Abstract
Astrocytes are the most abundant cells in the central nervous system (CNS). It appears that astrocytes are as diverse as neurons, having different phenotypes in various regions throughout the brain and participating in intercellular communication that involves signaling to neurons. It is not surprising then that astrocytes in the hypothalamus have an active role in the CNS regulation of reproduction. In addition to the traditional mechanism involving ensheathment of neurons and processes, astrocytes may have a critical role in regulating estrogen-positive feedback. Work in our laboratory has focused on the relationship between circulating estradiol and progesterone synthesized de novo in the brain. We have demonstrated that circulating estradiol stimulates the synthesis of progesterone in adult hypothalamic astrocytes, and this neuroprogesterone is critical for initiating the LH surge. Estradiol cell signaling is initiated at the cell membrane and involves the transactivation of metabotropic glutamate receptor type 1a (mGluR1a) leading to the release of intracellular stores of calcium. We used surface biotinylation to demonstrate that estrogen receptor-α (ERα) is present in the cell membrane and has an extracellular portion. Like other membrane receptors, ERα is inserted into the membrane and removed via internalization after agonist stimulation. This trafficking is directly regulated by estradiol, which rapidly and transiently increases the levels of membrane ERα, and upon activation, increases internalization that finally leads to ERα degradation. This autoregulation temporally limits membrane-initiated estradiol cell signaling. Thus, neuroprogesterone, the necessary signal for the LH surge, is released when circulating levels of estradiol peak on proestrus and activate progesterone receptors whose expression has been induced by the gradual rise of estradiol during follicular development.
Key Words: Astrocytes, Surface biotinylation, Estrogen receptor-alpha trafficking
Introduction
Astrocytes are found in abundance throughout the central nervous system (CNS). The relative percentage of astrocytes varies by species with an increasing astrocyte-to-neuron ratio with increasing brain size and complexity [1]. Initially astrocytes were named for their stellate appearance and distribution: protoplasmic in the grey matter, fibrous in the white matter and adult forms of radial glia (i.e. tanicytes of the median eminence, pituicytes of the posterior pituitary, Müller cells of the retina and Bergmann glia of the cerebellar cortex). Later, astrocytes were typically identified by their expression of the intermediate filament, glial fibrillary acidic protein (GFAP). Traditionally, astrocytes have been considered to provide the structural framework that organizes neurons and helps maintain the blood-brain barrier. Over the years, our view of astrocytes has evolved beyond a static role as the CNS stroma. Astrocytes are vital for the maintenance of local ionic concentration, neurotransmitter milieu, and regulation of electrical and chemical synaptic transmission through dynamically changing ensheathment of neuronal somata and processes [2]. One of the best-studied examples of the changing relationship between astrocytes and neurons is in the supraoptic nucleus of the hypothalamus. Activation of oxytocin magnocellular neurons leads to decreased astrocytic coverage allowing electrical coupling and synchronous firing that results in the pulsatile release of oxytocin [3,4,5,6,7,8,9]. Similar effects of dynamic astrocytic ensheathment have been reported in the arcuate nucleus and median eminence of the hypothalamus in response to fluctuations of sex steroid hormones [10,11]. These changes in astrocytic coverage appear to coincide with an increase in dendritic spines and their synapses [12,13,14].
It has become clear that astrocytes express a wide diversity of phenotypes. As with neurons, astrocytes appear to have different phenotypes throughout the brain [10,15,16,17,18,19,20,21], including differential expression of receptors for extracellular signaling molecules such as estradiol, ATP, glutamate, melanocortin, and norepinephrine [22,23,24,25,26,27,28,29,30]. This variation of receptors allows astrocytes to sense and respond to appropriate extracellular signals in specific brain regions leading to the modulation of calcium and other signaling pathways [22,31,32].
A variety of transmitters increase free cytosolic calcium ([Ca2+]i) through the release of intracellular calcium stores in astrocytes. In distinction to neurons, however, astrocytes are able to propagate Ca2+ waves over long distances since they are coupled through gap junctions [33,34,35,36,37,38,39]. Calcium waves activate glial synaptic mechanisms to trigger the release of glial transmitters (e.g. glutamate, serine, ATP and taurine), which has been termed ‘gliotransmission’ [9,40,41,42,43]. Thus, by responding to neurotransmitters and releasing their own gliotransmitters, astrocytes engage in a dynamic bidirectional cross talk with neurons and participate in the intercellular signaling of the CNS [9,44].
It is not surprising then that astrocytes have an active role in CNS regulation of reproductive physiology. Similar to the other types of glial-neuronal interaction, astrocytes help regulate the function of gonadotropin-releasing hormone (GnRH) neurons, including modulation of synaptic input [14,45,46,47,48,49] and access of GnRH terminals to the hypothalamo-hypophyseal portal capillaries in the median eminence [50,51,52]. In addition, astrocytes secrete a number of factors that stimulate GnRH release, including TGF-α, TGF-β, IGF-1, progesterone and 3α,5α-tetrahydro-progesterone (3α, 5α-THP) [[52,53,54,55], reviewed in [11], [56]]. We have been interested in studying how circulating estradiol regulates the activity of astrocytes that are involved in the central control of reproduction.
Astrocytes and Reproduction in Females
The Luteinizing Hormone Surge
The central event in female reproduction is the surge release of LH from anterior pituitary gonadotropes. These cells are controlled by a network of GnRH neurons that project to the median eminence where they make a neurohemal contact with portal capillaries serving the anterior pituitary. The resulting surge release of LH induces ovulation and subsequent luteinization of the ruptured ovarian follicles. The hormonal event that triggers increased GnRH activity to signal the surge release of LH is estrogen-positive feedback. This is a well-known but somewhat ill-defined process through which increasing levels of circulating estradiol, derived from developing follicles, act on the hypothalamus to induce the surge release of GnRH. This is dependent upon high levels of estradiol and progesterone, which activate their respective cognate receptors to stimulate the GnRH network [[57,58,59,60,61], reviewed in [43], [62]].
Since GnRH neurons do not express ERα, estradiol must activate a CNS network of astrocytes and neurons, which then indirectly stimulate GnRH neurons and the surge release of LH. A large number of peptide and non-peptide transmitters have been implicated in this process, but they do not appear critical [[63], reviewed in [64]]. The exception may be kisspeptin, a neuropeptide that has been suggested as the proximal neuronal signal for GnRH release [65,66,67]. Indeed, kisspeptin-containing neurons express ERα, progesterone receptor (PR), and the G protein-coupled receptor (GPCR) GPR54, a receptor shown to be essential for normal GnRH secretion [65,68,69].
While progesterone has been demonstrated to be necessary for the LH surge, its source has been debated. A telling experiment showed that in ovariectomized and adrenalectomized (ovx/adx) rats, estradiol priming without exogenous progesterone induced a physiological LH surge [61,70]. However, the LH surge was blocked if progesterone synthesis was disrupted with trilostane, a 3β-hydroxysteroid dehydrogenase inhibitor, in ovx/adx rats. When progesterone was given back to the estradiol-primed, trilostane-treated ovx/adx rats, the LH surge was restored. These results are congruent with a critical role for progesterone in the LH surge [71,72,73,74], and suggested that in ovx/adx rats progesterone synthesis continued – most likely in the CNS. This was verified by measuring an estradiol-induced increase of hypothalamic progesterone in the face of low to undetectable circulating levels of progesterone. Since the early 1980s, it has been accepted that the brain is a steroidogenic organ [reviewed in [75], [76]] and that astrocytes may be the most active steroidogenic cells in the CNS [77,78].
Work in our laboratory has focused on the relationship between circulating estradiol and neuroprogesterone, progesterone synthesized de novo in the brain. To demonstrate the importance of hypothalamic progesterone, a P450 side-chain cleavage (P450scc) enzyme inhibitor, aminoglutethimide (AGT), was infused into the third ventricle on the morning of proestrus to block hypothalamic steroidogenesis in gonadally intact rats with normal estrous cycles. Central AGT prevented the LH surge and ovulation, but did not disrupt peripheral steroidogenesis [78]. In AGT-treated rats, hypothalamic levels of neuroprogesterone were significantly reduced compared to vehicle treated rats – who continued to cycle as assessed by vaginal cytology. Moreover, the AGT-treated rats did not transition from proestrus to estrus, which requires the LH surge. When the AGT was metabolized after several days, the rats ovulated and resumed their estrous cycle. These results suggested a sequence of events in which peripheral estradiol (of ovarian origin) stimulates neuroprogesterone synthesis [79,80]. In the intact rat, as follicles develop in the ovary, circulating levels of estradiol increase and induce PRs in hypothalamic neurons, including kisspeptin neurons that lie along the rostral third ventricle [81]. When estradiol peaks during proestrus, it induces progesterone synthesis in hypothalamic astrocytes. This hypothalamic neuroprogesterone then acts as a trigger: stimulating estrogen-induced PRs, activating kisspeptin neurons that excite GnRH neurons, and initiating the LH surge [81,82]. Based on such observations of neuroprogesterone action, progesterone synthesized in the brain, like other neurosteroids, is a fourth-generation transmitter. Such fourth-generation transmitters are a family of diverse molecules that are regulated at the level of synthesis rather than release. Other examples include nitric oxide, carbon monoxide, prostaglandins and endocannabinoids [78].
Astrocytes and Neuroprogesterone
Neurons, oligodendrocytes and astrocytes are all capable of steroidogenesis [77]. However, the most probable source of neuroprogesterone is from astrocytes [[32], [55], reviewed in [43]]. Both whole hypothalamus in vivo and hypothalamic astrocyte cultures from postpubertal female rats express the enzymes and associated proteins needed for progesterone synthesis: P450scc, 3β-hydroxysteroid dehydrogenase (3β-HSD), steroid acute regulatory protein (StAR), and sterol carrier protein-2 (SCP-2) [32,59]. Estradiol has been shown to stimulate progesterone synthesis in hypothalamic astrocytes and whole hypothalamus, which corresponded with an increased expression of 3β-HSD mRNA and enzyme activity [reviewed in [83]]. Interestingly, estradiol did not increase hypothalamic progesterone levels in acyclic female rats with persistent estrus suggesting that reproductive aging may be the result of a lack of estrogen-induced neuroprogesterone synthesis [83]. Furthermore, no increase in hypothalamic progesterone was measured in male rats, consistent with the inability of male rodents to display an estrogen-positive feedback surge of LH [61].
Parallel studies in vitro showed that estradiol rapidly increased [Ca2+]i flux in astrocytes through a phospholipase C (PLC)/inositol triphosphate (IP3) receptor-mediated pathway [22]. This effect was stereospecific, reproduced with E-6-BSA and blocked with ICI 182,780, indicating that estradiol is signaling via a classical ER associated with the cell membrane. Further evidence for classical ER-mediated membrane signaling is supported by astrocytic expression of ERα and ERβ in both the cytoplasmic and membrane fractions. In subsequent experiments, physiological levels of estradiol or E-6-BSA increased [Ca2+]i flux through membrane ER activation [43]. Since calcium is a general signal in astrocytes, we needed to determined whether the increase in [Ca2+]i flux was the intracellular signal through which estradiol acted to rapidly increase progesterone synthesis. To this end, thapsigargin, a Ca2+-ATPase inhibitor that mobilizes IP3 receptor-sensitive calcium stores, was used [32]. Thapsigargin was as effective as estradiol at facilitating progesterone synthesis in astrocytes, indicating that de novo progesterone synthesis in astrocytes is dependent on the estradiol-induced [Ca2+]i flux.
A great deal of evidence now exists that the same ER proteins that interact with the estrogen response element (ERE) on DNA in the nucleus is also trafficked to and associated with the cell membrane. In addition to the classic ERα and ERβ, several other estrogen-binding proteins have been proposed including ER-X, GPR30 and a STX-activated protein [reviewed in [84]]. To date, we have concentrated on ERα and ERβ because: (1) transfection of ER-negative cells with ERα or ERβ mRNA produce cognate proteins in the membrane fraction that are functional [85]; (2) Western blots with ER-specific antibodies demonstrate both full-length and splice variants of the ER in the membrane fraction of neurons and astrocytes [22,86,87]; (3) the binding affinity (KD) for the membrane ER and nuclear ER is similar, but there are fewer ERs on the membrane (BMAX) [88], and (4) ERα and ERβ proteins are trafficked and attached to the membrane through palmitoylation and in association with calveolin proteins [89,90,91,92,93].
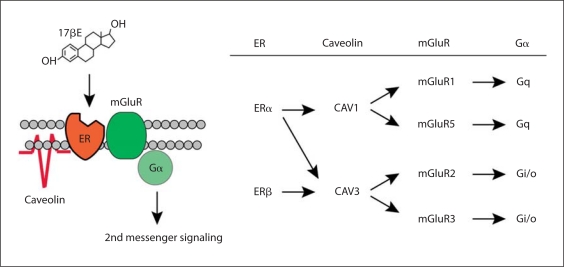
Membrane-initiated estradiol signaling can activate a number of GPCR associated pathways, including [Ca2+]i flux, cAMP, DAG, IP3, protein kinase C (PKC), PKA, MAPK/ERK and phosphorylation of cAMPresponse element-binding protein (CREB) [94,95,96], reviewed in [97]. ERα and ERβ activate intracellular signaling cascades by interacting with bona fide membrane receptors such as growth factor tyrosine kinase receptors or metabotropic glutamate receptors (mGluRs) to initiate cell signaling [[98,99,100,101,102,103,104], reviewed in [75], [79]]. In the CNS, membrane ERs interaction with mGluRs has been reported in many different brain regions [reviewed in [79]]. The mGluRs are glutamate-binding proteins grouped according to sequence homology and second messenger linkage: mGluR1 and mGluR5, coupled to Gq, are group I mGluRs; mGluR2 and mGluR3, activating Gi/Go signaling, are group II mGluRs; and mGluR4, mGluR6, mGluR7 and mGluR8, also Gi/Go coupled, comprise group III mGluRs. Co-immunoprecipitation demonstrated the probable physiological interaction of ERs and mGluRs in both neurons and astrocytes (fig. 1) [99,100,105].
Fig. 1.
ER activation of mGluR signaling through interactions with caveolin proteins. a Model framework of estradiol-induced activation of mGluRs via caveolin-based caveolae. b Summary of previous findings demonstrating ER activation of group I (mGluR1/5) and group II (mGluR2/3) metabotropic glutamate receptors is mediated by caveolin 1 and caveolin 3, respectively. From Micevych and Mermelstein [75].
While the details of the transactivation of mGluRs by ERs have not been elucidated, the downstream actions of estradiol-initiated transactivation of mGluR2/3 leads to inhibition of Ca2+ influx through the L-type voltage-gated calcium channel (VGCC) [35,98,106,107], while ERα transactivation of the mGluR1a activates the PLC pathway, increasing [Ca2+]i flux, activating PKC, and phosphorylating CREB [98,100,105]. In terms of reproduction, ERα interacts with mGluR1a to initiate lordosis behavior though activation of a novel PKC and increases neuroprogesterone synthesis, which is a necessary step for estrogen positive feedback [100,108]. Membrane-initiated activation of cell signaling can be blocked with the specific ER antagonist ICI 182,780 or the selective mGluR1a antagonist LY367385. Activating the mGluR1a with (S)-3,5-dihydroxyphenylglycine (DHPG) without estradiol mimicked the steroid-induced [Ca2+]i flux [100].
ERα in the Membrane
Evidence that reproduction is dependent on ERα, including the membrane ERα, is very strong [60], [61], [109], [110], but see [111]]. Membrane impermeable estradiol constructs (e.g. E-6-BSA) and studies showing ERα protein in the membrane fraction of native or transfected cells indicate an ERα association with the membrane [35,85,99,105,106,112,113,114,115,116,117,118]. Since ERα does not have the typical structure of a classic membrane protein, this association has remained unresolved. It is not clear whether ERα is anchored to the inner leaflet of the membrane or an integral membrane protein with an exposed extracellular portion. A method that has been useful in identifying integral membrane proteins is surface biotinylation. Proteins that have an extracellular portion are labeled with biotin, subsequently concentrated using an avidin column, eluted and characterized by Western blotting [119].
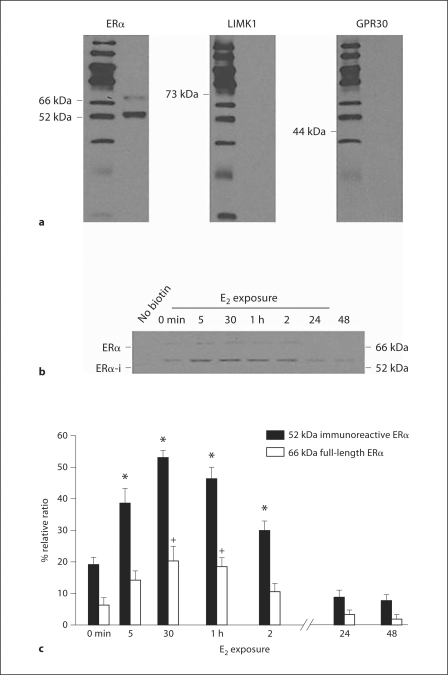
Using this technique, membrane ERs were labeled with the membrane-impermeable biotin reagent in primary cultures of hypothalamic neurons and astrocytes (fig. 2) [86,87,120]. These studies demonstrated surface biotinylated ERα-immunoreactive proteins, indicating that a portion of the ERα is exposed on the cell surface and implying that ERα is a membrane protein. Together with the functional E-6-BSA studies that showed activation of PKC and [Ca2+]i flux, these results suggest that the exposed portion of the ERα contained the ligand-binding site. Interestingly, while both groups identified a lower molecular weight (MW) form (50–55 kDa), as the major ERα-immunoreactive protein in the membrane, only our group detected a 66-kDa protein that was assumed to be the full-length ERα (fig. 2).
Fig. 2.
Postpubertal hypothalamic astrocytes were incubated with vehicle (0 min) or in the presence of 1 nM estradiol (E2) for 5, 30 min, 1, 2, 24 and 48 h. Astrocytes were then surface biotinylated, excess biotin removed, and the labeled proteins separated and detected with ERα, GPR30 and LIM domain kinase 1 (LIMK1) antibodies. a Two ERα-immunoreactive (ERα-i) ER bands were identified: 66 and 52 kDa. The cytoplasmic protein LIMK1 and the putative membrane ER GPR30 were not labeled with surface biotinylation. b Estradiol treatment (1 nM) increased both the 66- and 52-kDa ERα-i. In the first lane, cells were not surface biotinylated (no biotin), thus no biotinylated ERα-i was labeled. Detection of the 66-kDA ERα required a 2-hour exposure compared with a 1- to 2-min exposure for the 52-kDa ERα-i. c Quantification of the 66- and 52-kDa ERα-i was calculated by comparing the optical density of the ERα-i bands with that of the β-actin bands. Both 66- and 52-kDa ERα-i are regulated in parallel by E2 treatment, but the amount of 66-kDa ERα was much less at each time point. Data are mean ± SEM (n = 4). *,+ Statistical differences at the p < 0.05 level compared with 0 min for each molecular weight species. From Bondar et al. [87].
To determine whether these proteins were derived from the ERα gene, astrocytes from wild-type and ERα-disrupted (ERKO) mice were surface biotinylated [87]. As in the experiments conducted with rat astrocytes, both 52- and 66-kDa ERα proteins were detected in wild-type mouse astrocytes. However, ERKO astrocytes had neither the 52- nor the 66-kDa proteins, indicating that both proteins were derived from the ERα gene. It is likely that the 66-kDa protein is the full-length ERα, but the identity of the 52-kDa protein has not been resolved. The 52-kDa protein may potentially be an alternatively spliced form of ERα. Numerous alternatively spliced forms of ERα mRNA have been identified in a variety of estrogen-sensitive tissues, including the brain [88,121,122,123,124,125,126]. Some of these alternatively spliced mRNAs are translated into proteins [88,121,127,128,129,130]. Based on the MW of the predominant membrane ERα, the most probable splice variant is the exon 7-deleted form (ERαΔ7), with a predicted MW of 52 kDa. The resulting protein, however, is truncated and missing the COOH-terminal end of the full-length ERα, which includes part of the ligand-binding domain [88,122,131,132]. Both NH2-terminal-directed, H-184, and COOH-terminal directed, MC-20, antibodies recognized the 52-kDa protein, and E-6-BSA stimulated cell signaling indicating that it is unlikely to be the truncated ERαΔ7 product [87]. Another possible splice variant, ERαΔ4, has an apparent MW of 54 kDa. This splice variant is missing exon 4 of the full-length ERα, which codes for the DNA-binding domain and the hinge region, but retains the COOH-terminal amino acid sequence recognized by MC-20. In addition to our studies, other groups have also identified ERα splice variants using specific antibodies directed against both the NH2- and COOH-terminal ERα domains [88,122,131,132], but mass spectrometry studies (MALDI-TOF) focusing on identifying the membrane ERα have not been successful [93,133].
Regardless of the identity of the ERα splice variant, it is important to note that the full-length ERα was demonstrated in the membrane since it is probably the ERα needed for signaling. A previous study identified both full-length 66- and 55-kDa ERα in an endothelial cell line from the hypothalamus, but only the full-length ERα-bound estradiol and increased [Ca2+]i flux [88]. Based on co-immunoprecipitation with mGluR1a, membrane-initiated estrogen signaling in astrocytes is mediated by the 66-kDa ERα [100]. No lower MW ERα variants co-immunoprecipitated with mGluR1a. Currently, the identity of the 52-kDa ERα is unknown and will require further experimentation to understand its function. Interestingly, GPR30, a putative G protein-coupled membrane ER, was not detected in surface-biotinylated fractions from astrocytes (fig. 2) [87], confirming previous results suggesting that GPR30 may not be present on the cell membrane [120,134,135].
ER Trafficking to and from the Cell Membrane
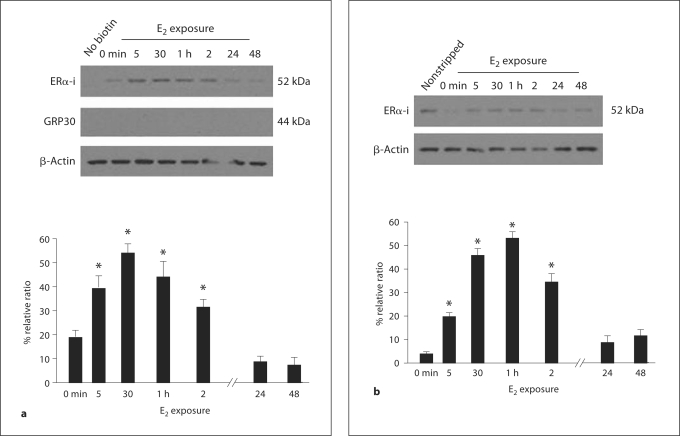
If ERα is a membrane receptor, its levels would be modulated at the cell surface. Membrane receptor populations are dynamic: inserted into and removed from the membrane. In addition, membrane receptors are massively internalized following agonist binding. To ascertain if ERα was trafficked to and/or internalized from the membrane, astrocyte cultures were exposed to estradiol for increasing intervals and then surface biotinylated. Estradiol treatment modulated the 52- and 66-kDa ERα proteins in parallel. In untreated astrocytes, levels of ERα in the membrane were low, but 5 min of estradiol treatment significantly increased the amount of ERα trafficked to the membrane and peak levels were reached after 30 min of estradiol exposure. Levels remained elevated with up to 2 h of estradiol exposure, but decreased to below basal concentration by 24 h, where they remained for the duration of the experiment (fig. 3) [87]. This transient increase of membrane ERα was dependent on estradiol as demonstrated by inhibition of trafficking with the ER antagonist ICI 182,780.
Fig. 3.
a Estradiol (E2) treatment transiently increases membrane ERα-i in postpubertal hypothalamic astrocytes. Basal levels of ERα-i were observed prior to E2 treatment (0 min). These levels were rapidly increased (5 min time point) (* p < 0.05), with a maximum at 30 min (* p < 0.05) and a slight depression after 1 h (* p < 0.05), and remained elevated at 2 h with 1 nM E2 stimulation (* p < 0.05). After 24–48 h of E2 treatment, ERα-i returned to basal levels (p > 0.05). GPR30 was not surface biotinylated despite incubating the astrocytes with estradiol for up to 48 h. b To track internalization, astrocytes were biotinylated, treated with estradiol (1 nM), and then biotin stripped from the cell surface with glutathione. Under these conditions, ERα-i is biotinylated, but after glutathione treatment, the only biotinylated receptors remaining are those that were internalized. The time course of internalization matched the time course of the estradiol-induced trafficking to the membrane. In the first lane, the biotin was not removed by glutathione (nonstripped). The amount of internalized ERα-i, with varying estradiol (1 nM) treatment, began increasing at 5 min (* p < 0.05) and reached its maximum at 30 min to 1 h (* p < 0.05). After 2 h of estradiol incubation, the level of internalized ERα decreased compared with the maximum but was still statistically significant from the 0 min time point (* p < 0.05). At the 24–48 h time points, internalized ERα-i levels reached basal levels comparable to 0 min (p > 0.05). All the data are mean ± SEM (n = 4). * Statistical differences at the p < 0.05 level compared with 0 min for each experiment. From Bondar et al. [87].
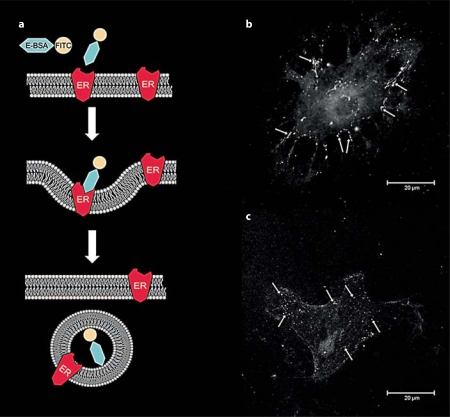
Estradiol not only increased trafficking of ERα to the membrane, but also internalization (fig. 3) [87]. In this experiment, astrocytes were surface biotinylated, treated with estradiol and then incubated with glutathione to remove the biotin from the cell surface. Any remaining biotinylated ERα must have been internalized and thus, protected from the glutathione. The time course of internalization, a marker for membrane receptor activation [reviewed in [136]], mimicked the pattern of membrane ERα insertion: estradiol increased the amount of internalized ERα at 5 min and remained elevated after 2 h. By 24 h, the amount of internalized ERα was below prestimulation levels. These observations of receptor internalization complement studies using a membrane-impermeable E-6-BSA-FITC [β-estradiol-6-(O-carboxymethyl) oxime-bovine serum albumin conjugated with fluorescein isothiocyanate] construct, which binds to and labels membrane ERs [84,114]. When cells are allowed to interact for a period of time (≥5 min) with the E-6-BSA-FITC, the fluorescent marker is seen within intracellular vesicles thought to be early endosomes, which is suggestive of internalization and is a morphological equivalent of the surface biotinylation experiments (fig. 4).
Fig. 4.
E-6-BSA-FITC is internalized in primary cultures of adult hypothalamic astrocytes. a Experiment in which binding of the membrane-impermeable E-6-BSA-FITC complex to membrane ER induces internalization. Estradiol-bound ERs are internalized and transported to endosomes in which the highly acidic environment facilitates the disassociation of the ligand from its receptor. b, c Confocal images of primary hypothalamic astrocytes grown on glass coverslips and treated with 100 μg/ml E-6-BSA-FITC for 5 min (b) and 30 min (c) at 37°C, then fixed with 4% paraformaldehyde. Arrows indicate binding of the E-6-BSA-FITC to ER on the cell membrane (b) and the internalized complex (c).
Proximal events in membrane-initiated cell signaling involve an ERα-mGluR1a interaction. The available evidence suggests that the ER and the mGluR are inserted together into the cell membrane as a complex [89]. To confirm this, we tested whether estradiol regulated ERα and mGluR1a trafficking in astrocytes. Indeed, ERα and the mGluR1a were trafficked to the cell membrane and internalized in tandem [87]. The rapidity of the estradiol-induced insertion into the cell membrane suggests a delivery mechanism consisting of exocytic vesicles loaded with the ERα-mGluR1a complex. In support of this, estradiol induces exocytosis of ERα-immunoreactive vesicles in hippocampal neurons and pituitary cells [137]. Trafficking of ERα to the membrane requires palmitoylation and association with calveolin proteins [91,92]. Calveolin proteins determine the association of ERα and ERβ with specific mGluRs (fig. 1) [75,89]. For example, ERα interaction with either mGluR1 or mGluR2/3 is dependent upon either caveolin 1 or caveolin 3, respectively [reviewed in [75]]. Disrupting calveolin synthesis prevented insertion into the membrane. Similarly, antagonizing mGluR1a with LY 367385 or ERα with ICI 182,780 prevented trafficking of both the mGluR1a and ERα.
In summary, these results indicate that astrocytes have an important function within the CNS network that regulates reproduction. These cells express a functional membrane ERα that associates with mGluR1a to activate GPCR cell signaling pathways. Circulating estradiol regulates the levels of membrane ERα on the astrocyte membrane thereby modulating its own membrane-initiated cell signaling [87]. In the context of estrogen-positive feedback, rising estradiol levels increase the concentration of membrane ERα and mGluR1a in hypothalamic astrocytes (fig. 5). The ERα-mGluR1a complex is activated by spiking estradiol levels on the morning of proestrus, releasing intracellular calcium stores that stimulate neuroprogesterone synthesis. The transient increase of neuroprogesterone stimulates local estradiol-induced PRs initiating the LH surge. Rapid estradiol signaling and progesterone synthesis are constrained by the internalization and eventual degradation of ERα, preventing continuous signaling. Thus, membrane-initiated estradiol signaling in astrocytes is an important step in the regulation of reproduction.
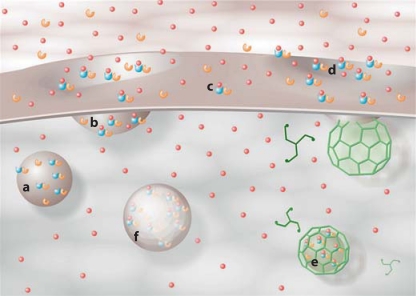
Fig. 5.
Schematic diagram illustrating estradiol-mediated ERα trafficking at the membrane. ERα-mGluR1a (blue-orange) complexes are inserted into the membrane of an exocytotic vesicle (a). These are associated through interactions with calveolin as depicted in figure 1. Estradiol (red spheres) induces vesicle docking with the membrane and insertion of the ERα-mGluR1a complex into the membrane (b). Colors refer to the online version only. Exposed to the extracellular space, estradiol binds to ERα (c), transactivating mGluR1a and initiating cell signaling. Estradiol-activated ERα-mGluR1a complexes are then internalized via a clathrin-mediated process (d) and fuse with early endosomes (e) where the estradiol is released from ERα. The ERα-mGluR1a complex can then be recycled to the membrane or degraded. Initially, there appears to be a recycling of the ERα-mGluR1a complex, but with continued stimulation, the process shifts toward degradation (fig. 2, 3).
Acknowledgements
We are grateful to Drs Kevin Sinchak and Phoebe Dewing, and Ms. Amy Christensen for their constructive comments on the manuscript. The research from our laboratory presented here was supported by NIH grants DA013185, HD042635 and HD001281.
References
- 1.Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- 3.Hatton GI, Tweedle CD. Magnocellular neuropeptidergic neurons in hypothalamus: increases in membrane apposition and number of specialized synapses from pregnancy to lactation. Brain Res Bull. 1982;8:197–204. doi: 10.1016/0361-9230(82)90046-6. [DOI] [PubMed] [Google Scholar]
- 4.Tweedle CD, Hatton GI. Ultrastructural changes in rat hypothalamic neurosecretory cells and their associated glia during minimal dehydration and rehydration. Cell Tissue Res. 1977;181:59–72. doi: 10.1007/BF00222774. [DOI] [PubMed] [Google Scholar]
- 5.Theodosis DT, Poulain DA. Evidence for structural plasticity in the supraoptic nucleus of the rat hypothalamus in relation to gestation and lactation. Neuroscience. 1984;11:183–193. doi: 10.1016/0306-4522(84)90222-7. [DOI] [PubMed] [Google Scholar]
- 6.Theodosis DT, Poulain DA, Vincent JD. Possible morphological bases for synchronisation of neuronal firing in the rat supraoptic nucleus during lactation. Neuroscience. 1981;6:919–929. doi: 10.1016/0306-4522(81)90173-1. [DOI] [PubMed] [Google Scholar]
- 7.Hawrylak N, Boone D, Salm AK. The surface density of glial fibrillary acidic protein immunopositive astrocytic processes in the rat supraoptic nucleus is reversibly altered by dehydration and rehydration. Neurosci Lett. 1999;277:57–60. doi: 10.1016/s0304-3940(99)00864-2. [DOI] [PubMed] [Google Scholar]
- 8.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 9.Theodosis DT, Poulain DA, Oliet SH. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev. 2008;88:983–1008. doi: 10.1152/physrev.00036.2007. [DOI] [PubMed] [Google Scholar]
- 10.Mong JA, Blutstein T. Estradiol modulation of astrocytic form and function: implications for hormonal control of synaptic communication. Neuroscience. 2006;138:967–975. doi: 10.1016/j.neuroscience.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Segura LM, McCarthy MM. Minireview: role of glia in neuroendocrine function. Endocrinology. 2004;145:1082–1086. doi: 10.1210/en.2003-1383. [DOI] [PubMed] [Google Scholar]
- 12.Csakvari E, Hoyk Z, Gyenes A, Garcia-Ovejero D, Garcia-Segura LM, Parducz A. Fluctuation of synapse density in the arcuate nucleus during the estrous cycle. Neuroscience. 2007;144:1288–1292. doi: 10.1016/j.neuroscience.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto A, Arai Y. Neuronal plasticity in the deafferented hypothalamic arcuate nucleus of adult female rats and its enhancement by treatment with estrogen. J Comp Neurol. 1981;197:197–205. doi: 10.1002/cne.901970203. [DOI] [PubMed] [Google Scholar]
- 14.Schwarz JM, Liang SL, Thompson SM, McCarthy MM. Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: a mechanism for organizational sex differences. Neuron. 2008;58:584–598. doi: 10.1016/j.neuron.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chneiweiss H, Glowinski J, Premont J. Modulation by monoamines of somatostatin-sensitive adenylate cyclase on neuronal and glial cells from the mouse brain in primary cultures. J Neurochem. 1985;44:1825–1831. doi: 10.1111/j.1471-4159.1985.tb07175.x. [DOI] [PubMed] [Google Scholar]
- 16.el-Etr M, Cordier J, Torrens Y, Glowinski J, Premont J. Pharmacological and functional heterogeneity of astrocytes: regional differences in phospholipase C stimulation by neuromediators. J Neurochem. 1989;52:981–984. doi: 10.1111/j.1471-4159.1989.tb02551.x. [DOI] [PubMed] [Google Scholar]
- 17.Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc Natl Acad Sci USA. 2005;102:5606–5611. doi: 10.1073/pnas.0408483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morga E, Faber C, Heuschling P. Regional heterogeneity of the astroglial immunoreactive phenotype: effect of lipopolysaccharide. J Neurosci Res. 1999;57:941–952. [PubMed] [Google Scholar]
- 19.Hansson E. Co-existence between receptors, carriers, and second messengers on astrocytes grown in primary cultures. Neurochem Res. 1989;14:811–819. doi: 10.1007/BF00964809. [DOI] [PubMed] [Google Scholar]
- 20.Porter JT, McCarthy KD. Astrocytic neurotransmitter receptors in situ and in vivo. Prog Neurobiol. 1997;51:439–455. doi: 10.1016/s0301-0082(96)00068-8. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz JP, Taniwaki T. Heterogeneity of expression of neuropeptide genes by astrocytes: functional implications. Perspect Dev Neurobiol. 1994;2:251–257. [PubMed] [Google Scholar]
- 22.Chaban VV, Lakhter AJ, Micevych P. A Membrane Estrogen Receptor Mediates Intracellular Calcium Release in Astrocytes. Endocrinology. 2004;145:3788–3795. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- 23.Langub MC, Jr, Watson RE., Jr Estrogen receptor-immunoreactive glia, endothelia, and ependyma in guinea pig preoptic area and median eminence: electron microscopy. Endocrinology. 1992;130:364–372. doi: 10.1210/endo.130.1.1727710. [DOI] [PubMed] [Google Scholar]
- 24.Donahue JE, Stopa EG, Chorsky RL, King JC, Schipper HM, Tobet SA, Blaustein JD, Reichlin S. Cells containing immunoreactive estrogen receptor-alpha in the human basal forebrain. Brain Res. 2000;856:142–151. doi: 10.1016/s0006-8993(99)02413-0. [DOI] [PubMed] [Google Scholar]
- 25.Kruijver FP, Balesar R, Espila AM, Unmehopa UA, Swaab DF. Estrogen receptor-alpha distribution in the human hypothalamus in relation to sex and endocrine status. J Comp Neurol. 2002;454:115–139. doi: 10.1002/cne.10416. [DOI] [PubMed] [Google Scholar]
- 26.Hosli L, Hosli E. Receptors for dopamine and serotonin on astrocytes of cultured rat central nervous system. J Physiol (Paris) 1987;82:191–195. [PubMed] [Google Scholar]
- 27.Sharma G, Vijayaraghavan S. Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores. Proc Natl Acad Sci USA. 2001;98:4148–4153. doi: 10.1073/pnas.071540198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verkhratsky A, Steinhauser C. Ion channels in glial cells. Brain Res Brain Res Rev. 2000;32:380–412. doi: 10.1016/s0165-0173(99)00093-4. [DOI] [PubMed] [Google Scholar]
- 29.Caruso C, Durand D, Schioth HB, Rey R, Seilicovich A, Lasaga M. Activation of melanocortin 4 receptors reduces the inflammatory response and prevents apoptosis induced by lipopolysaccharide and interferon-gamma in astrocytes. Endocrinology. 2007;148:4918–4926. doi: 10.1210/en.2007-0366. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Segura LM, Chowen JA, Naftolin F. Endocrine glia: roles of glial cells in the brain actions of steroid and thyroid hormones and in the regulation of hormone secretion. Front Neuroendocrinol. 1996;17:180–211. doi: 10.1006/frne.1996.0005. [DOI] [PubMed] [Google Scholar]
- 31.Dhandapani KM, Brann DW. Role of astrocytes in estrogen-mediated neuroprotection. Exp Gerontol. 2007;42:70–75. doi: 10.1016/j.exger.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 32.Micevych PE, Chaban V, Ogi J, Dewing P, Lu JK, Sinchak K. Estradiol stimulates progesterone synthesis in hypothalamic astrocyte cultures. Endocrinology. 2007;148:782–789. doi: 10.1210/en.2006-0774. [DOI] [PubMed] [Google Scholar]
- 33.Hansson E, Ronnback L. Glial neuronal signaling in the central nervous system. Faseb J. 2003;17:341–348. doi: 10.1096/fj.02-0429rev. [DOI] [PubMed] [Google Scholar]
- 34.Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 35.Chaban VV, Mayer EA, Ennes HS, Micevych PE. Estradiol inhibits ATP-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003;118:941–948. doi: 10.1016/s0306-4522(02)00915-6. [DOI] [PubMed] [Google Scholar]
- 36.Micevych PE, Abelson L. Distribution of mRNAs coding for liver and heart gap junction proteins in the rat central nervous system. J Comp Neurol. 1991;305:96–118. doi: 10.1002/cne.903050110. [DOI] [PubMed] [Google Scholar]
- 37.Spray DC: Gap junctions in the nervous system; in Spray DC, Dermietzel R (eds): Gap Junctions in the Nervous System. Georgetown, Landes, 1996, pp 1–11.
- 38.Giaume C, McCarthy KD. Control of gap-junctional communication in astrocytic networks. Trends Neurosci. 1996;19:319–325. doi: 10.1016/0166-2236(96)10046-1. [DOI] [PubMed] [Google Scholar]
- 39.Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. J Neurosci. 2001;21:2215–2223. doi: 10.1523/JNEUROSCI.21-07-02215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parpura V, Haydon PG. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci USA. 2000;97:8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 42.Santello M, Volterra A. Synaptic modulation by astrocytes via Ca2+-dependent glutamate release. Neuroscience. 2009;158:253–259. doi: 10.1016/j.neuroscience.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 43.Micevych P, Kuo J, Christensen A. Physiology of membrane oestrogen receptor signalling in reproduction. J Neuroendocrinol. 2009;21:249–256. doi: 10.1111/j.1365-2826.2009.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mantyh PW, Johnson DJ, Boehmer CG, Catton MD, Vinters HV, Maggio JE, Too HP, Vigna SR. Substance P receptor binding sites are expressed by glia in vivo after neuronal injury. Proc Natl Acad Sci USA. 1989;86:5193–5197. doi: 10.1073/pnas.86.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mong JA, McCarthy MM. Steroid-induced developmental plasticity in hypothalamic astrocytes: implications for synaptic patterning. J Neurobiol. 1999;40:602–619. doi: 10.1002/(sici)1097-4695(19990915)40:4<602::aid-neu14>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 46.Rozovsky I, Wei M, Stone DJ, Zanjani H, Anderson CP, Morgan TE, Finch CE. Estradiol (E2) enhances neurite outgrowth by repressing glial fibrillary acidic protein expression and reorganizing laminin. Endocrinology. 2002;143:636–646. doi: 10.1210/endo.143.2.8615. [DOI] [PubMed] [Google Scholar]
- 47.Jennes L, Stumpf WE, Sheedy ME. Ultrastructural characterization of gonadotropin-releasing hormone (GnRH)-producing neurons. J Comp Neurol. 1985;232:534–547. doi: 10.1002/cne.902320410. [DOI] [PubMed] [Google Scholar]
- 48.Lehman MN, Karsch FJ, Robinson JE, Silverman AJ. Ultrastructure and synaptic organization of luteinizing hormone-releasing hormone (LHRH) neurons in the anestrous ewe. J Comp Neurol. 1988;273:447–458. doi: 10.1002/cne.902730402. [DOI] [PubMed] [Google Scholar]
- 49.Baroncini M, Allet C, Leroy D, Beauvillain JC, Francke JP, Prevot V. Morphological evidence for direct interaction between gonadotrophin-releasing hormone neurones and astroglial cells in the human hypothalamus. J Neuroendocrinol. 2007;19:691–702. doi: 10.1111/j.1365-2826.2007.01576.x. [DOI] [PubMed] [Google Scholar]
- 50.Kozlowski GP, Coates PW. Ependymoneuronal specializations between LHRH fibers and cells of the cerebroventricular system. Cell Tissue Res. 1985;242:301–311. doi: 10.1007/BF00214542. [DOI] [PubMed] [Google Scholar]
- 51.King JC, Letourneau RJ. Luteinizing hormone-releasing hormone terminals in the median eminence of rats undergo dramatic changes after gonadectomy, as revealed by electron microscopic image analysis. Endocrinology. 1994;134:1340–1351. doi: 10.1210/endo.134.3.8119174. [DOI] [PubMed] [Google Scholar]
- 52.Ojeda SR, Lomniczi A, Sandau US. Glial-gonadotrophin hormone (GnRH) neurone interactions in the median eminence and the control of GnRH secretion. J Neuroendocrinol. 2008;20:732–742. doi: 10.1111/j.1365-2826.2008.01712.x. [DOI] [PubMed] [Google Scholar]
- 53.Rage F, Lee BJ, Ma YJ, Ojeda SR. Estradiol enhances prostaglandin E2 receptor gene expression in luteinizing hormone-releasing hormone (LHRH) neurons and facilitates the LHRH response to PGE2 by activating a glia-to-neuron signaling pathway. J Neurosci. 1997;17:9145–9156. doi: 10.1523/JNEUROSCI.17-23-09145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma YJ, Berg-von der Emde K, Rage F, Wetsel WC, Ojeda SR. Hypothalamic astrocytes respond to transforming growth factor-alpha with the secretion of neuroactive substances that stimulate the release of luteinizing hormone-releasing hormone. Endocrinology. 1997;138:19–25. doi: 10.1210/endo.138.1.4863. [DOI] [PubMed] [Google Scholar]
- 55.Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK, Micevych P. Estrogen induces de novo progesterone synthesis in astrocytes. Dev Neurosci. 2003;25:343–348. doi: 10.1159/000073511. [DOI] [PubMed] [Google Scholar]
- 56.Mahesh VB, Dhandapani KM, Brann DW. Role of astrocytes in reproduction and neuroprotection. Mol Cell Endocrinol. 2006;246:1–9. doi: 10.1016/j.mce.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 57.Chappell PE, Levine JE. Stimulation of gonadotropin-releasing hormone surges by estrogen. I. Role of hypothalamic progesterone receptors. Endocrinology. 2000;141:1477–1485. doi: 10.1210/endo.141.4.7428. [DOI] [PubMed] [Google Scholar]
- 58.MacLusky NJ, McEwen BS. Oestrogen modulates progestin receptor concentrations in some rat brain regions but not in others. Nature. 1978;274:276–278. doi: 10.1038/274276a0. [DOI] [PubMed] [Google Scholar]
- 59.Soma KK, Sinchak K, Lakhter A, Schlinger BA, Micevych PE. Neurosteroids and female reproduction: estrogen increases 3beta-HSD mRNA and activity in rat hypothalamus. Endocrinology. 2005;146:4386–4390. doi: 10.1210/en.2005-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Micevych P, Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK. The luteinizing hormone surge is preceded by an estrogen-induced increase of hypothalamic progesterone in ovariectomized and adrenalectomized rats. Neuroendocrinology. 2003;78:29–35. doi: 10.1159/000071703. [DOI] [PubMed] [Google Scholar]
- 62.Couse JF, Korach KS. Contrasting phenotypes in reproductive tissues of female estrogen receptor null mice. Ann NY Acad Sci. 2001;948:1–8. doi: 10.1111/j.1749-6632.2001.tb03981.x. [DOI] [PubMed] [Google Scholar]
- 63.Scorticati C, Fernandez-Solari J, De Laurentiis A, Mohn C, Prestifilippo JP, Lasaga M, Seilicovich A, Billi S, Franchi A, McCann SM, Rettori V. The inhibitory effect of anandamide on luteinizing hormone-releasing hormone secretion is reversed by estrogen. Proc Natl Acad Sci USA. 2004;101:11891–11896. doi: 10.1073/pnas.0404366101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herbison AE. Physiology of the GnRH neuronal network. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. 3rd ed. San Diego: Academic Press; 2006. pp. 1415–1482. [Google Scholar]
- 65.Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28:8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V) Brain Res Rev. 2008;57:277–287. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett. 2006;401:225–230. doi: 10.1016/j.neulet.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 69.Richard N, Galmiche G, Corvaisier S, Caraty A, Kottler ML. KiSS-1 and GPR54 genes are co-expressed in rat gonadotrophs and differentially regulated in vivo by oestradiol and gonadotrophin-releasing hormone. J Neuroendocrinol. 2008;20:381–393. doi: 10.1111/j.1365-2826.2008.01653.x. [DOI] [PubMed] [Google Scholar]
- 70.Mann DR, Korowitz CD, Barraclough CA. Adrenal gland involvement in synchronizing the preovulatory release of LH in rats. Proc Soc Exp Biol Med. 1975;150:115–120. doi: 10.3181/00379727-150-38985. [DOI] [PubMed] [Google Scholar]
- 71.Hibbert ML, Stouffer RL, Wolf DP, Zelinski-Wooten MB. Midcycle administration of a progesterone synthesis inhibitor prevents ovulation in primates. Proc Natl Acad Sci USA. 1996;93:1897–1901. doi: 10.1073/pnas.93.5.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Remohi J, Balmaceda JP, Rojas FJ, Asch RH. The role of pre-ovulatory progesterone in the midcycle gonadotrophin surge, ovulation and subsequent luteal phase: studies with RU486 in rhesus monkeys. Hum Reprod. 1988;3:431–435. doi: 10.1093/oxfordjournals.humrep.a136722. [DOI] [PubMed] [Google Scholar]
- 73.DePaolo LV. Attenuation of preovulatory gonadotrophin surges by epostane: a new inhibitor of 3beta-hydroxysteroid dehydrogenase. J Endocrinol. 1988;118:59–68. doi: 10.1677/joe.0.1180059. [DOI] [PubMed] [Google Scholar]
- 74.DePaolo LV, Barraclough CA. Dose dependent effects of progesterone on the facilitation and inhibition of spontaneous gonadotropin surges in estrogen treated ovariectomized rats. Biol Reprod. 1979;21:1015–1023. doi: 10.1095/biolreprod21.4.1015. [DOI] [PubMed] [Google Scholar]
- 75.Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Mol Neurobiol. 2008;38:66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schumacher M, Robert F. Progesterone synthesis, metabolism, mechanisms of action, and effects in the nervous system. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Amsterdam: Academic Press; 2002. pp. 683–745. [Google Scholar]
- 77.Zwain IH, Yen SS. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinology. 1999;140:3843–3852. doi: 10.1210/endo.140.8.6907. [DOI] [PubMed] [Google Scholar]
- 78.Micevych P, Sinchak K. Estradiol regulation of progesterone synthesis in the brain. Mol Cell Endocrinol. 2008;290:44–50. doi: 10.1016/j.mce.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mermelstein PG, Micevych PE. Nervous system physiology regulated by membrane estrogen receptors. Rev Neurosci. 2008;19:413–424. doi: 10.1515/revneuro.2008.19.6.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Micevych P, Sinchak K. Synthesis and function of hypothalamic neuroprogesterone in reproduction. Endocrinology. 2008;149:2739–2742. doi: 10.1210/en.2008-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clarkson J, Herbison AE. Oestrogen, kisspeptin, GPR54 and the pre-ovulatory luteinising hormone surge. J Neuroendocrinol. 2009;21:305–311. doi: 10.1111/j.1365-2826.2009.01835.x. [DOI] [PubMed] [Google Scholar]
- 82.Aparicio SA. Kisspeptins and GPR54 – the new biology of the mammalian GnRH axis. Cell Metab. 2005;1:293–296. doi: 10.1016/j.cmet.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 83.Micevych P, Soma KK, Sinchak K. Neuroprogesterone: key to estrogen positive feedback? Brain Res Rev. 2008;57:470–480. doi: 10.1016/j.brainresrev.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front Neuroendocrinol. 2009;30:315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 86.Dominguez R, Micevych P: Estradiol rapidly regulates membrane ERα levels in hypothalamic neurons. J Neurosci. In review. [DOI] [PMC free article] [PubMed]
- 87.Bondar G, Kuo J, Hamid N, Micevych P. Estradiol-induced estrogen receptor-alpha trafficking. J Neurosci. 2009;29:15323–15330. doi: 10.1523/JNEUROSCI.2107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deecher DC, Swiggard P, Frail DE, O'Connor LT. Characterization of a membrane-associated estrogen receptor in a rat hypothalamic cell line (D12) Endocrine. 2003;22:211–223. doi: 10.1385/ENDO:22:3:211. [DOI] [PubMed] [Google Scholar]
- 89.Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci. 2007;27:9941–9950. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grove-Strawser D, Mermelstein PG. Estrogen receptors activate different mGluRs across distinct brain regions. Soc Neuroscience Ann Meet, San Diego, 2007.
- 91.Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282:22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- 92.Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Mol Biol Cell. 2005;16:231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER. Identification of a structural determinant necessary for the localization and function of estrogen receptor alpha at the plasma membrane. Mol Cell Biol. 2003;23:1633–1646. doi: 10.1128/MCB.23.5.1633-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gu G, Rojo AA, Zee MC, Yu J, Simerly RB. Hormonal regulation of CREB phosphorylation in the anteroventral periventricular nucleus. J Neurosci. 1996;16:3035–3044. doi: 10.1523/JNEUROSCI.16-09-03035.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou Y, Watters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 1996;137:2163–2166. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]
- 96.Carlstrom L, Ke ZJ, Unnerstall JR, Cohen RS, Pandey SC. Estrogen modulation of the cyclic AMP response element-binding protein pathway: effects of long-term and acute treatments. Neuroendocrinology. 2001;74:227–243. doi: 10.1159/000054690. [DOI] [PubMed] [Google Scholar]
- 97.Kelly MJ, Ronnekleiv OK. Membrane-initiated estrogen signaling in hypothalamic neurons. Mol Cell Endocrinol. 2008;290:14–23. doi: 10.1016/j.mce.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dewing P, Boulware MI, Sinchack K, Christensen A, Mermelstein PG, Micevych P. Membrane ERα interacts with mGluR1a to modulate female sexual receptivity. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kuo J, Hariri OR, Bondar G, Ogi J, Micevych P. Membrane estrogen receptor-alpha interacts with metabotropic glutamate receptor type 1a to mobilize intracellular calcium in hypothalamic astrocytes. Endocrinology. 2009;150:1369–1376. doi: 10.1210/en.2008-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kahlert S, Nuedling S, van Eickels M, Vetter H, Meyer R, Grohe C. Estrogen receptor alpha rapidly activates the IGF-1 receptor pathway. J Biol Chem. 2000;275:18447–18453. doi: 10.1074/jbc.M910345199. [DOI] [PubMed] [Google Scholar]
- 102.Etgen AM, Ansonoff MA, Quesada A. Mechanisms of ovarian steroid regulation of norepinephrine receptor-mediated signal transduction in the hypothalamus: implications for female reproductive physiology. Horm Behav. 2001;40:169–177. doi: 10.1006/hbeh.2001.1676. [DOI] [PubMed] [Google Scholar]
- 103.Quesada A, Micevych PE. Estrogen interacts with the IGF-1 system to protect nigrostriatal dopamine and maintain motoric behavior after 6-hydroxdopamine lesions. J Neurosci Res. 2004;75:107–116. doi: 10.1002/jnr.10833. [DOI] [PubMed] [Google Scholar]
- 104.Stefanova I, Horejsi V, Ansotegui IJ, Knapp W, Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991;254:1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- 105.Dewing P, Christensen A, Bondar G, Micevych P. Protein kinase C signaling in the hypothalamic arcuate nucleus regulates sexual receptivity in female rats. Endocrinology. 2008;149:5934–5942. doi: 10.1210/en.2008-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chaban V, Li J, McDonald J, Rapkin A, Micevych P: Estradiol attenuates ATP-induced increase of intracellular calcium through group II metabotropic glutamate receptors in rat DRG neurons. J Neurosci Res. In review. [DOI] [PMC free article] [PubMed]
- 108.Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P: Membrane estrogen receptors stimulate intracellular calcium flux and progesterone synthesis in hypothalamic astrocytes. J Neurosci. In review. [DOI] [PMC free article] [PubMed]
- 109.Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 110.Rissman EF, Wersinger SR, Taylor JA, Lubahn DB. Estrogen receptor function as revealed by knockout studies: neuroendocrine and behavioral aspects. Horm Behav. 1997;31:232–243. doi: 10.1006/hbeh.1997.1390. [DOI] [PubMed] [Google Scholar]
- 111.Chu Z, Andrade J, Shupnik MA, Moenter SM. Differential regulation of gonadotropin-releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype. J Neurosci. 2009;29:5616–5627. doi: 10.1523/JNEUROSCI.0352-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chaban VV, Micevych PE. Estrogen receptor-alpha mediates estradiol attenuation of ATP-induced Ca2+ signaling in mouse dorsal root ganglion neurons. J Neurosci Res. 2005;81:31–37. doi: 10.1002/jnr.20524. [DOI] [PubMed] [Google Scholar]
- 113.Ramirez VD, Kipp JL, Joe I. Estradiol, in the CNS, targets several physiologically relevant membrane-associated proteins. Brain Res Brain Res Rev. 2001;37:141–152. doi: 10.1016/s0165-0173(01)00114-x. [DOI] [PubMed] [Google Scholar]
- 114.Dominguez R, Hu E, Zhou M, Baudry M. 17Beta-estradiol-mediated neuroprotection and ERK activation require a pertussis toxin-sensitive mechanism involving GRK2 and beta-arrestin-1. J Neurosci. 2009;29:4228–4238. doi: 10.1523/JNEUROSCI.0550-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Razandi M, Pedram A, Levin ER. Estrogen signals to the preservation of endothelial cell form and function. J Biol Chem. 2000;275:38540–38546. doi: 10.1074/jbc.M007555200. [DOI] [PubMed] [Google Scholar]
- 116.Russell KS, Haynes MP, Sinha D, Clerisme E, Bender JR. Human vascular endothelial cells contain membrane binding sites for estradiol, which mediate rapid intracellular signaling. Proc Natl Acad Sci USA. 2000;97:5930–5935. doi: 10.1073/pnas.97.11.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc Natl Acad Sci USA. 2004;101:12354–12357. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Morales A, Diaz M, Ropero AB, Nadal A, Alonso R. Estradiol modulates acetylcholine-induced Ca2+ signals in LHRH-releasing GT1–7 cells through a membrane binding site. Eur J Neurosci. 2003;18:2505–2514. doi: 10.1046/j.1460-9568.2003.02997.x. [DOI] [PubMed] [Google Scholar]
- 119.Lin JW, Ju W, Foster K, Lee SH, Ahmadian G, Wyszynski M, Wang YT, Sheng M. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat Neurosci. 2000;3:1282–1290. doi: 10.1038/81814. [DOI] [PubMed] [Google Scholar]
- 120.Gorosito SV, Lorenzo AG, Cambiasso MJ. Estrogen receptor alpha is expressed on the cell-surface of embryonic hypothalamic neurons. Neuroscience. 2008;154:1173–1177. doi: 10.1016/j.neuroscience.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 121.Friend KE, Ang LW, Shupnik MA. Estrogen regulates the expression of several different estrogen receptor mRNA isoforms in rat pituitary. Proc Natl Acad Sci USA. 1995;92:4367–4371. doi: 10.1073/pnas.92.10.4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bollig A, Miksicek RJ. An estrogen receptor-alpha splicing variant mediates both positive and negative effects on gene transcription. Mol Endocrinol. 2000;14:634–649. doi: 10.1210/mend.14.5.0460. [DOI] [PubMed] [Google Scholar]
- 123.Poola I, Abraham J, Baldwin K. Identification of ten exon deleted ERbeta mRNAs in human ovary, breast, uterus and bone tissues: alternate splicing pattern of estrogen receptor beta mRNA is distinct from that of estrogen receptor alpha. FEBS Lett. 2002;516:133–138. doi: 10.1016/s0014-5793(02)02521-8. [DOI] [PubMed] [Google Scholar]
- 124.Moriarty K, Kim KH, Bender JR. Minireview: estrogen receptor-mediated rapid signaling. Endocrinology. 2006;147:5557–5563. doi: 10.1210/en.2006-0729. [DOI] [PubMed] [Google Scholar]
- 125.Al-Bader MD, El-Abdallah AA, Redzic ZB. Ontogenic profile of estrogen receptor alpha and beta mRNA and protein expression in fetal rat brain. Neurosci Lett. 2008;440:222–226. doi: 10.1016/j.neulet.2008.05.061. [DOI] [PubMed] [Google Scholar]
- 126.Ishunina TA, Swaab DF. Estrogen receptor-alpha splice variants in the human brain. Gynecol Endocrinol. 2008;24:93–98. doi: 10.1080/09513590701705148. [DOI] [PubMed] [Google Scholar]
- 127.Ishunina TA, Swaab DF. Age-dependent ERalpha MB1 splice variant expression in discrete areas of the human brain. Neurobiol Aging. 2008;29:1177–1189. doi: 10.1016/j.neurobiolaging.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 128.Karas RH, Baur WE, van Eickles M, Mendelsohn ME. Human vascular smooth muscle cells express an estrogen receptor isoform. FEBS Lett. 1995;377:103–108. doi: 10.1016/0014-5793(95)01293-1. [DOI] [PubMed] [Google Scholar]
- 129.Fasco MJ, Keyomarsi K, Arcaro KF, Gierthy JF. Expression of an estrogen receptor alpha variant protein in cell lines and tumors. Mol Cell Endocrinol. 2000;166:156–169. [PubMed] [Google Scholar]
- 130.Shupnik MA, Pitt LK, Soh AY, Anderson A, Lopes MB, Laws ER., Jr Selective expression of estrogen receptor alpha and beta isoforms in human pituitary tumors. J Clin Endocrinol Metab. 1998;83:3965–3972. doi: 10.1210/jcem.83.11.5236. [DOI] [PubMed] [Google Scholar]
- 131.Fuqua SA, Fitzgerald SD, Allred DC, Elledge RM, Nawaz Z, McDonnell DP, O'Malley BW, Greene GL, McGuire WL. Inhibition of estrogen receptor action by a naturally occurring variant in human breast tumors. Cancer Res. 1992;52:483–486. [PubMed] [Google Scholar]
- 132.Perlman WR, Matsumoto M, Beltaifa S, Hyde TM, Saunders RC, Webster MJ, Rubinow DR, Kleinman JE, Weickert CS. Expression of estrogen receptor alpha exon-deleted mRNA variants in the human and non-human primate frontal cortex. Neuroscience. 2005;134:81–95. doi: 10.1016/j.neuroscience.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 133.Heberden C, Reine F, Grosse B, Henry C, Zagar Y, Chaumaz G, Lieberherr M. Detection of a raft-located estrogen receptor-like protein distinct from ER alpha. Int J Biochem Cell Biol. 2006;38:376–391. doi: 10.1016/j.biocel.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 134.Matsuda K, Sakamoto H, Mori H, Hosokawa K, Kawamura A, Itose M, Nishi M, Prossnitz ER, Kawata M. Expression and intracellular distribution of the G protein-coupled receptor 30 in rat hippocampal formation. Neurosci Lett. 2008;441:94–99. doi: 10.1016/j.neulet.2008.05.108. [DOI] [PubMed] [Google Scholar]
- 135.Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzemeier KH. GPR30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008;149:4846–4856. doi: 10.1210/en.2008-0269. [DOI] [PubMed] [Google Scholar]
- 136.Sinchak K, Micevych P. Visualizing activation of opioid circuits by internalization of G protein-coupled receptors. Mol Neurobiol. 2003;27:197–222. doi: 10.1385/MN:27:2:197. [DOI] [PubMed] [Google Scholar]
- 137.Gonzalez M, Reyes R, Damas C, Alonso R, Bello AR. Oestrogen receptor alpha and beta in female rat pituitary cells: an immunochemical study. Gen Comp Endocrinol. 2008;155:857–868. doi: 10.1016/j.ygcen.2007.10.007. [DOI] [PubMed] [Google Scholar]