Abstract
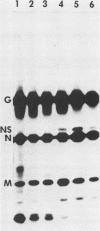
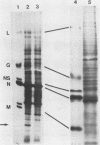
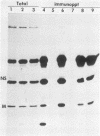
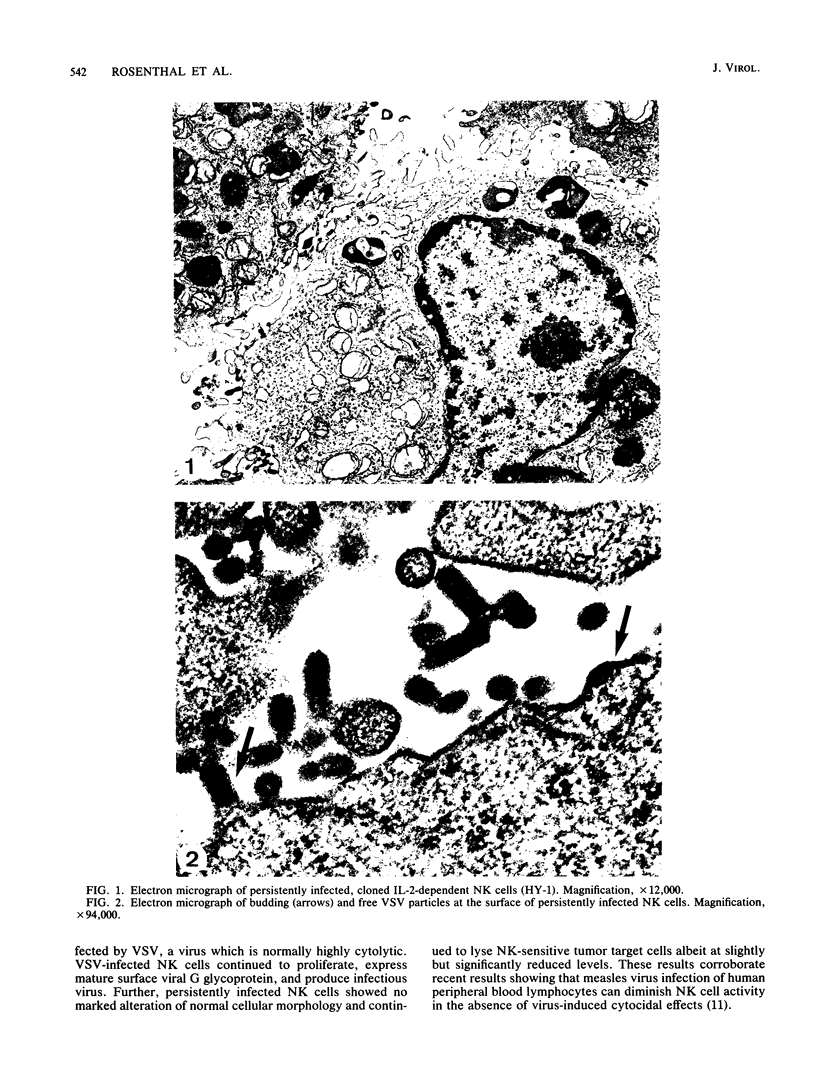
We have investigated virus-lymphocyte interactions by using cloned subpopulations of interleukin-2-dependent effector lymphocytes maintained in vitro. Cloned lines of H-2-restricted hapten- or virus-specific cytotoxic T lymphocytes (CTL) and alloantigen-specific CTL were resistant to productive infection by vesicular stomatitis virus (VSV). In contrast, cloned lines of natural killer (NK) cells were readily and persistently infected by VSV, a virus which is normally highly cytolytic. VSV-infected NK cells continued to proliferate, express viral surface antigen, and produce infectious virus. Furthermore, persistently infected NK cells showed no marked alteration of normal cellular morphology and continued to lyse NK-sensitive target cells albeit at a slightly but significantly reduced level. The persistence of VSV in NK cells did not appear to be caused by the generation of temperature-sensitive viral mutants, defective interfering particles, or interferon. Consequently, studies comparing the intracellular synthesis and maturation of VSV proteins in infected NK and mouse L cells were conducted. In contrast to L cells, in which host cell protein synthesis was essentially totally inhibited by infection, the infection of NK cells caused no marked diminution in the synthesis of host cell proteins. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of immunoprecipitates of viral proteins from infected cells showed that the maturation rate and size of VSV surface G glycoprotein were comparable in L cells and NK cells. Nucleocapsid (N) protein synthesis also appeared to be unaffected in NK cells. In contrast, the viral proteins NS and M appeared to be selectively degraded in NK cell extracts. Mixing experiments suggested that a protease in NK cells was responsible for the selective breakdown of VSV NS protein. Finally, VSV-infected NK cells were resistant to lysis by virus-specific CTL, suggesting that persistently infected NK cells may harbor virus and avoid cell-mediated immune destruction in an immunocompetent host.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Banerjee A. K. Sequential transcription of the genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 May;73(5):1504–1508. doi: 10.1073/pnas.73.5.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acha-Orbea H., Groscurth P., Lang R., Stitz L., Hengartner H. Characterization of cloned cytotoxic lymphocytes with NK-like activity. J Immunol. 1983 Jun;130(6):2952–2959. [PubMed] [Google Scholar]
- Acha-Orbea H., Zinkernagel R. M., Hengartner H. Cytotoxic T cell clone-specific monoclonal antibodies used to select clonotypic antigen-specific cytotoxic T cells. Eur J Immunol. 1985 Jan;15(1):31–36. doi: 10.1002/eji.1830150107. [DOI] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Order of transcription of genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):442–446. doi: 10.1073/pnas.73.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Bell J. C., Brown E. G., Takayesu D., Prevec L. Protein kinase activity associated with immunoprecipitates of the vesicular stomatitis virus phosphoprotein NS. Virology. 1984 Jan 30;132(2):229–238. doi: 10.1016/0042-6822(84)90030-8. [DOI] [PubMed] [Google Scholar]
- Bland C. E., Rosenthal K. L., Pluznik D. H., Dennert G., Hengartner H., Bienenstock J., Metcalfe D. D. Glycosaminoglycan profiles in cloned granulated lymphocytes with natural killer function and in cultured mast cells: their potential use as biochemical markers. J Immunol. 1984 Apr;132(4):1937–1942. [PubMed] [Google Scholar]
- Bloom B. R., Senik A., Stoner G., Ju G., Nowakowski M., Kano S., Jimenez L. Studies on the interactions between viruses and lymphocytes. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):73–83. doi: 10.1101/sqb.1977.041.01.011. [DOI] [PubMed] [Google Scholar]
- Brown E., Prevec L. Proteins of vesicular stomatitis virus. IV. A comparison of tryptic peptides of the vesicular stomatitis group of rhabdoviruses. Virology. 1978 Aug;89(1):7–21. doi: 10.1016/0042-6822(78)90035-1. [DOI] [PubMed] [Google Scholar]
- Casali P., Rice G. P., Oldstone M. B. Viruses disrupt functions of human lymphocytes. Effects of measles virus and influenza virus on lymphocyte-mediated killing and antibody production. J Exp Med. 1984 May 1;159(5):1322–1337. doi: 10.1084/jem.159.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creager R. S., Cardamone J. J., Jr, Youngner J. S. Human lymphoblastoid cell lines of B- and T-cell origin: different responses to infection with vesicular stomatitis virus. Virology. 1981 May;111(1):211–222. doi: 10.1016/0042-6822(81)90666-8. [DOI] [PubMed] [Google Scholar]
- Creager R. S., Whitaker-Dowling P., Frey T. K., Youngner J. S. Varied responses of human B-lymphoblastoid cell lines to infection with vesicular stomatitis virus. Virology. 1982 Sep;121(2):414–419. doi: 10.1016/0042-6822(82)90179-9. [DOI] [PubMed] [Google Scholar]
- Dennert G., Yogeeswaran G., Yamagata S. Cloned cell lines with natural killer activity. Specificity, function, and cell surface markers. J Exp Med. 1981 Mar 1;153(3):545–556. doi: 10.1084/jem.153.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent P. B. Immunodepression by oncogenic viruses. Prog Med Virol. 1972;14:1–35. [PubMed] [Google Scholar]
- Edelman R., Wheelock E. F. Specific role of each human leukocyte type in viral infections. II. Phytohemagglutinin-treated lymphocytes as host cells for vesicular stomatitis virus replication in vitro. J Virol. 1968 May;2(5):440–448. doi: 10.1128/jvi.2.5.440-448.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar J. J., Fuller-Farrar J., Simon P. L., Hilfiker M. L., Stadler B. M., Farrar W. L. Thymoma production of T cell growth factor (Interleukin 2). J Immunol. 1980 Dec;125(6):2555–2558. [PubMed] [Google Scholar]
- Geiger B., Rosenthal K. L., Klein J., Zinkernagel R. M., Singer S. J. Selective and unidirectional membrane redistribution of an H-2 antigen with an antibody-clustered viral antigen: relationship to mechanisms of cytotoxic T-cell interactions. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4603–4607. doi: 10.1073/pnas.76.9.4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Gresser I., Lang D. J. Relationships between viruses and leucocytes. Prog Med Virol. 1966;8:62–130. [PubMed] [Google Scholar]
- Hale A. H., Ruebush M. J., Lefrançois L. Cross-reactive anti-vesicular stomatitis virus (VSV) cytotoxic T lymphocytes recognize the major surface glycoprotein. Eur J Immunol. 1981 May;11(5):434–436. doi: 10.1002/eji.1830110517. [DOI] [PubMed] [Google Scholar]
- Hale A. H., Witte O. N., Baltimore D., Eisen H. N. Vesicular stomatitis virus glycoprotein is necessary for H-2-restricted lysis of infected cells by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1978 Feb;75(2):970–974. doi: 10.1073/pnas.75.2.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W. W., Choppin P. W. Evidence for lack of synthesis of the M polypeptide of measles virus in brain cells in subacute sclerosing panencephalitis. Virology. 1979 Dec;99(2):443–447. doi: 10.1016/0042-6822(79)90026-6. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Lamb R. A., Choppin P. W. Measles and subacute sclerosing panencephalitis virus proteins: lack of antibodies to the M protein in patients with subacute sclerosing panencephalitis. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2047–2051. doi: 10.1073/pnas.76.4.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman R. B., Ortaldo J. R. Natural killer cells: their roles in defenses against disease. Science. 1981 Oct 2;214(4516):24–30. doi: 10.1126/science.7025208. [DOI] [PubMed] [Google Scholar]
- Hoxie J. A., Haggarty B. S., Rackowski J. L., Pillsbury N., Levy J. A. Persistent noncytopathic infection of normal human T lymphocytes with AIDS-associated retrovirus. Science. 1985 Sep 27;229(4720):1400–1402. doi: 10.1126/science.2994222. [DOI] [PubMed] [Google Scholar]
- Johnson G. P., Herman R. C. Nonpermissive infection of lymphoblastoid cells by vesicular stomatitis virus. I. Synthesis and function of the viral transcripts. Virus Res. 1984;1(3):259–274. doi: 10.1016/0168-1702(84)90043-1. [DOI] [PubMed] [Google Scholar]
- Katsura Y., Takaoki M., Kono Y., Minato N. Augmentation of delayed-type hypersensitivity by vesicular stomatitis virus infection in mice. J Immunol. 1980 Oct;125(4):1459–1462. [PubMed] [Google Scholar]
- Katsura Y., Takaoki Y., Minato N. Augmentation of delayed-type hypersensitivity to serum proteins by vesicular stomatitis virus infection in mice: virus-suppressor cell interactions. J Immunol. 1982 Jul;129(1):362–365. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller G., Enders J. F. Vaccinia virus replication and cytopathic effect in cultures in phytohemagglutinin-treated human peripheral blood leukocytes. J Virol. 1968 Aug;2(8):787–792. doi: 10.1128/jvi.2.8.787-792.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minato N., Katsura Y. Virus-replicating T cells in the immune response of mice. II. Characterization of T cells capable of replicating vesicular stomatitis virus. J Exp Med. 1978 Oct 1;148(4):837–849. doi: 10.1084/jem.148.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minato N., Katsura Y. Virus-replicating T cells in the immune response of mice. III. Role of vesicular stomatitis virus-replicating T cells in the antibody response. J Exp Med. 1978 Oct 1;148(4):850–861. doi: 10.1084/jem.148.4.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller J. R., Rager-Zisman B., Quan P. C., Schattner A., Panush D., Rose J. K., Bloom B. R. Natural killer cell recognition of target cells expressing different antigens of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2456–2459. doi: 10.1073/pnas.82.8.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notkins A. L., Mergenhagen S. E., Howard R. J. Effect of virus infections on the function of the immune system. Annu Rev Microbiol. 1970;24:525–538. doi: 10.1146/annurev.mi.24.100170.002521. [DOI] [PubMed] [Google Scholar]
- Nowakowski M., Bloom B. R., Ehrenfeld E., Summers D. F. Restricted replication of vesicular stomatitis virus in human lymphoblastoid cells. J Virol. 1973 Dec;12(6):1272–1278. doi: 10.1128/jvi.12.6.1272-1278.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowski M., Feldman J. D., Kano S., Bloom B. R. The production of vesicular stomatitis virus by antigen- or mitogen-stimulated lymphocytes and continuous lymphoblastoid lines. J Exp Med. 1973 Apr 1;137(4):1042–1059. doi: 10.1084/jem.137.4.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher H., Hämmerling G., Hengartner H. Restriction fine specificity of long-term, hapten-specific cytotoxic T cell clones: analysis with H-2Kbm-mutant mice and H-2Kb-specific monoclonal antibodies. Eur J Immunol. 1984 Feb;14(2):144–152. doi: 10.1002/eji.1830140208. [DOI] [PubMed] [Google Scholar]
- Pircher H., Zinkernagel R. M., Hengartner H. Inhibition of hapten-specific cytotoxic T cell recognition by monoclonal anti-hapten antibodies. Eur J Immunol. 1985 Mar;15(3):228–235. doi: 10.1002/eji.1830150305. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Rosenthal K. L., Ishizaka T., Befus D., Dennert G., Hengartner H., Bienenstock J. Expression of IgE receptors and histamine in cloned natural killer cell lines. J Immunol. 1984 Aug;133(2):642–646. [PubMed] [Google Scholar]
- Rosenthal K. L., Oldstone M. B., Hengartner H., Zinkernagel R. M. Specificity of in vitro cytotoxic T cell clones directed against vesicular stomatitis virus. J Immunol. 1983 Jul;131(1):475–478. [PubMed] [Google Scholar]
- Rosenthal K. L., Zinkernagel R. M. Cross-reactive cytotoxic T cells to serologically distinct vesicular stomatitis virus. J Immunol. 1980 May;124(5):2301–2308. [PubMed] [Google Scholar]
- Sheppard R. D., Raine C. S., Bornstein M. B., Udem S. A. Measles virus matrix protein synthesized in a subacute sclerosing panencephalitis cell line. Science. 1985 Jun 7;228(4704):1219–1221. doi: 10.1126/science.4001938. [DOI] [PubMed] [Google Scholar]
- Sy M. S., Tsurufuji M., Finberg R., Benacerraf B. Effect of vesicular stomatitis virus (VSV) infection on the development and regulation of T cell-mediated immune responses. J Immunol. 1983 Jul;131(1):30–36. [PubMed] [Google Scholar]
- Takahashi C., Katsura Y. Hapten-specific virus-replication T cells: analysis of the functional role in contact sensitivity. J Immunol. 1980 Jun;124(6):2721–2727. [PubMed] [Google Scholar]
- Villarreal L. P., Breindl M., Holland J. J. Determination of molar ratios of vesicular stomatitis virus induced RNA species in BHK21 cells. Biochemistry. 1976 Apr 20;15(8):1663–1667. doi: 10.1021/bi00653a012. [DOI] [PubMed] [Google Scholar]
- Warner J. F., Dennert G. Effects of a cloned cell line with NK activity on bone marrow transplants, tumour development and metastasis in vivo. Nature. 1982 Nov 4;300(5887):31–34. doi: 10.1038/300031a0. [DOI] [PubMed] [Google Scholar]
- Wethers J. A., Johnson G. P., Schumacher C. L., Herman R. C. Nonpermissive infection of lymphoblastoid cells by vesicular stomatitis virus. II. Effect on viral morphogenesis. Virus Res. 1985 Jun;2(4):345–358. doi: 10.1016/0168-1702(85)90030-9. [DOI] [PubMed] [Google Scholar]
- Wheelock E. F., Edelman R. Specific role of each human leukocyte type in viral infections. 3. 17D yellow fever virus replication and interferon production in homogeneous leukocyte cultures treated with phytohemagglutinin. J Immunol. 1969 Sep;103(3):429–436. [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. T lymphocyte interaction with viruses and virus-infected tissues. Prog Med Virol. 1975;19:120–160. [PubMed] [Google Scholar]
- Yanagi Y., Caccia N., Kronenberg M., Chin B., Roder J., Rohel D., Kiyohara T., Lauzon R., Toyonaga B., Rosenthal K. Gene rearrangement in cells with natural killer activity and expression of the beta-chain of the T-cell antigen receptor. Nature. 1985 Apr 18;314(6012):631–633. doi: 10.1038/314631a0. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Mackett M., Lefrancois L., Lyles D. S., Moss B. Recognition of cloned vesicular stomatitis virus internal and external gene products by cytotoxic T lymphocytes. J Exp Med. 1986 Jun 1;163(6):1529–1538. doi: 10.1084/jem.163.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Althage A., Holland J. Target antigens for H-2-restricted vesicular stomatitis virus-specific cytotoxic T cells. J Immunol. 1978 Aug;121(2):744–748. [PubMed] [Google Scholar]