Abstract
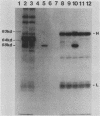
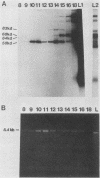
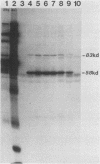
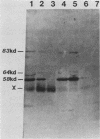
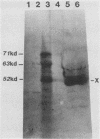
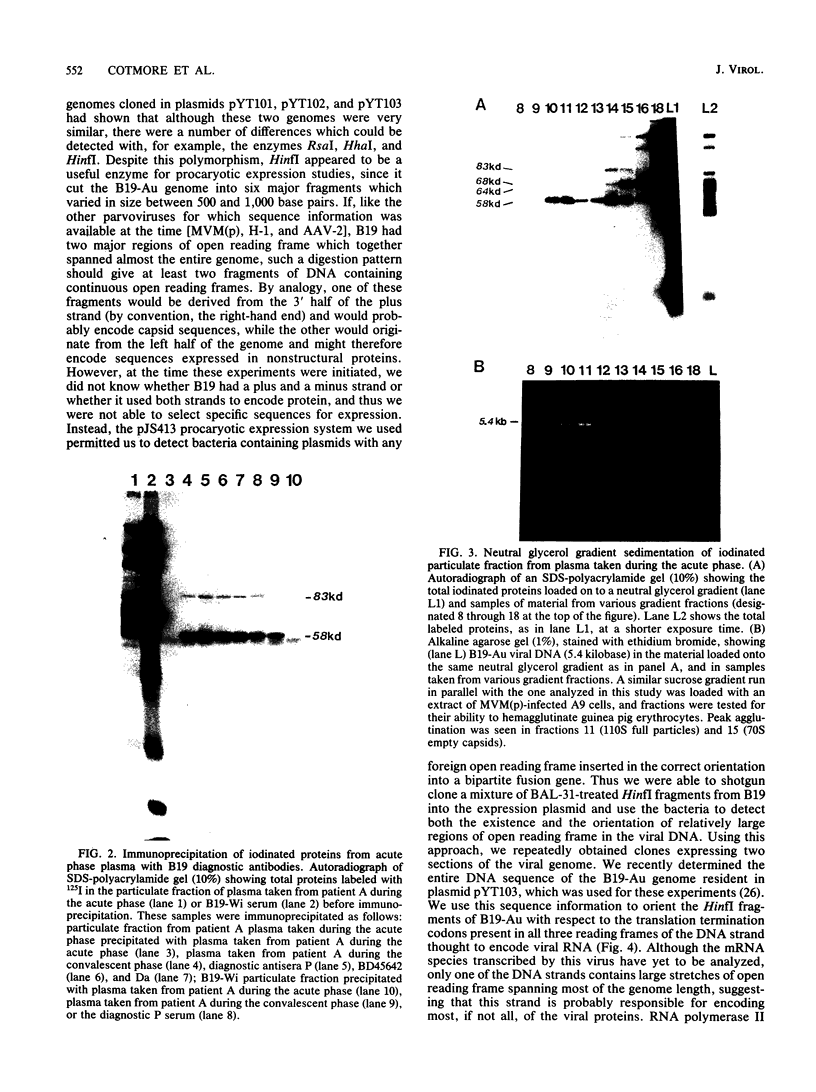
Plasma from a child with homozygous sickle-cell disease, sampled during the early phase of an aplastic crisis, contained human parvovirus B19 virions. Plasma taken 10 days later (during the convalescent phase) contained both immunoglobulin M and immunoglobulin G antibodies directed against two viral polypeptides with apparent molecular weights of 83,000 and 58,000 which were present exclusively in the particulate fraction of the plasma taken during the acute phase. These two protein species comigrated at 110S on neutral sucrose velocity gradients with the B19 viral DNA and thus appear to constitute the viral capsid polypeptides. The B19 genome was molecularly cloned into a bacterial plasmid vector. Restriction endonuclease fragments of this cloned B19 genome were treated with BAL 31 and shotgun cloned into the open reading frame expression vector pJS413. Two expression constructs containing B19 sequences from different halves of the viral genome were obtained, which directed the synthesis, in bacteria, of segments of virally encoded protein. These polypeptide fragments were then purified and used to immunize rabbits. Antibodies against a protein sequence specified between nucleotides 2897 and 3749 recognized both the 83- and 58-kilodalton capsid polypeptides in aplastic plasma taken during the acute phase and detected similar proteins in the tissues of a stillborn fetus which had been infected transplacentally with B19. Antibodies against a protein sequence encoded in the other half of the B19 genome (nucleotides 1072 through 2044) did not react specifically with any protein in plasma taken during the acute phase but recognized three nonstructural polypeptides of 71, 63, and 52 kilodaltons present in the liver and, at lower levels, in some other tissues of the transplacentally infected fetus.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Davis L. R., Jones S. E., Pattison J. R., Serjeant G. R. The development and use of an antibody capture radioimmunoassay for specific IgM to a human parvovirus-like agent. J Hyg (Lond) 1982 Apr;88(2):309–324. doi: 10.1017/s0022172400070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. J., Higgins P. G., Davis L. R., Willman J. S., Jones S. E., Kidd I. M., Pattison J. R., Tyrrell D. A. Experimental parvoviral infection in humans. J Infect Dis. 1985 Aug;152(2):257–265. doi: 10.1093/infdis/152.2.257. [DOI] [PubMed] [Google Scholar]
- Anderson M. J., Lewis E., Kidd I. M., Hall S. M., Cohen B. J. An outbreak of erythema infectiosum associated with human parvovirus infection. J Hyg (Lond) 1984 Aug;93(1):85–93. doi: 10.1017/s0022172400060964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T., Anand A., Ritchie L. D., Clewley J. P., Reid T. M. Intrauterine parvovirus infection associated with hydrops fetalis. Lancet. 1984 Nov 3;2(8410):1033–1034. doi: 10.1016/s0140-6736(84)91126-7. [DOI] [PubMed] [Google Scholar]
- Clewley J. P. Biochemical characterization of a human parvovirus. J Gen Virol. 1984 Jan;65(Pt 1):241–245. doi: 10.1099/0022-1317-65-1-241. [DOI] [PubMed] [Google Scholar]
- Cohen B. J., Mortimer P. P., Pereira M. S. Diagnostic assays with monoclonal antibodies for the human serum parvovirus-like virus (SPLV). J Hyg (Lond) 1983 Aug;91(1):113–130. doi: 10.1017/s0022172400060095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart Y. E., Field A. M., Cant B., Widdows D. Parvovirus-like particles in human sera. Lancet. 1975 Jan 11;1(7898):72–73. doi: 10.1016/s0140-6736(75)91074-0. [DOI] [PubMed] [Google Scholar]
- Cotmore S. F., Sturzenbecker L. J., Tattersall P. The autonomous parvovirus MVM encodes two nonstructural proteins in addition to its capsid polypeptides. Virology. 1983 Sep;129(2):333–343. doi: 10.1016/0042-6822(83)90172-1. [DOI] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. Characterization and molecular cloning of a human parvovirus genome. Science. 1984 Dec 7;226(4679):1161–1165. doi: 10.1126/science.6095448. [DOI] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. Organization of nonstructural genes of the autonomous parvovirus minute virus of mice. J Virol. 1986 Jun;58(3):724–732. doi: 10.1128/jvi.58.3.724-732.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The NS-1 polypeptide of the autonomous parvovirus MVM is a nuclear phosphoprotein. Virus Res. 1986 May;4(3):243–250. doi: 10.1016/0168-1702(86)90003-1. [DOI] [PubMed] [Google Scholar]
- Duncan J. R., Potter C. B., Cappellini M. D., Kurtz J. B., Anderson M. J., Weatherall D. J. Aplastic crisis due to parvovirus infection in pyruvate kinase deficiency. Lancet. 1983 Jul 2;2(8340):14–16. doi: 10.1016/s0140-6736(83)90005-3. [DOI] [PubMed] [Google Scholar]
- Edwards J. M., Kessel I., Gardner S. D., Eaton B. R., Pollock T. M., Fleck D. G., Gibson P., Woodroof M., Porter A. D. A search for a characteristic illness in children with serological evidence of viral or toxoplasma infection. J Infect. 1981 Dec;3(4):316–323. doi: 10.1016/s0163-4453(81)91863-6. [DOI] [PubMed] [Google Scholar]
- Kelleher J. F., Luban N. L., Mortimer P. P., Kamimura T. Human serum "parvovirus": a specific cause of aplastic crisis in children with hereditary spherocytosis. J Pediatr. 1983 May;102(5):720–722. doi: 10.1016/s0022-3476(83)80243-1. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mortimer P. P., Cohen B. J., Buckley M. M., Cradock-Watson J. E., Ridehalgh M. K., Burkhardt F., Schilt U. Human parvovirus and the fetus. Lancet. 1985 Nov 2;2(8462):1012–1012. doi: 10.1016/s0140-6736(85)90558-6. [DOI] [PubMed] [Google Scholar]
- Mortimer P. P., Humphries R. K., Moore J. G., Purcell R. H., Young N. S. A human parvovirus-like virus inhibits haematopoietic colony formation in vitro. 1983 Mar 31-Apr 6Nature. 302(5907):426–429. doi: 10.1038/302426a0. [DOI] [PubMed] [Google Scholar]
- Paradiso P. R., Williams K. R., Costantino R. L. Mapping of the amino terminus of the H-1 parvovirus major capsid protein. J Virol. 1984 Oct;52(1):77–81. doi: 10.1128/jvi.52.1.77-81.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattison J. R., Jones S. E., Hodgson J., Davis L. R., White J. M., Stroud C. E., Murtaza L. Parvovirus infections and hypoplastic crisis in sickle-cell anaemia. Lancet. 1981 Mar 21;1(8221):664–665. doi: 10.1016/s0140-6736(81)91579-8. [DOI] [PubMed] [Google Scholar]
- Rao K. R., Patel A. R., Anderson M. J., Hodgson J., Jones S. E., Pattison J. R. Infection with parvovirus-like virus and aplastic crisis in chronic hemolytic anemia. Ann Intern Med. 1983 Jun;98(6):930–932. doi: 10.7326/0003-4819-98-6-930. [DOI] [PubMed] [Google Scholar]
- Reid D. M., Reid T. M., Brown T., Rennie J. A., Eastmond C. J. Human parvovirus-associated arthritis: a clinical and laboratory description. Lancet. 1985 Feb 23;1(8426):422–425. doi: 10.1016/s0140-6736(85)91146-8. [DOI] [PubMed] [Google Scholar]
- Salacinski P. R., McLean C., Sykes J. E., Clement-Jones V. V., Lowry P. J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen). Anal Biochem. 1981 Oct;117(1):136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serjeant G. R., Topley J. M., Mason K., Serjeant B. E., Pattison J. R., Jones S. E., Mohamed R. Outbreak of aplastic crises in sickle cell anaemia associated with parvovirus-like agent. Lancet. 1981 Sep 19;2(8247):595–597. doi: 10.1016/s0140-6736(81)92739-2. [DOI] [PubMed] [Google Scholar]
- Shade R. O., Blundell M. C., Cotmore S. F., Tattersall P., Astell C. R. Nucleotide sequence and genome organization of human parvovirus B19 isolated from the serum of a child during aplastic crisis. J Virol. 1986 Jun;58(3):921–936. doi: 10.1128/jvi.58.3.921-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J., Jones S. E., Anderson M. J. Characterization of the genome of the agent of erythrocyte aplasia permits its classification as a human parvovirus. J Gen Virol. 1983 Nov;64(Pt 11):2527–2532. doi: 10.1099/0022-1317-64-11-2527. [DOI] [PubMed] [Google Scholar]
- Tattersall P., Shatkin A. J., Ward D. C. Sequence homology between the structural polypeptides of minute virus of mice. J Mol Biol. 1977 Apr 25;111(4):375–394. doi: 10.1016/s0022-2836(77)80060-0. [DOI] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Weis J. H., Salstrom J. S., Enquist L. W. Herpes simplex virus type-1 glycoprotein D gene: nucleotide sequence and expression in Escherichia coli. Science. 1982 Oct 22;218(4570):381–384. doi: 10.1126/science.6289440. [DOI] [PubMed] [Google Scholar]
- Weis J. H., Enquist L. W., Salstrom J. S., Watson R. J. An immunologically active chimaeric protein containing herpes simplex virus type 1 glycoprotein D. Nature. 1983 Mar 3;302(5903):72–74. doi: 10.1038/302072a0. [DOI] [PubMed] [Google Scholar]
- White D. G., Woolf A. D., Mortimer P. P., Cohen B. J., Blake D. R., Bacon P. A. Human parvovirus arthropathy. Lancet. 1985 Feb 23;1(8426):419–421. doi: 10.1016/s0140-6736(85)91145-6. [DOI] [PubMed] [Google Scholar]
- Yen T. S., Webster R. E. Bacteriophage f1 gene II and X proteins. Isolation and characterization of the products of two overlapping genes. J Biol Chem. 1981 Nov 10;256(21):11259–11265. [PubMed] [Google Scholar]
- de Foresta F., Deleys-Hertoghs J., Cornelis J., Klein B., Rommelaere J. La transformation par le virus SV40 sensibilise les fibroblastes de peau humaine à l'action lytique du parvovirus H-1. C R Seances Soc Biol Fil. 1985;179(2):276–282. [PubMed] [Google Scholar]