Abstract
Objective To test predictors of medication adherence in high-risk racial or ethnic minority youth living with HIV (YLH) using a conceptual model of social cognitive predictors including a continuous measure of motivational readiness. Methods Youth were participants in a multi-site clinical trial examining the efficacy of a motivational intervention. Racial-minority YLH (primarily African American) who were prescribed antiretroviral medication were included (N = 104). Data were collected using computer-assisted personal interviewing method via an Internet-based application and questionnaires. Results Using path analysis with bootstrapping, most youth reported suboptimal adherence, which predicted higher viral load. Higher motivational readiness predicted optimal adherence, and higher social support predicted readiness. Decisional balance was indirectly related to adherence. Conclusions The model provided a plausible framework for understanding adherence in this population. Culturally competent interventions focused on readiness and social support may be helpful for improving adherence in YLH.
Keywords: Adherence, adolescents, HIV, minority populations, young adults
Adolescents and young adults have one of the fastest growing rates of new HIV infection (Centers for Disease Control and Prevention [CDC], 2007). The rates of new and existing infections continue to be disproportionately higher in youth of color, particularly among African American and Latino adolescents and young adults (CDC, 2008). Recent advances in antiretroviral medications have allowed for dramatic improvements in life expectancy and health outcomes for youth living with HIV (YLH; Watson & Farley, 1999). However, these medications are only effective with near perfect rates (i.e., 90–95%) of adherence to the regimen. Suboptimal adherence to medication can lead to further complications such as infections and drug resistance (Mullen et al., 2002), and is associated with higher viral loads. The few studies of adherence to HIV medication regimens in adolescents and young adults suggest suboptimal adherence (59% in Murphy, Wilson, Durako, Muenz, & Belzer, 2001; 63% in Naar-King et al., 2006).
Studies of the factors related to medication adherence among YLH are limited, and studies specifically focused on medication adherence in racial or ethnic minority YLH are even more limited (Belzer, Fuchs, Luftman, & Tucker, 1999; Murphy et al., 2001). Murphy et al. (2001) conducted the REACH study, the first large-scale disease progression study of behaviorally infected YLH. In this sample, only 41% of youth reported full adherence to highly active antiretrorival therapy (HAART). Youth depression was strongly associated with nonadherence; however, there was no relationship between social support and adherence. In a study of much smaller scale, Belzer et al. (1999) found that 61% of YLH reported >90% adherence in the last 90 days. Youth who perceived that medications would improve and prolong their lives were more likely to be adherent.
In general, health behavior change during late adolescence and early adulthood has received little attention compared to other periods of lifespan development (e.g., childhood, early and middle adolescence, and adulthood). This lack of attention is problematic given that adolescence and early adulthood are developmental periods marked by experimentation and risk taking (Elkind, 1998). Emerging adults of color face unique challenges due to societal stereotypes regarding the competencies of racial or ethnic minority youth (Arnett, 2003). The transition into adulthood can be even more difficult for youth with chronic medical conditions, as many have to negotiate a transition of care as their parents become decreasingly involved and they shift from pediatric/adolescent medicine to adult-care settings (Weissberg-Benchell, Wolpert & Anderson, 2007).
In the few quantitative studies specifically focused on YLH, frequent substance use, advanced stage of HIV infection, younger age, life stressors (Murphy et al., 2005), psychological symptoms (Hosek, Harper, & Domanico, 2005; Naar-King et al., 2006; Williams et al., 2006), low self-efficacy (Naar-King et al., 2006), and perceptions of the effects of HIV medications (Belzer et al., 1999) have been linked to poor medication adherence. Conceptual models of adherence in YLH that are empirically tested are needed to understand the interrelationships among these factors. Researchers have called for theoretically driven and scientifically sound empirical adherence studies in pediatric psychology to integrate research and practice (Riekert & Drotar, 2000). These studies should address a range of risk and resiliency factors among specific groups of youth living with chronic illnesses (such as racial or ethnic minority YLH) in order to best inform future interventions (Harper & Hosek, 2003).
The Transtheoretical Model (TTM; Prochaska et al., 1994) has been suggested as a plausible framework for understanding medication adherence (Riekert & Drotar, 2000), though it has not been tested in pediatric/adolescent medicine populations to date. The TTM posits that motivational readiness to change behavior, or a continuum of an individual's perception of how ready he/she is to change, precedes actual behavior change. While originally conceptualized as stages of change, critiques of the stage model (e.g., Littel & Girvin, 2002) suggest a more continuous conceptualization of motivational readiness (Migneault, Adams, & Read, 2005). Beyond motivational readiness, there are cognitive factors from the TTM that may be applicable to understanding adherence behavior. Cognitive factors that relate to increases in motivational readiness in the TTM include self-efficacy (confidence and avoiding temptation) and decisional balance (weighing the pros and the cons of behavior change). The purpose of the present study is to test a conceptual model of adherence behavior in YLH using variables from the TTM and from existing research on YLH.
We hypothesized that motivational readiness to adhere to medication regimens would be associated with better adherence. Higher self-efficacy and higher decisional balance scores (with pros of adherence outweighing cons) would predict increased motivational readiness and better adherence. Low social support and high levels of psychological symptoms were expected to relate to lower self-efficacy, lower decisional balance (cons of adherence outweighing pros), and suboptimal adherence. Higher decisional balance was expected to be associated with higher self-efficacy. Substance use was also assessed because of the high prevalence among YLH and its relationship to adherence in one study (Murphy et al., 2005). High rate of substance use was expected to be related to poorer adherence. Finally, optimal adherence was expected to predict lower viral load, the marker of disease progression most immediately impacted by adherence to HIV medications (Murphy et al., 2001).
Methods
Participants
Youth were participants in “Healthy Choices,” a randomized, multi-site clinical trial examining the efficacy of a motivational intervention aimed at reducing risk and promoting healthy behaviors in YLH. Inclusion criteria included HIV-positive status, aged 16–24 years, and ability to complete questionnaires in English. Because the study targeted high-risk HIV-positive youth, they had to have engaged in at least one of the three problem behaviors: a sexual risk problem (at least one unprotected sex act in the previous month); an alcohol or illicit drug use problem based on an adolescent medicine screener (CRAFFT; Knight et al., 1999); or a medication adherence problem (self-report of <90% adherence in the last month). Youth with an active thought disorder were excluded due to an inability to complete questionnaires. Youth who were currently involved in behavioral research (assessment or intervention) targeting adherence, sexual risk, or alcohol and/or drug substance abuse or who were involved in a substance abuse treatment program were also excluded from the study.
Youth were recruited from five study sites across the USA. All five sites offered comprehensive, multidisciplinary care including social work and case management services and access to mental health services. The Adolescent Trials Network (ATN) sites were located in Fort Lauderdale, FL; Philadelphia, PA; Baltimore, MD; and Los Angeles, CA. Additionally, a non-ATN site was located in Detroit, Michigan. The sites were chosen to represent a regional cross-section of the population to promote the ability to test the effectiveness of the intervention and assure transportability. Indeed, the sample demographics were consistent with the national HIV/AIDS epidemic (CDC, 2008).
Only racial or ethnic minority youth (self-identified as African American, Latino, or mixed race) that were prescribed the HIV antiretroviral medication (N = 104 of 186) were included in the present study. Demographic information overall and by site is presented in Table I. Total 85% of youth self-identified as African American, 14% Latino, and 1% mixed race. From the sample 65% identified themselves as heterosexual, 11% as bisexual, 23% as gay, and 1% as other. Sexual orientation was treated as a dichotomous variable (gay, bisexual, or other vs. heterosexual) for subsequent analyses.
Table I.
Sample Demographics by Study Site
| Los Angeles | Philadelphia | Baltimore | Fort Lauderdale | Detroit | Total | |
|---|---|---|---|---|---|---|
| n | 26 | 11 | 21 | 23 | 23 | 104 |
| Age (mean) | 21.5 (1.9) | 19.9 (1.8) | 21.2 (2.5) | 19.6 (2.1) | 20.3 (2.6) | 20.6 (2.3) |
| Females (%) | 50.0 | 54.5 | 52.4 | 82.6 | 21.7 | 51.9 |
| African Americans (%) | 46.2 | 100.0 | 100.0 | 91.3 | 100.0 | 84.6 |
| Sexual minorities (%) | 30.8 | 54.5 | 38.1 | 4.3 | 56.5 | 34.6 |
| Perinatally infected (%) | 15.4 | 18.2 | 14.3 | 52.2 | 34.8 | 27.9 |
Procedure
The protocol was approved by each site's institutional review board and a certificate of confidentiality was obtained from the National Institutes of Health. Participants were approached during a regularly scheduled clinic visit or during supportive activities. All participants were screened to confirm seropositivity or provided documented test results from a prior HIV screening. Informed consent was obtained from all participants, and a waiver of parental permission was obtained for youth aged 16 and 17 years. This waiver was approved by each site's IRB board as part of the consent packet. Interviewers collected participant data using a computer-assisted personal interviewing (CAPI) method via an Internet-based application. Responses to CAPI questions were entered into the computer by the research interviewer. Upon completion of the baseline interview, the participants received $30 for their time. Food, childcare, and transportation to and from visits were also available for participants at no cost.
Measures
Adherence to Medications
Adherence was assessed using a visual analog scale from 0 to 100 (Giordano et al., 2004). Participants indicated the percent of time they took HIV medications, the percent of time they took HIV medications as directed (e.g., food restrictions), and how often they took all doses as prescribed for the day. Responses to these questions were averaged to form a composite percent adherence score for the past month. Adherence as assessed by this measure has been strongly correlated to plasma HIV RNA levels in prior studies (Naar-King et al., 2006).
Viral Load
Viral load (in HIV copies/ml of blood) was tested at the initial clinic visit unless a recent (within 1 month) test result was available from the participant. Because of a highly skewed distribution, viral load was log transformed.
Decisional Balance
To understand the decision-making process, attitudes toward adherence were measured using a 22-item measure that includes perceived pros and cons of adhering to HIV medications. This measure was based on the Decisional Balance Inventory (Velicer, DiClemente, Prochaska, & Brandenburg, 1985) and adapted for pros and cons of taking HIV medication (Parsons, Rosof & Mustanski, 2007). Respondents are given a number of reasons for taking or not taking their HIV medications and asked to rate the importance of each from 1 (“not at all important”) to 5 (“extremely important”). Higher decisional balance scores indicate more importance placed on reasons to take medications as prescribed. Cronbach's α was .89 for this measure in the present sample.
Self-efficacy
Youth completed a 25-item instrument consisting of a combination of a temptation measure (22 items, reverse-coded) and a confidence measure (3 items). The temptation measure asks participants to use a 5-point Likert Scale to rate how confident they are that they could take their HIV medications on time under 11 circumstances (e.g., on vacation; out at night). The measure also asks about their temptation to miss their HIV medications under those same 11 circumstances. The measure was shown to have strong reliability and validity in previous studies of HIV medication adherence (e.g., Parsons, Rosof, & Mustanski, 2008), and had excellent reliability in the current study with Cronbach's α = .92. The confidence measure assessed confidence to take medications as prescribed on a 5-point scale. The measure showed adequate reliability (α = .81). Cronbach's α for the combined self-efficacy measure was .88.
Motivational Readiness to Adhere to Medications
The adherence to medications item from Rollnick's Readiness Ruler (Stott, Rollnick, Rees, & Pill, 1995) was used to assess motivation for adherence. Respondents rated how ready they are to take HIV medications as prescribed on a scale from 1 (“not ready”) to 10 (“ready to change or already changed”). This measure has been recommended for use by clinicians to determine readiness to change in HIV care (CDC, 1993), and has been used for other behaviors in YLH (S. Naar-King, J. Parsons, D. Murphy, R. Harris, & K. Kolmodin, manuscript submitted for publication).
Symptoms of Emotional Distress
The Brief Symptom Inventory (BSI) measures nine primary symptom dimensions of physical and mental status combined to form the Global Severity Index (GSI; Derogatis & Spencer, 1982). The BSI asks respondents to rate how much they are distressed by a series of issues (e.g., lack of appetite, thoughts of ending your life) based on a 5-point response scale ranging from 1 (“not at all”) to 5 (“extremely”). Internal consistency for the BSI–GSI was excellent with Cronbach's α = .98.
Social Support for Taking Medications
A single item asking about social support specific to medication adherence (“There are people in my life that are supportive about taking HIV medication”) was rated on a 5-point Likert Scale from “strongly agree” to “strongly disagree”. This item was used because social support specific for reducing risk behaviors has been shown to be related to risk behaviors more than has general social support (Naar-King et al., 2006).
Substance Use
Actual alcohol and illicit drug use in the past 30 days was assessed using the Timeline Follow-Back (TLFB) procedure. A calendar assists participants in recalling when they used a particular substance and the amount used on each occasion. The TLFB procedure has demonstrated excellent psychometric properties in a number of studies (Carney, Tennen, Affleck, del Boca, & Kranzler, 1998) including correlation with urine drug screens (Fals-Stewart et al., 2000). Number of standard drinks (8 ounces beer, 4 ounces of wine, or 1 ounce hard liquor) and number of illicit drug use episodes were used for analysis.
Data Analysis
Descriptive statistics and bivariate analyses assessed simple associations between variables. Chi-square and t-tests were used to assess demographic differences across variables. Path analysis with bootstrapping was conducted (AMOS v7.0; Arbuckle, 2006) to examine the relations between the variables in the model and adherence to HIV medication. Path analysis rather than latent variable modeling was used because of the relatively small sample size. While SEM is often utilized with large samples (N > 200), bootstrap analyses allow model testing with small samples by utilizing the actual data to estimate standard error (Bollen & Stine, 1993). There is evidence that bootstrapping increases the power of the statistical results and is a particularly useful technique when sample sizes are small (Shrout & Bolger, 2002). Our sample size of 104 is reasonable for estimation of model effects (Kline, 1998), but certainly caution should be used in interpreting results due to the relatively small sample size.
In the path model, we tested whether social support, self-efficacy, and decisional balance predicted motivational readiness. We also tested if the associations between these three variables and our outcome variables, adherence and viral load, were mediated by motivational readiness. In addition to standardized root mean square residual (SRMR), comparative fit index (CFI) and root mean square error of approximation (RMSEA), and the Tucker-Lewis index (TLI) were used as fit indices as they are less sensitive to small sample size (n < 200) than other indices (Fan, Thompson, & Wang, 1999).
Results
Descriptive Analyses and Bivariate Correlations
Seventy-nine percent of youth (N = 82) reported taking their prescribed antiretroviral therapy medications <90% of the time in the last month. Viral load ranged from 0 (below detection) to 750,000 copies/ml (M = 65,316.89, SD = 144,005.58) and a median of 8,500 copies/ml. Substance use did not vary by adherence status [χ2(1, n = 91) = 1.65, p > .10] and was excluded from subsequent analyses. Decisional balance scores ranged from –4.0 to 40.0, M = 18.07 (11.48). Average self-efficacy score was 7.91 (1.51; range 4.12–10.00). Motivational readiness ranged from 1.0 to 10.0, M = 8.44 (1.82). Social support ranged from 1.0 to 5.0 with M = 4.35 (0.99). Youth BSI scores averaged 56.82 (15.44; range 25.0–80.0). There were no significant differences between biological males and females on any variable. Youth who identified as gay, bisexual, or other than heterosexual scored higher than heterosexuals on the BSI GSI, t(102) = –3.60, p < .01. Sample demographics across the different study sites are detailed in Table I.
Motivational readiness (.21, p < .05) and viral load (−.53, p < .01) were significantly correlated with adherence. Higher motivational readiness (–.27, p < .01) and optimal adherence (–.53, p < .01) were strongly associated with lower viral load. There were also a number of significant correlations among predictor variables. Youth BSI was not associated with any outcome or proposed predictor variable and was not included in the model.
Path Analysis
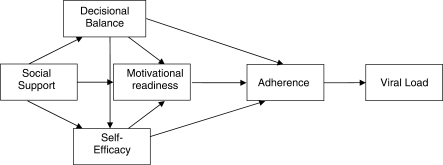
Both direct and indirect relations were evaluated using standardized regression weights. Model testing involved consideration of the hypothesized model (Figure 1) followed by modifications to improve parsimony. The hypothesized model resulted in an adequate fit, χ2(5, 104) = 9.41, p = .094, RMSEA = .093, CFI = .939, SRMR = .051, TLI = .816, but a number of paths were not significant. A reduced model was estimated with non-significant paths (p > .10) deleted from the originally hypothesized model. Figure 2 shows the final model. Table II details the progression from the hypothesized model to the final, most parsimonious model. The final model had an acceptable fit with the data (χ2 = 12.42; df = 9; p = .19; CFI = .95; TLI = .92; RMSEA = .06; SRMR = .07). The model accounted for approximately 29% of the variance in viral load.
Figure 1.
Hypothesized path model of adherence.
Figure 2.
Final path model of adherence (standardized regression weights).
Table II.
Progression of the Path Model
| Step | Path removed | χ2 | p | df | CFI | TLI | RMSEA | SRMR |
|---|---|---|---|---|---|---|---|---|
| 1 | – | 9.4 | .09 | 5 | .94 | .82 | .09 | .05 |
| 2 | Social support → self-efficacy | 9.5 | .15 | 6 | .95 | .88 | .08 | .05 |
| 3 | Self-efficacy → adherence | 9.7 | .21 | 7 | .96 | .92 | .06 | .05 |
| 4 | Social support → decisional balance | 10.2 | .25 | 8 | .97 | .94 | .05 | .06 |
| 5 (Final) | Decisional balance → adherence | 12.4 | .19 | 9 | .95 | .92 | .06 | .07 |
The reduced model demonstrated a number of significant associations among variables. Direct effects are detailed in Table III. Indirect effect significance levels were obtained through bootstrapping. As expected, optimal adherence predicted lower viral load. Higher motivational readiness significantly predicted optimal adherence to medications. Motivational readiness also has a significant indirect effect on viral load (–.11, p < .05). Higher self-efficacy was associated with higher motivational readiness. There was a trend towards higher decisional balance predicting higher motivational readiness (p = .06). Decisional balance was also significantly related to self-efficacy, and it had significant indirect effects on both adherence (.05, p < .05) and viral load (–.03, p < .05). In turn, higher levels of social support predicted higher motivational readiness. Social support also had trend-level indirect effects on adherence (.05, p = .07) and viral load (–.03, p = .07).
Table III.
Final Path Model (Bootstrapping Results)
| Path | Standardized B | Unstandardized B | SE | Biasa | p |
|---|---|---|---|---|---|
| Social support → motivational readiness | 0.26 | 0.48 | 0.16 | −0.01 | .003 |
| Decisional balance → self-efficacy | 0.36 | 0.05 | 0.01 | 0.001 | <.01 |
| Decisional balance → motivational readiness | 0.18 | 0.03 | 0.02 | 0.001 | .06 |
| Self-efficacy → motivational readiness | 0.22 | 0.26 | 0.12 | −0.004 | .02 |
| Motivational readiness → adherence | 0.21 | 4.36 | 2.01 | −0.005 | .03 |
| Adherence → viral load | −0.53 | −0.03 | 0.004 | 0.001 | <.01 |
aBias refers to the difference between the average of estimates obtained from bootstrap samples and the estimate obtained from the original sample.
Discussion
The current study was among the first to explore predictors of adherence to medications in a multi-site sample of racial or ethnic-minority YLH. Youth in this study were in emerging adulthood, a developmental period often characterized by high rates of risk-taking behavior. The present study illustrated that risk-taking may extend to health behaviors. YLH reported suboptimal medication adherence despite the benefits of taking antiretroviral medications and the potential complications of not following the medication regimen. Our results support the link between self-reported adherence and health outcome in this population, as youth report of adherence was strongly associated with higher viral load.
Our conceptual model of adherence was a good fit for the data and provided a plausible framework for understanding adherence behavior in youth of color living with HIV. Our model may also guide the development of adherence interventions. Youth who reported higher motivational readiness were likely to have optimal adherence and lower viral load. Decisional balance was related to motivational readiness at trend-level. Social support to take medications was also included in the model. While it was not associated with self-efficacy or decisional balance, it was associated with the construct directly related to adherence, motivational readiness. Future research further assessing decisional balance and self-efficacy as well as other change processes would improve our understanding of the utility of this model for understanding adherence behavior.
Contrary to expectations, psychological symptoms were not associated with medication adherence, viral load, or any of the predictors included in the model. Previous research has found links between depression or psychological symptoms and decreased adherence (Hosek et al., 2005; Murphy et al., 2001). Perhaps we did not see associations with psychological symptoms because of our restricted range, as almost half the youth had clinical levels of symptoms. The REACH sample had lower rates of psychological symptoms (as measured by depression), but used a different measure of psychological symptoms (CES-D). Unlike our project, REACH did not include perinatally infected youth or youth older than 18 years, and had a majority female participants.
Similarly, while the REACH study found a relationship between substance use and adherence, our study did not. High rates of substance use have been found in the general population of YLH (Etzel, Lightfoot, Rotheram-Borus, & Swendeman, 2002). This may indicate that although substance use is a risky behavior, by itself it does not significantly influence medication adherence. Rather, underlying motivation and factors that influence motivation have the most impact on medication adherence and health outcomes. However, the presence of multiple risk factors, such as psychological symptoms and substance use, could potentially have a cumulative impact on medication adherence (e.g., Koinis-Mitchell et al., 2007).
Although this study represents one of the first investigations of medication adherence in high-risk youth of color living with HIV, there are several limitations. This sample may be at higher risk than the broader population of YLH as they were recruited based on risky behaviors, and this may have influenced our findings regarding depression and substance use and their lack of association with adherence. This study was also conducted with a small clinic-based convenience sample, and may not represent community samples. The model should be tested using a larger sample size. Another limitation includes the reliance on self-report measures, particularly for adherence and substance use. An additional limitation is that social support was measured using a single-item, and given the complexity of this construct the findings regarding social support should be viewed with caution. Finally, longitudinal data are necessary to truly predict and understand adherence in this population.
Future research should continue to explore adherence to medications in youth of color living with HIV. Interventions to improve adherence and decrease health risks should be designed using culturally sensitive and developmentally specific frameworks, and address the range of factors that may compromise the health and well being of racial or ethnic minority YLH. Specific recommendations that have been offered for how to address issues of culture in primary and secondary HIV risk reduction programs (Harper, 2007) may also be applicable to adherence programs for racial or ethnic minority YLH. Our model provides a feasible framework for understanding adherence in older adolescents and young adults of color with other chronic medical conditions. Future research could further explore these factors beyond our at-risk group of racial or ethnic minority youth living with HIV and in the broader population of youth with chronic medical conditions.
Funding
The ATN for HIV/AIDS Interventions [U01-HD040533 from the National Institutes of Health through the National Institute of Child Health and Human Development (B. Kapogiannis, S. Lee)], with supplemental funding from the National Institutes on Drug Abuse (N. Borek) and Mental Health (P. Brouwers, S. Allison).
Conflict of interest: None declared.
Acknowledgments
The study was scientifically reviewed by the ATN's Behavioral Leadership Group. Network, scientific and logistical support was provided by the ATN Coordinating Center (C. Wilson, C. Partlow) at The University of Alabama at Birmingham Network operations and data management support was provided by the ATN Data and Operations Center at Westat, Inc. (J. Korelitz, J. Davidson, B. Harris). We acknowledge the contribution of the investigators and staff at the following ATN 004 sites that participated in this study: Children's Diagnostic and Treatment Center (Ana Puga, MD, Esmine Leonard, BSN, Zulma Eysallenne, RN); Children's Hospital of Los Angeles (Marvin Belzer, MD, Cathy Salata, RN, Diane Tucker, RN, MSN); University of Maryland (Ligia Peralta, MD, Leonel Flores, MD, Esther Collinetti, BA); University of Pennsylvania and the Children's Hospital of Philadelphia (Bret Rudy, MD, Mary Tanney, MPH, MSN, CPNP, Adrienne DiBenedetto, BSN); University of Southern California (Andrea Kovacs, MD,), and Wayne State University Horizons Project (K. Wright, DO, P. Lam, MA, V. Conners, BA). We sincerely thank the youth who participated in this project.
References
- Arbuckle JL. Amos (Version 7.0) [Computer Program] Chicago: SPSS; 2006. [Google Scholar]
- Arnett J. Conceptions of the transition to adulthood among emerging adults in American ethnic groups. New Directions in Child & Adolescent Development. 2003;100:63–75. doi: 10.1002/cd.75. [DOI] [PubMed] [Google Scholar]
- Belzer M, Fuchs D, Luftman D, Tucker D. Antiretroviral adherence issues among HIV-positive adolescents and young adults. Journal of Adolescent Health. 1999;25:316–319. doi: 10.1016/s1054-139x(99)00052-x. [DOI] [PubMed] [Google Scholar]
- Bollen K, Stine R. Bootstrapping goodness-of-fit measures in structural equation models. In: Bollen KA, Scott Long J, editors. Testing structural equation models. Newbury Park: International Educational and Professional Publisher; 1993. pp. 111–135. [Google Scholar]
- Carney MA, Tennen H, Affleck G, del Boca FK, Kranzler HR. Levels and patterns of alcohol consumption using timeline follow-back, daily diaries and real-time “electronic interviews”. Journal of Studies on Alcohol. 1998;59:447–454. doi: 10.15288/jsa.1998.59.447. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Recommendations for HIV testing services for inpatients and outpatients in acute-care hospital settings and technical guidance on HIV counseling. Morbidity & Mortality Weekly Report. 1993;42 No. RR-2. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cases of HIV/AIDS, HIV incidence, and cases of AIDS in the United States and dependent areas, 2007. HIV/AIDS Surveillance Report. 2007;19:1–54. [Google Scholar]
- Centers for Disease Control and Prevention. Subpopulation estimates from the HIV Incidence Surveillance System—United States, 2006. Morbidity & Mortality Weekly Report. 2008;57:985–989. [PubMed] [Google Scholar]
- Derogatis L, Spencer P. Brief Symptom Inventory: Administration, scoring, and procedures manual-I. Baltimore, MD: Clinical Psychometric Research; 1982. [Google Scholar]
- Elkind D. Teenagers in crisis. All grown up and no place to go. Reading, MA: Perseus Books; 1998. [Google Scholar]
- Etzel M, Lightfoot M, Rotheram-Borus M, Swendeman D. Presentation at the 14th annual International Conference on AIDS. Challenges facing HIV positive youth, post HAART. San Fransciso, CA: Health Initiatives for Youth; 2002. [Google Scholar]
- Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: Psychometric properties. Journal of Consulting and Clinical Psychology. 2000;68:134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- Fan X, Thompson B, Wang L. Effects of sample size, estimation methods, and model specification on structural equation modeling fit indexes. Structural Equation Modeling. 1999;6:56–83. [Google Scholar]
- Giordano TP, D. Guzman, R. Clark ED, Charlebois, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clin. 2004;5:74–79. doi: 10.1310/JFXH-G3X2-EYM6-D6UG. [DOI] [PubMed] [Google Scholar]
- Harper GW. Sex isn’t that simple: Culture and context in HIV prevention interventions for gay and bisexual male adolescents. American Psychologist. 2007;62:803–819. doi: 10.1037/0003-066X.62.8.806. [DOI] [PubMed] [Google Scholar]
- Harper GW, Hosek S. Promoting healthy development with youth who are chronically ill. In: Gullotta T, Bloom M, editors. The encyclopedia of primary prevention and health promotion. New York: Kluwer Academic/Plenum Publishers; 2003. pp. 292–300. [Google Scholar]
- Henderson R, Colgrove J, Lusk H. Presentation at the 12th annual International Conference on AIDS. A survey of the mental health needs of HIV positive adolescents and young adults. San Fransciso, CA: Health Initiatives for Youth; 1998. [Google Scholar]
- Hosek SG, Harper GW, Domanico R. Psychological predictors of medication adherence among HIV-infected youth. Psychology, Health, and Medicine. 2005;10:166–179. doi: 10.1080/1354350042000326584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. New York: Guilford Press; 1998. [Google Scholar]
- Knight JR, Shrier LA, Bravender TD, Farrell M, Vander Bilt J, Shafer HJ. A new brief screen for adolescent substance abuse. Archives of Pediatric and Adolescent Medicine. 1999;153:591–596. doi: 10.1001/archpedi.153.6.591. [DOI] [PubMed] [Google Scholar]
- Koinis-Mitchell D, McQuaid E, Kopel S, Esteban C, Canino G, Garcia-Coll C, et al. Multiple urban and asthma-related risks and their association with asthma morbidity in children. Journal of Pediatric Psychology. 2007;32:582–595. doi: 10.1093/jpepsy/jsl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littell J, Girvin H. Stages of change: A critique. Behavior Modification. 2002;26:223–273. doi: 10.1177/0145445502026002006. [DOI] [PubMed] [Google Scholar]
- Long J, Freese J. Regression models for categorical dependent variables using stata. College Station: Stata Press; 2006. [Google Scholar]
- Migneault JP, Adams TB, Read JP. Application of the Transtheoretical Model to subatance abuse: historical development and future directions. Drug and Alcohol Review. 2005;24:437–448. doi: 10.1080/09595230500290866. [DOI] [PubMed] [Google Scholar]
- Mullen J, Leech S, O'Shea S, Chrystie I, du Mont G, Ball C, et al. Antiretroviral drug resistance among HIV-1 infected children failing treatment. Journal of Medical Virology. 2002;68:299–304. doi: 10.1002/jmv.10203. [DOI] [PubMed] [Google Scholar]
- Murphy D, Belzer M, Durako S, Sarr M, Wilson C, Muenz L. Longitudinal antiretroviral adherence among adolescents infected with Human Immunodeficiency Virus. Archives of Pediatric & Adolescent Medicine. 2005;159:764–770. doi: 10.1001/archpedi.159.8.764. [DOI] [PubMed] [Google Scholar]
- Murphy DA, Wilson C, Durako S, Muenz L, Belzer M. Antiretroviral medication adherence among the REACH HIV-infected adolescent cohort. AIDS Care. 2001;13:27–40. doi: 10.1080/09540120020018161. [DOI] [PubMed] [Google Scholar]
- Naar-King S, Parsons J, Murphy D, Harris R, Kolmodin K. A multisite randomized trial of Healthy Choices: Design and preliminary outcomes of Motivational Enhancement Therapy targeting multiple risk behaviors in youth living with HIV. AIDS & Behavior. Under review. Manuscript submitted. [Google Scholar]
- Naar-King S, Templin T, Wright K, Frey M, Parsons J, Lam P. Psychosocial factors and medication adherence in HIV-positive youth. AIDS Patient Care & STDs. 2006;20:44–47. doi: 10.1089/apc.2006.20.44. [DOI] [PubMed] [Google Scholar]
- Naar-King S, Wright K, Parsons J, Frey M, Templin T, Ondersma S. Transtheoretical model and substance use in HIV+ youth. AIDS Care. 2006;18:839–845. doi: 10.1080/09540120500467075. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Rosof E, Mustanski B. Medication adherence mediates the relationship between adherence self-efficacy and biological assessments of HIV health among those with alcohol use disorders. AIDS and Behavior. 2008;12:95–103. doi: 10.1007/s10461-007-9241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Rosof E, Mustanski B. Patient related factors predicting HIV medication adherence among men and women with alcohol problems. Journal of Health Psychology. 2007;12:357–370. doi: 10.1177/1359105307074298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson D, Swindells S, Mohr J, et al. How much adherence is enough? A prospective study of adherence to protease inhibitor therapy using MEMS Caps. 1999. Paper presented at the 6th Conference on Retroviruses and Opportunistic Infections. Chicago, IL. [Google Scholar]
- Prochaska JO, Velicer WF, Rossi JS, Goldstein MG, Marcus BH, Rakowski W, et al. Stages of change and decisional balance for 12 problem behaviors. Health Psychology. 1994;13:39–46. doi: 10.1037//0278-6133.13.1.39. [DOI] [PubMed] [Google Scholar]
- Riekert K, Drotar D. Adherence to medical treatment in pediatric chronic illness: Critical issues and answered questions. In: Drotar D, editor. Promoting adherence to medical treatment in chronic childhood illness: Concepts, methods, and interventions. New Jersey: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychological Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- Stott NC, Rollnick S, Rees MR, Pill RM. Innovation in clinical method: Diabetes care and negotiating skills. Family Practice. 1995;12:413–418. doi: 10.1093/fampra/12.4.413. [DOI] [PubMed] [Google Scholar]
- Velicer WF, DiClemente CC, Prochaska JO, Brandenburg N. Decisional balance measure for assessing and predicting smoking status. Journal of Personality & Social Psychology. 1985;48:1279–1289. doi: 10.1037//0022-3514.48.5.1279. [DOI] [PubMed] [Google Scholar]
- Watson D, Farley J. Efficacy of and adherence to highly active antiretroviral therapy in children infected with human immunodeficiency virus type 1. The Pediatric Infectious Disease Journal. 1999;18:682–696. doi: 10.1097/00006454-199908000-00006. [DOI] [PubMed] [Google Scholar]
- Weissberg-Benchell J, Wolpert H, Anderson B. Transitioning from pediatric to adult care: A new approach to the post-adolescent young person with type 1 diabetes. Diabetes Care. 2007;30:2441–2446. doi: 10.2337/dc07-1249. [DOI] [PubMed] [Google Scholar]