Pattern 4: Impairment at the level of the graphemic buffer | |||
|---|---|---|---|
| Patient (PPA Variant) | % PPE | % PIN | % PSW |
| BKK (SD) | 17.9% | 63.5% | 11.1% |
| GAR (LPA) | 46.9% | 51.9% | 0.0% |
| ENN (SD) | 10.6% | 74.5% | 4.3% |
| DNN (PNFA) | 14.5% | 73.5% | 6.0% |
| LBY (UN) | 12.5% | 70.3% | 7.8% |
3.1 Pattern 1: Impaired access to lexical representations with reliance on intact sublexical phonology-to-orthography conversion
We identified a general pattern of performance that could best be explicated by proposing relatively selective damage at the level of accessing orthographic lexical representations for output from the semantic representation or damage to the semantic or orthographic lexical representations themselves (see Figure 1). This pattern was characterized by a predominance of phonologically plausible errors (PPEs), effect of orthographic probability (often referred to as “regularity”; correct spelling by applying the most common phonology to orthography mappings, with high probability words more accurate than low probability words), and effect of lexicality (with pseudowords spelled more accurately than words, see Table 1 for evidence). This pattern is sometimes referred to as surface dysgraphia. A total of 8 patients showed this general pattern with all of the trends listed above; 3/9 showed significant effects of orthographic probability (regularity) and 3/9 showed significant effects of lexicality, and 7/9 produced PPEs for more than 60% of their errors (at least one of these was significant in all patients). However, all patients showed trends in the expected direction if we assume reliance on POC mechanisms. Moreover, even their PIN’s were at “almost” phonologically plausible (e.g. palace-> ballice; sincere-> synschere), and many of their visually/phonologically similar word errors were also broadly phonologically plausible (e.g. pierce-> pears). Furthermore, the majority of the PPE’s could not be explained easily except by proposing reliance on sublexical POC mechanisms (e.g., sparrow-> spero; into-> entue; sought-> sawt; ruin-> rewen; machine-> misheen; pity-> pittie; leopard-> lepeard; knife-> niphe; vague-> vaige; jerk-> jurck; phase-> fayze, all of which were errors by a patient with a doctoral degree who was premordidly an excellent speller by all reports). One patient (JAN) showed significant effects of concreteness, with concrete words spelled more accurately than abstract words, indicating some impairment in accessing lexical or lexical-semantic representations as well (Hillis et al., 1999). None of these patients showed a significant effect of word length. Because they had spared word comprehension, their deficit appeared to be due to impaired access to lexical orthographic representations, rather than impaired lexical-semantics.
Three of these patients (JAN, MJE, JGD) had unclassifiable PPA with spared motor speech, sentence repetition, and word comprehension. Four (BNR, JRH, LLD, RPN) were diagnosed with lvPPA, and the other two patients (RLP, RMR) had svPPA. Interestingly, both of these svPPA patients exhibited a spelling pattern that varied slightly from the general Pattern 1. Though RLP had a significant effect of lexicality and RMR had a significant effect of regularity, and both patients produced some unambiguous PPEs (e.g., sauce->sos; effort-> efarte; cattle-> catole; afraid-> afrade; grief-> grefe; faith-> fathe; carry-> carie; greet-> greate by RLP; college-> colig; sister-> syster; though-> thow; speak-> speek by RMR) indicating use of POC mechanisms in spelling, under half (38.0% and 40.4%, respectively) of their errors were PPEs due to a large number of PIN responses (55.7% and 47.7%, respectively) which were “mostly” phonologically plausible (e.g. decent-> deasone by RLP; pierce-> pearch by RMR). Since this variation is associated with a different PPA subtype than most of the other patients with this spelling pattern, it is likely that these patients have a different distribution of brain atrophy than is seen in the lvPPA patients with this pattern of spelling. Alternatively, it is also possible that these two patients were in a more advanced stage of the disease and that the others will produce more PINs over time.
3.2 Pattern 2: Impaired access to lexical representations and complete disruption of sublexical phonology-to-orthography conversion (POC) mechanisms
We identified a pattern that could best be explicated by damage to POC mechanisms, along with some impairment in access to lexical orthographic representations for output. This pattern was characterized by the absence of PPEs and the inability to spell pseudowords. Errors on words and pseudowords consisted of omissions, unrelated words, phonologically similar words, semantically related words, and PINs. These patients had spared word comprehension on the WAB, indicating that their problem was in accessing lexical representations rather than in lexical-semantic representations, and they spelled nouns better than verbs in written naming. These patients (VBN and SBN) had nfvPPA. The performance of SBN is not shown in the Table 1, because he had no correct responses on the dysgraphia battery (although he correctly spelled names of some pictured objects (but not actions) on previous testing. His distribution of 3 error types is given in Table 2.
3.3 Pattern 3: Impaired access to lexical-semantic representations and/or lexical representations with partially spared sublexical POC mechanisms
We identified a general pattern of performance that could be explained by assuming BOTH impaired access to lexical orthographic representations from semantics and impaired POC mechanisms. These patients seemed to rely on partial information from POC mechanisms, and partial information from semantics to attempt to access orthographic representations for output. This pattern was characterized by a predominance of phonologically related word errors (e.g., cheap->sheep), with some PPEs, some mixed errors that seemed like attempts at using POC (e.g., grief->greeve, jury->jeror), some PINs, and some unrelated words. Nonword errors were predominantly lexicalizations or phonologically similar words with some PINs. This pattern was observed in one patient who had svPPA (TEY, with poor word comprehension), four patients with lvPPA (FSE, JBH, FHY, and SRR with good word comprehension but poor sentence repetition), and two patients with unclassifiable PPA (MRN, EMY, with good repetition, word comprehension, and fluency). These patients also performed better on words than nonwords, which is the opposite pattern of that seen in Pattern 1.
3.4 Pattern 4: Impairment at the level of the graphemic buffer
We identified a pattern of performance that is best accounted for by assuming relatively selective damage at the level of the graphemic buffer. This pattern was characterized by mostly PINs and an effect of word length, with short words spelled more accurately than long words. There were similar error rates and types on all spelling tasks (oral and written spelling to dictation, written naming, written narrative).
This pattern was seen in one patient with nfvPPA (DNN, whose speech was characterized by agrammatic sentence production and apraxia of speech, with spared word comprehension), two patients with svPPA (BKK and ENN), one patient with lvPPA (GAR), and one patient with unclassifiable PPA (LBY). These patients showed no significant effects of regularity, lexicality, or concreteness on spelling accuracy.
These four patterns of spelling errors, and the data from each patient are summarized in Tables 1 and 2. Note that the letter codes for patients in the tables and text do not correspond to patients’ real initials.
3.5 Longitudinal Evaluation
Nine patients were at least partially evaluated twice, in sessions that ranged from 2 to 10 months apart. Three patients (RLP, FHY, and FSE) maintained relatively constant performance over time, but for each of these patients the interval between testing sessions was less than four months. The rest of the patients (GAR, LBY, VBN, TEY, LLD, and SRR) showed a marked increase in overall spelling errors in the second testing session. As PPA is a progressive disorder, it is unsurprising that performance tends to worsen over time.
However, despite the worsening of overall performance, the pattern of errors observed for each patient almost always remained constant over time. Only two patients (SRR, LBY) were reclassified into different spelling patterns after a second testing session, but in both cases the initial testing results were ambiguous, as described below. Both patients showed a decline in the percentage of PPEs over time.
The majority (54.1%) of SRR’s errors in the first testing session were PPEs with only 5.3% PSW, which made her spelling errors most consistent with Pattern 1.
Unfortunately, SRR did not complete enough testing at this session to calculate whether she had effects of regularity or lexicality at this time. When she was tested again 10 months later, her errors were 21.5% PPEs and 17.8% PSWs, which is more consistent with Pattern 3. In this session, her performance on words was significantly better (p<0.01) than on nonwords, and there was no effect of regularity. An abbreviated testing session that occurred 6 months after the original session was also consistent with Pattern 3 and showed no effect of regularity. In short, SRR first showed impaired access to lexical representations with reliance on intact sublexical phonology-to-orthography conversion (POC), and later showed deterioration on POC (and relied on information from partially spared POC and partially spared lexical-semantic information to access lexical representations for spelling).
In LBY’s initial testing session, 29.9% of errors were PPEs, 16.9% were PSWs, and 48.1% were PINs with no effect of word length, which was most consistent with Pattern 3. However, during a testing session 10 months later, her errors were 12.5% PPEs, 70.3% PINs, and 7.8% PSWs with no effect of lexicality, which is most consistent with Pattern 4. It is likely that more than one component of LBY’s spelling system was affected (e.g., impaired lexical access, partially impaired POC, and impaired graphemic buffer at least by the later testing).
Of the remaining seven patients, only two showed any notable change in error patterns. FSE, who has lvPPA and is classified as Pattern 3, demonstrated a gradual increase in phonologically similar word errors from 1.3% to 25% over a span of approximately 10 months. This change may represent a disproportionate worsening of her phonological input processing. TEY, who has svPPA and is classified as Pattern 3, showed an increase in PPEs between testing sessions, which may be due to a worsening deficit in her lexical-semantic system which allowed her sublexical system to contribute to the response in a greater percentage of trials.
The other five patients not discussed in the previous paragraphs experienced no significant changes in error type pattern between testing sessions, even 10 months apart, depite overall increase in errors.
3.6 Imaging
Figure 2A illustrates the distribution of brain atrophy in our sample of 16 patients with PPA. Figure 2B illustrates the distribution of atrophy in the six patients with Pattern 1 spelling errors. Of note, 5/6 patients with Pattern 1 spelling errors had left anterior temporal damage suggesting that atrophy in this region may be associated with PPE errors. There was no region of atrophy overlap in each of the other groups of patients, when thresholded such that a minimum of three patients had damage according to the method described in Seghier et al. (Seghier et al., 2008).
4. Discussion
Detailed analysis of spelling performance across tasks and stimuli demonstrates that any of the cognitive processes underlying spelling can be affected in PPA. Although there were some differences in the patterns of spelling across PPA subtypes discussed below, there was not a tight relationship between the PPA variant and the component of the spelling process that was impaired.
Patients with lvPPA showed relatively spared sublexical POC mechanisms and graphemic buffer, at least early in the course of their condition. Only one patient with lvPPA showed a predominance of PINs or a significant word length effect, two of the prominent features of damage to the graphemic buffer, despite the fact that impaired (phonological) working memory is thought to underlie lvPPA. This finding indicates a possible dissociation between phonological working memory (impaired) and an orthographic working memory system, the graphemic buffer(spared), in some cases of lvPPA. All of the patients with lvPPA, but none of the patients with nfvPPA, showed a substantial number of PPEs. Thus, despite limited working memory that interferes with sentence repetition, they might be able to rely on a phonological buffer to hold the sequence of phonemes while each is converted to a grapheme in spelling to dictation.
Patients with svPPA did not cluster into any of our spelling pattern types, but it is interesting to note that four out of the five svPPA patients we tested produced a large percentage of PINs (approximately 50% or greater). We expected to find that many patients with svPPA would make a predominance of PPEs, because they frequently make PPEs in reading (i.e., show surface dyslexia (Patterson et al., 1994; Noble et al., 2000)). However, we did not confirm this expectation, indicating that patients with svPPA might have a deficit at the level of the graphemic buffer, superimposed on their semantic deficits, or that PPEs break down as sublexical mechanisms deteriorate. However, we did not see a reduction in PPEs over time in patients with svPPA who were studied longitudinally. Another possible explanation is that these PINs represented partial lexical representations because only one of the svPPA patients showed a significant effect of word length.
Patients with nfvPPA produced few or no PPEs in spelling to dictation or narrative writing. In two cases, errors were predominantly omissions or unrelated word responses indicating poor access to orthographic (or phonological) representations and severely disrupted POC mechanisms that would otherwise help compensate for poor lexical access. The other patient with nfvPPA made mostly PINs in all spelling tasks and showed a marked word length effect on spelling accuracy in all spelling tasks, consistent with damage to the graphemic buffer.
The majority (5/7) of unclassifiable patients, who had mostly anomic speech and impaired spelling, showed a marked predominance of PPEs (along with higher accuracy on “regular” words and pseudowords), indicating that this pattern might be seen early in PPA, before the appearance of speech and language characteristics that distinguish subtypes. Whether or not this early pattern predicts eventual PPA variant is a matter for future studies.
One patient with svPPA, three with lvPPA, and one with unclassifiable PPA showed a predominance of PSW, along with some PPEs, mixed errors, and morphological errors. They also showed better performance on words than nonwords and poor repetition and/or comprehension, consistent with damage to the phonological loop and/or phonological input lexicon. We hypothesize that they utilize the damaged input lexicon or partially preserved POC mechanisms to access orthographic representations for output (resulting in PSW) or partially preserved lexical and lexical-semantic representations plus partially preserved POC mechanisms to access or assemble responses (resulting in morphological errors and mixed errors, see Hillis, Rapp & Caramazza, 1999 for discussion).
The failure to confirm our hypotheses about the level of deficit in the spelling process that would be likely be affected in each subtype of PPA (based on studies of stroke patients with dysgraphia) was sobering. Similar sobering results were reported from a study of oral reading in dementia, in which it was found that semantic deficits in patients with Alzheimer’s Disease, Frontotemporal Dementia, and Progressive Non-Fluent Aphasia did not invariably lead to the disruption of the orthographic and phonological lexicons as expected from previous studies (Noble, et al., 2000).In an earlier study, patients with probable Alzheimer’s disease showed a progressive deterioration in spelling pseudowords over the course of several months (Hillis et al., 1995). Although Alzheimer’s disease is associated with bilateral temporoparietal atrophy, while lvPPA is associated with predominantly left temporoparietal atrophy, both are usually associated with the Alzheimer-type pathology (neurofibrillary tangles and amyloid plaques) (Josephs et al., 2008; Gorno-Tempini et al., 2008; Rabinovici et al., 2008), suggesting that these clinical syndromes are different manifestations of the same disease. Deterioration in pseudoword spelling is likely to be a reflection of the left temporoparietal atrophy because the right hemisphere likely has little role in POC mechanisms (Coltheart, 1980).
Other longitudinal studies of frontotemporal dementia (or frontotemporal lobar degeneration) and Alzheimer’s disease have not included the study of spelling. These longitudinal studies have shown that patients with frontotemporal lobar degeneration (including behavioral variant FTD as well as the semantic and nonfluent variants of PPA) show faster decline in language (Blair et al., 2007) and faster decline on all subscales of the Mattis Dementia Rating Scale (Rascovsky et al., 2008) compared to patients with Alzheimer’s disease. In another large longitudinal study of 441 patients diagnosed with Alzheimer’s disease or one of four subtypes of frontotemporal lobar degeneration (including those referred to here as the nonfluent and semantic variants of PPA and behavioral variant FTD, and also corticobasal degeneration), distinct patterns of language and cognitive impairments were maintained longitudinally rather than converging on a single pattern (Libon et al., 2009). It was hypothesized that the persistently divergent patterns reflect the unique anatomic distributions of disease burden in AD and frontotemporal dementia.
Most of our patients demonstrated a pattern of spelling errors that is consistent with damage to one or two components of the spelling process. However, as we would expect with a neurodegenerative disorder that causes somewhat diffuse cortical damage, some patients exhibited spelling patterns that can best be explained by damage to more than two cognitive processes. For example, LBY’s errors consisted of primarily PINs, suggesting that the primary source of her problem is damage to the graphemic buffer, but her nonword performance was qualitatively better than her performance with words, suggesting that she also has a coexisting lexical deficit. Interestingly, these co-deficits sometimes occur in more than one patient. For example, both RMR and RLP had a significant number of PIN responses and likely have graphemic buffer impairment along with their lexical deficits. Both FSE and TEY showed better performance with oral than written spelling and some orthographically similar word errors, which suggests the possibility of a mild letter shape selection deficit superimposed on impaired POC mechanisms. If these co-deficits are seen in additional patients in the future, they may provide clues regarding the localization of neural correlates of cognitive spelling processes, relative to one another.
The only apparent relationship we found between spelling error pattern and regional atrophy was that 5/6 patients with Pattern 1 spelling errors had left anterior temporal damage suggesting that atrophy in this region may be associated with PPE errors. It is an area of atrophy commonly associated with svPPA, and Pattern 1 was one of the patterns observed in svPPA. In fact, we expected a stronger relationship between Pattern 1 and svPPA. Further studies, for example using VBM analyses, are necessary to statistically address this interesting question. Because there is substantial white matter disease in the proteinopathies that cause PPA, disease in white matter fasciculi also might interfere with large-scale neural networks that support these distributed spelling processes, so that longitudinal diffusion tensor imaging might also provide important information.
When our data are considered together with studies that show prominent atrophy in posterior frontal cortex and insula in patients with nfvPPA, our results indicate that POC mechanisms and the graphemic buffer depend at least in part on these regions. Recent functional imaging and lesion studies provide evidence that both of these cognitive processes are distributed systems that rely on several brain regions including posterior frontal cortex (Rapp and Hsieh; Rapcsak and Beeson, 2004; Philipose et al., 2007; Cloutman et al., 2009). The appearance of PINs, PSWs, and unrelated word errors in patients with evidence of lvPPA and svPPA is consistent with the hypothesis that the graphemic buffer and POC mechanisms are eventually affected by more posterior atrophy. When there are many types of errors and effects of many variables, as seen in some cases of lvPPA and svPPA, it is impossible to determine whether the graphemic buffer, lexical representation, POC mechanisms, or some combination, are affected. These components of the spelling system are likely to interact, such that damage to the system can affect operation of several components (Rapp et al., 2002).
In short, dysgraphia can be an early sign of PPA. Predominance of PPEs, more accurate spelling of regular words than irregular words, and more accurate spelling of pseudowords than words (indicating spared POC mechanisms) may indicate a low probability of progression to nfvPPA. Additional longitudinal studies are necessary to test this hypothesis.
Acknowledgements
This research was supported by NIH (NIDCD), RO1 DC 05375 to AH and a MRC, UK grant to JC. We gratefully acknowledge this support and the cheerful participation of the patients. We are also grateful to Dr. Brenda Rapp and Dr. Simon Fischer-Baum for helpful discussion of the spelling performance of our patients, and to two anonymous reviewers for helpful suggestions in revising the paper.
Addendum. Criteria for Subtypes of Primary Progressive Aphasia (from Gorno-Tempini et al, submitted)
|
Clinical Diagnosis of svPPA: |
| Both of the following core features must be present: |
| 1. Poor confrontation naming (of pictures or objects), particularly for low familiarity or low frequency items |
| 2. Impaired single word comprehension |
| At least three of the following other diagnostic features must be present: |
| 1. Poor object and/or person knowledge, particularly for low frequency or low familiarity items |
| 2. Surface dyslexia and/or dysgraphia |
| Clinical Diagnosis of nfvPPA |
| At least one of the following core features must be present: |
| 1. Grammatical errors and simplification in language production |
| 2. Effortful, halting speech with inconsistent distortions, deletions, substitutions, insertions, or transpositions of speech sounds, particularly in polysyllabic words (often considered to reflect “apraxia of speech”) |
| At least two of three of the following other features must be present: |
| 1. Impaired comprehension of syntactically complex sentences, with relatively spared comprehension of syntactically simpler sentences |
| 2. Spared content, single word comprehension |
| 3. Spared object knowledge |
| 3. Spared single word comprehension |
| 4. Spared object knowledge |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Beeson PM, Hillis AE. Comprehension and production of written words. In: Chapey R, editor. Language intervention strategies in adult aphasia. Lippencott, Williams & Wilkins; Baltimore: 2001. pp. 572–595. [Google Scholar]
- Berndt RS, Mitchum CC, Haendiges AN, Sandson J. Verb retrieval in aphasia. 1. Characterizing single word impairments. Brain and Language. 1997;56:68–106. doi: 10.1006/brln.1997.1727. [DOI] [PubMed] [Google Scholar]
- Blair M, Marczinski CA, Davis-Faroque N, Kertesz A. A longitudinal study of language decline in Alzheimer’s disease and frontotemporal dementia. Journal of the International Neuropsychological Society. 2007;13:237–245. doi: 10.1017/S1355617707070269. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Miceli G, Villa G, Romani C. The role of the Graphemic Buffer in spelling: evidence from a case of acquired dysgraphia. Cognition. 1987;26:59–85. doi: 10.1016/0010-0277(87)90014-x. [DOI] [PubMed] [Google Scholar]
- Cloutman L, Gingis L, Newhart M, Davis C, Heidler-Gary J, Crinion J, Hillis AE. A neural network critical for spelling. Annals of Neurology. 2009 doi: 10.1002/ana.21693. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltheart M. Deep dyslexia: A right hemisphere hypothesis. In: Coltheart M, Patteron KE, Marshall JC, editors. Deep Dyslexia. Routledge and Kegan Paul; London: 1980. pp. 326–380. [Google Scholar]
- Dabul B. The Apraxia Battery for Adults. Pro-Ed; Austin: 1979. [Google Scholar]
- Davies RR, Hodges JR, Kril JJ, Patterson K, Halliday GM, Xuereb JH. The pathological basis of semantic dementia. Brain: a journal of neurology. 2005;128:1984–1995. doi: 10.1093/brain/awh582. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. The assessment of aphasia and related disorders. Lea & Febiger; Philadelphia: 1972. [Google Scholar]
- Goodglass H, Kaplan E, Weintraub S. The Revised Boston Naming Test. Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, Perani D, Garibotto V, Cappa SF, Miller BL. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71:1227–1234. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL. Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology. 2004;55:335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa S, Ogar J, Rohrer J, Black S, Boeve B, Manes F, Dronkers N, Vandenberghe R, Rascovsky K, Patterson K, Miller B, Knopman D, Hodges JR, Mesulam M, Grossman M. International guidelines for the diagnosis of primary progressive aphasia and its variants. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham K, Patterson K, Hodges JR. Progressive pure anomia: Insufficient activation of phonology by meaning. Neurocase. 1995;1:25–38. [Google Scholar]
- Hillis AE. Lost for words. Neurology. 2008;71:1218–1219. doi: 10.1212/01.wnl.0000327100.41096.b3. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Caramazza A. Converging evidence for the interaction of semantic and sublexical phonological information in accessing lexical representations for spoken output. Cognitive Neuropsychology. 1995;12:187–227. [Google Scholar]
- Hillis AE, Caramazza A. Mechanisms for accessing lexical representations for output: evidence from a category-specific semantic deficit. Brain and language. 1991;40:106–144. doi: 10.1016/0093-934x(91)90119-l. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Caramazza A. The graphemic buffer and attentional mechanisms. Brain and language. 1989;36:208–235. doi: 10.1016/0093-934x(89)90062-x. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Oh S, Ken L. Deterioration of naming nouns versus verbs in primary progressive aphasia. Annals of Neurology. 2004;55:268–275. doi: 10.1002/ana.10812. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Rapp BC, Caramazza A. When a rose is a rose in speech but a tulip in writing. Cortex; a journal devoted to the study of the nervous system and behavior. 1999;35:337–356. doi: 10.1016/s0010-9452(08)70804-9. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Tuffiash E, Caramazza A. Modality-specific deterioration in naming verbs in nonfluent primary progressive aphasia. Journal of cognitive neuroscience. 2002;14:1099–1108. doi: 10.1162/089892902320474544. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Benzing L, Epstein C, Lyketsos C. Cognitive Gains and Losses of Patients With Alzheimer’s Disease During Frequent Practice. American Journal of Speech-Language Pathology. 1995;4:152–158. [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain: a journal of neurology. 1992;115(Pt 6):1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Holland AL, McBurney DH, Moossy J, Reinmuth OM. The dissolution of language in Pick’s disease with neurofibrillary tangles: a case study. Brain and language. 1985;24:36–58. doi: 10.1016/0093-934x(85)90096-3. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Duffy JR, Vanvoorst WA, Strand EA, Hu WT, Boeve BF, Graff-Radford NR, Parisi JE, Knopman DS, Dickson DW, Jack CR, Jr, Petersen RC. Progressive aphasia secondary to Alzheimer disease vs FTLD pathology. Neurology. 2008;70:25–34. doi: 10.1212/01.wnl.0000287073.12737.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A. The Western Aphasia Battery. Grune & Stratton; New York: 1982. [Google Scholar]
- Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain. 2005;128:1996–2005. doi: 10.1093/brain/awh598. [DOI] [PubMed] [Google Scholar]
- Libon DJ, Xie SX, Wang X, Massimo L, Moore P, Vesely L, Khan A, Chatterjee A, Coslett HB, Hurtig HI, Liang TW, Grossman M. Neuropsychological decline in frontotemporal lobar degeneration: a longitudinal analysis. Neuropsychology. 2009;23:337–346. doi: 10.1037/a0014995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, Weintraub S, Bigio EH. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Annals of Neurology. 2008;63:709–719. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia--a language-based dementia. The New England Journal of Medicine. 2003;349:1535–1542. doi: 10.1056/NEJMra022435. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Annals of Neurology. 2000;47:36–45. [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Noble K, Glosser G, Grossman M. Oral reading in dementia. Brain and Language. 2000;74:48–69. doi: 10.1006/brln.2000.2330. [DOI] [PubMed] [Google Scholar]
- Osterrieth G. Le test de copie d’une figure complex. Archives de Psychologie. 1944;30:206–356. [Google Scholar]
- Partington J, Leiter R. Partington’s Pathway Test. The Psychological Service Center Bulletin. 1949;1:9–20. [Google Scholar]
- Patterson K, Graham N, Hodges JR. The Impact of Semantic Memory Loss on Phonological Representations. Journal of cognitive neuroscience. 1994;6:57–69. doi: 10.1162/jocn.1994.6.1.57. [DOI] [PubMed] [Google Scholar]
- Philipose LE, Gottesman RF, Newhart M, Kleinman JT, Herskovits EH, Pawlak MA, Marsh EB, Davis C, Heidler-Gary J, Hillis AE. Neural regions essential for reading and spelling of words and pseudowords. Annals of Neurology. 2007;62:481–492. doi: 10.1002/ana.21182. [DOI] [PubMed] [Google Scholar]
- Posteraro L, Zinelli P, Mazzucchi A. Selective impairment of the graphemic buffer in acquired dysgraphia: a case study. Brain and language. 1988;35:274–286. doi: 10.1016/0093-934x(88)90112-5. [DOI] [PubMed] [Google Scholar]
- Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, O’Neil JP, Lal RA, Dronkers NF, Miller BL, Gorno-Tempini ML. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Annals of Neurology. 2008;64:388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapcsak SZ, Beeson PM. The role of left posterior inferior temporal cortex in spelling. Neurology. 2004;62:2221–2229. doi: 10.1212/01.wnl.0000130169.60752.c5. [DOI] [PubMed] [Google Scholar]
- Rapp B. Uncovering the cognitive architecture of spelling. In: Hillis AE, editor. Handbook on adult language disorders: Integrating cognitive neuropsychology, neurology, and rehabilitation. Psychology Press; Philadelphia: 2002. pp. 47–70. [Google Scholar]
- Rapp B, Epstein C, Tainturier MJ. The integration of information across lexical and sublexical processes in spelling. Cognitive Neuropsychology. 2002;19:1–29. doi: 10.1080/0264329014300060. [DOI] [PubMed] [Google Scholar]
- Rapp B, Hsieh L. Functional magnetic resonance imaging of the cognitive components of the spelling process. [Google Scholar]
- Rascovsky K, Salmon DP, Hansen LA, Galasko D. Distinct cognitive profiles and rates of decline on the Mattis Dementia Rating Scale in autopsy-confirmed frontotemporal dementia and Alzheimer’s disease. Journal of the International Neuropsychological Society. 2008;14:373–383. doi: 10.1017/S135561770808051X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. L’examen psychologique dans les cas d’encephalopathie tramatique. Archives de Psychologie. 1941;28:286–340. [Google Scholar]
- Rhee J, Antiquena P, Grossman M. Verb comprehension in frontotemporal degeneration: the role of grammatical, semantic and executive components. Neurocase: case studies in neuropsychology, neuropsychiatry, and behavioural neurology. 2001;7:173–184. doi: 10.1093/neucas/7.2.173. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Ramlackhansingh A, Crinion J, Leff AP, Price CJ. Lesion identification using unified segmentation-normalisation models and fuzzy clustering. NeuroImage. 2008;41:1253–1266. doi: 10.1016/j.neuroimage.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden J, Neary D, Mann D. Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathologica. 2007;114:31–38. doi: 10.1007/s00401-007-0236-3. [DOI] [PubMed] [Google Scholar]
- Trenerry M, Crosson B, DeBoe J, Leber W. Stroop Neuropsychological Screening Test manual. Psychological Assessment Resources; Odessa: 1989. [Google Scholar]
- Zingeser LB, Berndt RS. Retrieval of nouns and verbs in agrammatism and anomia. Brain and language. 1990;39:14–32. doi: 10.1016/0093-934x(90)90002-x. [DOI] [PubMed] [Google Scholar]
- Zingeser LB, Berndt RS. Grammatical class and context effects in a case of pure anomia: Implications for models of language production. Cognitive Neuropyschology. 1988;5:473–516. [Google Scholar]



