Abstract
Background/Aims
To investigate associations of the Mediterranean diet (MeDi) components and the MeDi score with mild cognitive impairment (MCI).
Methods
Participants (aged 70–89 years) were clinically evaluated to assess MCI and dementia, and completed a 128-item food frequency questionnaire.
Results
163 of 1,233 nondemented persons had MCI. The odds ratio of MCI was reduced for high vegetable intake [0.66 (95% CI = 0.44–0.99), p = 0.05] and for high mono-plus polyunsaturated fatty acid to saturated fatty acid ratio [0.52 (95% CI = 0.33–0.81), p = 0.007], adjusted for confounders. The risk of incident MCI or dementia was reduced in subjects with a high MeDi score [hazard ratio = 0.75 (95% CI = 0.46–1.21), p = 0.24].
Conclusion
Vegetables, unsaturated fats, and a high MeDi score may be beneficial to cognitive function.
Key Words: Mild cognitive impairment, Dietary intake, Moderate alcohol intake, Unsaturated fatty acids, Mediterranean diet, Longitudinal, Prevalence studies, Incidence studies, Population-based
Introduction
Mild cognitive impairment (MCI), a transitional stage between normal aging and dementia, offers an opportunity for reducing the public health burden of Alzheimer disease (AD) and other dementias through early detection and prevention [1,2]. Several studies suggest that in part because of the association with vascular risk factors, certain dietary patterns may be associated with the risk of dementia and cognitive impairment [3,4,5,6,7]. One such diet is the Mediterranean diet (MeDi), a diet high in intake of vegetables, legumes, fruit, and nuts, and low in intake of meats and dairy products. Greater adherence to the MeDi was inversely associated with total mortality and death from coronary heart disease in a Greek population [8]. In addition, the MeDi has been associated with incident MCI and AD in a multiethnic community [9,10]. However, there is little information on adherence to the MeDi in the US Midwest, or the association of adherence to the MeDi with MCI and its subtypes, in this setting. It has also been suggested that the MeDi approach may not be transferrable to communities that do not typically eat a MeDi [11]. The objective of this study is to describe adherence to the MeDi in a sample of subjects randomly selected from the Olmsted County, Minn., USA, community and evaluated for MCI, and to assess the association of the MeDi score and components of this diet with prevalent MCI and its subtypes, and with incident MCI and dementia during follow-up.
Materials and Methods
Study Participants
The study design and methodology have been previously published in detail [12]. Briefly, all residents from Olmsted County, Minn., USA, aged 70–89 years on October 1, 2004, were identified using the medical records-linkage system of the Rochester Epidemiology Project [13]. Of the 9,953 persons identified, 5,233 were randomly selected for participation. We excluded subjects who died before they could be contacted (n = 263), and subjects who were terminally ill and in hospice (n = 56); subjects with previously diagnosed confirmed dementia (n = 402), and subjects who could not be contacted (n = 114) were considered ineligible. The remaining 4,398 subjects were eligible; of the eligible subjects, 2,719 agreed to participate (61.8% response) by telephone (n = 669) or via a face-to-face evaluation (n = 2,050) that allowed characterization of dementia, MCI, and normal cognition. Information obtained from the medical records-linkage system showed that nonparticipants were more likely to be older, male, less educated, and to have greater comorbidity [12]. Subjects who participated by telephone only were not included in any nontelephone assessments. Of the 2,050 subjects who participated in the face-to-face evaluation, 67 were found to be demented at the time of evaluation, and 14 did not complete the evaluation and could not be assigned a diagnosis; these subjects were not eligible for future studies. The remaining subjects (n = 1,969) had a diagnosis of cognitively normal (n = 1,640) or had MCI (n = 329). All study protocols were approved by the Mayo Clinic and Olmsted Medical Center institutional review boards.
Measurements
Measurements included an interview by a nurse or study coordinator, a neurological evaluation by a physician, and neuropsychological testing. The interview included administration of the Clinical Dementia Rating (CDR) Scale to the participant and an informant and the Functional Activities Questionnaire to an informant [14]. The neurological evaluation included the Short Test of Mental Status [15], the Hachinski Scale, and a complete neurological examination. Neuropsychological testing was performed using 9 cognitive tests to assess 4 cognitive domains: memory [Logical Memory II (delayed recall) and Visual Reproduction II (delayed recall) from Wechsler Memory Scale-Revised, Auditory Verbal Learning Test [16]]; executive function (Trail Making Test B [17], Digit Symbol Substitution from Wechsler Adult Intelligence Scale-Revised); language (Boston Naming Test, Category Fluency [18]), and visuospatial skills (Picture Completion and Block Design from Wechsler Adult Intelligence Scale-Revised [19]). A neuropsychologist assessed impairment in cognition by comparing the age-corrected scaled scores for each participant with the norms determined for the same population from which subjects were recruited [20] taking into account the level of education, prior occupation, and other information. Scores of 1.0 standard deviation or more below the mean were considered potentially impaired, but a final decision about impairment in a domain was made by consensus. An expert panel of physicians, neuropsychologists, and the nurse or study coordinators who evaluated the participant reviewed all the information collected for each participant in order to reach a diagnosis of cognitively normal, MCI, or dementia by consensus.
Diagnostic Criteria
Cases. MCI was defined according to the following criteria: cognitive concern by participant, physician, nurse, or informant (from CDR); impairment in 1 or more of the 4 cognitive domains (including nonmemory domains) from the cognitive testing battery; essentially normal functional activities from the CDR and Functional Activities Questionnaire; and absence of dementia as previously published [1]. Participants were characterized as having amnestic MCI (a-MCI) if they had impairment in the memory domain and nonamnestic MCI (na-MCI) if they had impairment in any 1 or more of the nonmemory cognitive domains.
Dementia. A diagnosis of dementia was made according to the DSM-IV criteria [21]. Subjects with dementia were excluded from the present study.
Controls. Subjects who did not meet the criteria for MCI or dementia were characterized as cognitively normal according to published criteria [20].
Measurement of Dietary Food Intake
We assessed dietary intake via the modified Block 1995 Revision of the Health Habits and History Questionnaire [22] that included 128 items: 103 food items and 25 beverages. The self-administered questionnaire was mailed to the homes of participants with an addressed, stamped envelope for its return. Participants were asked to provide information on usual eating habits during the previous year. For each food item, respondents were asked to indicate (1) their usual portion size consumed (small, medium, large), with the medium serving provided as a specific amount, and (2) how often they had consumed each food (never or <1 per month, 1–3 per month, 1 per week, 2–4 per week, 5–6 per week, 1 per day, 2–3 per day, 4–5 per day, 6+ per day). The Food Processor SQL nutrition analysis software program (version 10.0.0, ESHA Research, Salem, Oreg., USA) was used to calculate the total nutrient, food group, and energy (caloric) intake per day, under the supervision of a registered dietician (H.M.O.).
MeDi Adherence
We assessed adherence to a MeDi by computing a MeDi score using a previously developed scale [8,10]. We calculated the energy-adjusted nutrient and food group values using the residual method by regressing the log(energy) on log(nutrient) to derive the residual [23]. We computed the daily intake of nutrients and food groups as the sum of the residual and the mean log(nutrient) value determined from the regression model. Using the sex-specific median from the distribution as the cutoff, we assigned a value of 0 for consumption below the median and 1 for consumption at or above the median for beneficial components (vegetables, legumes, fruits, cereal, and fish). For components presumed to have adverse effects (meat, dairy products), we assigned a value of 1 for consumption below the median and 0 for consumption at or above the median. For fat intake, we used the ratio of monounsaturated fatty acids (MUFA) to saturated fatty acids (SFA), with a value of 1 for high and 0 for low intake. We scored alcohol intake as 0 for intake of 0 or ≥30 g per day and 1 for >0 to <30 g per day. The total possible MeDi score ranged from 0 (minimal) to 9 (maximal adherence).
Covariates
We ascertained date of birth, years of education, marital status, and vascular comorbidities (diabetes, hypertension, coronary heart disease, stroke) by interview. We assessed medication use from medication bottles that participants were asked to bring to the evaluation. We validated self-report of vascular comorbidities from the medical record [13], and considered the medical record the gold standard. A history of depressive symptoms was assessed by interview of an informant using the Neuropsychiatric Inventory Questionnaire [24]. We assessed the frequency of moderate physical exercise in the year prior to recruitment (≤1 per month, 2–3 times per month, 1–2 times per week, 3–4 times per week, 5–6 times per week, and daily) [25]. Weight and height were measured and body mass index (BMI) was calculated (kg/m2). A blood draw was performed to assess Apolipoprotein E (ApoE) ∊4 genotype, and biomarkers including HbA1c, total cholesterol, triglycerides, and C-reactive protein were also measured using standard methods.
Statistical Analyses
We investigated the associations between components of the MeDi score, MeDi score tertiles, and MCI using multiple logistic regression models. We determined cutpoints for the MeDi score from the distribution of scores for cases and controls combined; these tertile cutpoints were consistent with cutpoints in other studies [9,10]. The base model was adjusted for age, years of education, and total caloric intake (as continuous variables), and sex; the fully adjusted model included variables in the base model and stroke, ApoE ∊4 allele status (ApoE ∊4+ vs. ApoE ∊4–), coronary heart disease, and depressive symptoms. BMI, diabetes, and hypertension were not associated with MCI in our sample, and since their inclusion in the analyses did not alter the results, those results are not presented. We included total caloric intake in all the logistic regression models to avoid spurious associations [26] and also examined potential confounding by physical exercise, and potential effect modification by age, sex, and years of education, and ApoE ∊4 allele. We also investigated associations of the MeDi with a-MCI and na-MCI.
To adjust for potential nonparticipation bias, we used a propensity score approach to develop a logistic regression model that predicted the probability of participation [27,28]. We included demographic (age, sex, years of education) and clinical (diabetes, stroke, depressive symptoms, MCI status) characteristics for subjects included and excluded from the study, and used the reciprocal of the predicted probabilities as weights in all the logistic regression models presented.
Secondary Analyses
Because polyunsaturated fatty acid (PUFA) is a primary source of beneficial unsaturated fatty acids in non-Mediterranean regions [29,30], we examined the use of the (MUFA + PUFA):SFA ratio as the measure of fat intake in the calculation of the MeDi score. We also examined the use of sex-specific cutpoints for moderate alcohol intake (5 to <25 g/day for women and 10 to <50 g/day for men), fruit and nut intake [8,31] in the calculation of the MeDi score. We conducted additional analyses with the MeDi score as a continuous variable or as quartiles on account of the skewness of the MeDi score distribution.
Longitudinal Associations
In subjects who were cognitively normal or had MCI at baseline, we used proportional hazards models to investigate the association of the baseline MeDi score with incident MCI or dementia; subjects were censored at the time of death or loss to follow-up. All analyses were performed using SAS® (SAS Institute, Cary, N.C., USA).
Results
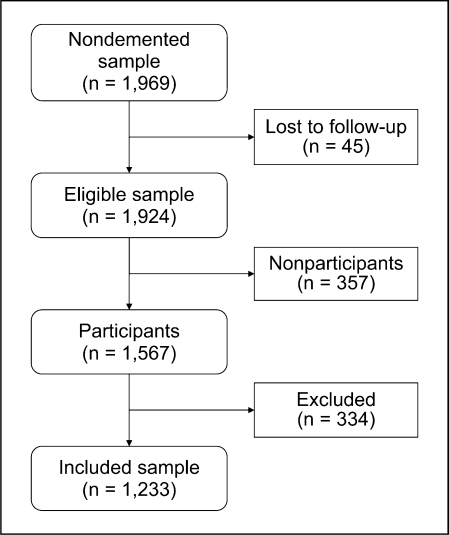
Of the 1,969 nondemented subjects who participated at baseline, 45 were lost to follow-up prior to the food frequency questionnaires being mailed out, 22 had died, 22 had refused participation since the baseline evaluation, and 1 person could not speak English and was therefore unable to complete the questionnaire (fig. 1). The food frequency questionnaire was mailed to the eligible 1,924 subjects. A total of 1,567 (81.4%) subjects returned the questionnaire; of these, we excluded subjects who had missing responses on more than 10 questions on frequency of consumption (n = 268), reported extreme caloric intake (<800 or >6,000 kcal per day for 32 men and <600 or >5,000 kcal per day for 24 women), or were demented at the time of the evaluation (n = 10). The remaining 1,233 subjects were included in the present study (fig. 1). Subjects who were not included (n = 691) were similar to those included in regard to sex, history of hypertension, coronary heart disease, BMI, and ApoE ∊4 allele status. However, there were modest differences: excluded subjects were older (median age = 82.2 vs. 80.1 years, p < 0.0001), had a higher frequency of stroke (14.2 vs. 10.1%, p = 0.007), diabetes (16.1 vs. 12.1%, p = 0.014), depressive symptoms (16.3 vs. 12.3%, p = 0.018), and MCI (23.9 vs. 13.2%, p < 0.0001), but were less likely to be married (52.5 vs. 64.9%, p < 0.0001) and had fewer years of education (median = 12 vs. 13 years, p < 0.0001). There were no differences between included and excluded MCI cases in regard to age, sex, years of education, and ApoE ∊4 allele.
Fig. 1.
Flow chart for study participants. Losses to follow-up: 22 died, 22 refused further participation, 1 was non-English speaking, and 357 did not return questionnaire. Exclusions: 268 had missing responses on more than 10 questions on frequency of consumption, 56 reported extreme caloric intake (<800 or >6,000 kcal per day for 32 men and <600 or >5,000 kcal per day for 24 women), and 10 were demented at the time of the evaluation.
Table 1 presents the baseline demographic and clinical characteristics of MCI cases and controls included in the analyses. Questionnaires for these subjects were completed at a median of 3.3 months (interquartile range = 1.5–6.1) from the time of the evaluation. MCI cases were more likely to be older, male, to have fewer years of education, a history of stroke, depressive symptoms, and an ApoE ∊4 allele. The baseline characteristics of the follow-up cohort by diagnostic status at follow-up are also presented.
Table 1.
Baseline characteristics of participants by diagnostic status at baseline and at follow-up, Mayo Clinic Study of Aging, 2004-2006
| Variable | Baseline sample |
Follow-up cohorta |
||||
|---|---|---|---|---|---|---|
| MCI (n = 163) | no MCI (n = 1,070) | P | event (n = 116) | no event (n = 1,025) | P | |
| Age | ||||||
| Median (Ql, Q3), years | 82.9 (78.3, 86.4) | 79.6 (75.5,84.1) | <0.0001 | 83.3 (78.7, 86.2) | 79.7 (75.6, 84.2) | <0.0001 |
| 70-79 years | 53 (32.5) | 558 (52.1) | <0.0001 | 35 (30.2) | 530 (51.7) | <0.0001 |
| ≥80 years | 110 (67.5) | 512 (47.9) | 81 (69.8) | 495 (48.3) | ||
| Sex | ||||||
| Male | 97 (59.5) | 544 (50.8) | 0.04 | 76 (65.5) | 527 (51.4) | 0.004 |
| Female | 66 (40.5) | 526 (49.2) | 40 (34.5) | 498 (48.6) | ||
| Education | ||||||
| Median (Ql, Q3), years | 12 (12, 15) | 13 (12, 16) | 0.001 | 13 (12,15.5) | 13 (12, 16) | 0.04 |
| <9 years | 22 (13.5) | 43 (4.0) | <0.0001 | 8 (6.9) | 51 (5.0) | 0.29 |
| 9-12 years | 63 (38.7) | 414 (38.7) | 49 (42.2) | 379 (37.0) | ||
| >12 years | 78 (47.9) | 613 (57.3) | 59 (50.9) | 595 (58.0) | ||
| Marital status | ||||||
| Married | 104 (63.8) | 696 (65.0) | 0.76 | 74 (63.8) | 673 (65.7) | 0.69 |
| Not married | 59 (36.2) | 374 (35.0) | 42 (36.2) | 352 (34.3) | ||
| Body mass indexb | ||||||
| <25 | 53 (35.1) | 336 (32.2) | 0.31 | 43 (37.4) | 328 (32.2) | 0.37 |
| 25-29 | 65 (43.0) | 419 (40.1) | 40 (34.8) | 421 (41.3) | ||
| ≥30 | 33 (21.9) | 290 (27.8) | 32 (27.8) | 270 (26.5) | ||
| Diabetes | ||||||
| Yes | 22 (13.5) | 127 (11.9) | 0.55 | 20 (17.2) | 116 (11.3) | 0.06 |
| No | 141 (86.5) | 943 (88.1) | 96 (82.8) | 909 (88.7) | ||
| Hypertension | ||||||
| Yes | 122 (74.8) | 754 (70.5) | 0.25 | 82 (70.7) | 720 (70.2) | 0.92 |
| No | 41 (25.2) | 316 (29.5) | 34 (29.3) | 305 (29.8) | ||
| Coronary heart disease | ||||||
| Yes | 51 (31.3) | 282 (26.4) | 0.19 | 28 (24.1) | 280 (27.3) | 0.46 |
| No | 112 (68.7) | 788 (73.6) | 88 (75.9) | 745 (72.7) | ||
| Stroke | ||||||
| Yes | 35 (21.5) | 89 (8.3) | <0.0001 | 16 (13.8) | 93 (9.1) | 0.10 |
| No | 128 (78.5) | 981 (91.7) | 100 (86.2) | 932 (90.9) | ||
| Depressionc | ||||||
| Yes | 41 (25.8) | 107 (10.3) | <0.0001 | 23 (20.7) | 106 (10.6) | 0.002 |
| No | 118 (74.2) | 935 (89.7) | 88 (79.3) | 895 (89.4) | ||
| ApoE ε4d | ||||||
| E3ε4/ε4ε4 | 47 (31.1) | 217 (21.5) | 0.008 | 30 (28.0) | 219 (22.6) | 0.20 |
| E2ε2/ε2ε3/ε3ε3 | 104 (68.9) | 793 (78.5) | 77 (72.0) | 752 (77.4) | ||
| Smoking | ||||||
| Never | 82 (50.3) | 552 (51.6) | 0.95 | 59 (50.9) | 534 (52.1) | 0.92 |
| Former | 76 (46.6) | 484 (45.2) | 54 (46.6) | 460 (44.9) | ||
| Current | 5 (3.1) | 34 (3.2) | 3 (2.6) | 31 (3.0) | ||
| Domain Z scores, median (Ql, Q3) | ||||||
| Memory domain scoree | −1.19 (−1.77, −0.72) | 0.48 (−0.19, 1.17) | <0.0001 | −0.80 (−1.22, −0.12) | 0.48 (−0.25, 1.20) | <0.0001 |
| Executive function domain scoref | −1.01 (−1.79, −0.17) | 0.42 (−0.13, 0.93) | <0.0001 | −0.47 (−1.02, 0.12) | 0.42 (−0.18, 0.93) | <0.0001 |
| Visuospatial domain scoreg | −0.73 (−1.39, −0.03) | 0.38 (−0.31, 0.94) | <0.0001 | −0.46 (−1.16, 0.15) | 0.38 (−0.27, 0.94) | <0.0001 |
| Language domain scoreh | −0.75 (−1.58, −0.24) | 0.32 (−0.20, 0.91) | <0.0001 | −0.36 (−1.08, 0.21) | 0.32 (−0.26, 0.91) | <0.0001 |
Q1 = Quartile 1; Q3 = quartile 3. Figures in parentheses are percentages unless indicated otherwise.
Event = MCI or dementia during follow-up; no event = no MCI or dementia during follow-up.
37 missing in the cross-sectional studies total cohort: 25 in the No MCI group, 12 in the MCI group; 7 missing in the longitudinal studies total cohort: 6 in the No event group, 1 in the Event group.
32 missing in the cross-sectional studies total cohort: 28 in the No MCI group, 4 in the MCI group; 29 missing in the longitudinal studies total cohort: 24 in the No event group, 5 in the Event group.
40 missing in the cross-sectional studies total cohort: 33 in the No MCI group, 7 in the MCI group; ε2ε4 was found in 5 (3.2%) in the MCI group, 27 (2.6%) in the No MCI group, and 32 (2.7%) among the total cohort. 34 missing in the longitudinal studies total cohort: 31 in the No event group, 3 in the Event group; ε2ε4 was found in 6 (5.3%) in the Event group, 23 (2.3%) in the No event group, and 29 (2.6%) in the total cohort. ε2ε4 was not included in the table due to small numbers.
52 missing in the baseline sample: 39 in the No MCI group, 13 in the MCI group; 51 missing in the follow-up cohort: 45 in the No event group, 6 in the Event group.
108 missing in the baseline sample: 81 in the No MCI group, 27 in the MCI group; 100 missing in the follow-up cohort: 89 in the No event group, 11 in the Event group.
100 missing in the baseline sample: 78 in the No MCI group, 22 in the MCI group; 91 missing in the follow-up cohort: 85 in the No event group, 6 in the Event group.
86 missing in the baseline sample: 67 in the No MCI group, 19 in the MCI group; 79 missing in the follow-up cohort: 75 in the No event group, 4 in the Event group.
Table 2 shows the distribution (median and interquartile range) of nutrient intakes for cases with MCI and controls overall and by sex. Among men and women combined, MCI cases had a slightly lower daily intake of vegetables (p = 0.06), a lower (MUFA + PUFA):SFA ratio (p = 0.05), and a lower frequency of moderate alcohol consumption (p = 0.05), but a higher daily caloric intake (p = 0.0005) than controls. The MeDi score did not differ between MCI cases and controls. Compared to women, men had a higher intake of legumes (p = 0.014), grains and cereals (p = 0.003), red meat (p < 0.001), and moderate alcohol (p < 0.01), but a lower intake of fruits and vegetables (p = 0.004). Among men, MCI cases had a marginally lower intake of fish (p = 0.06), but a higher daily caloric intake (p = 0.0001) than controls. Among women, the frequency of moderate alcohol intake was lower in MCI cases compared to controls (p = 0.005).
Table 2.
Distribution of components of MeDi and MeDi score by MCI status and by sex
| Variable | MCI cases (n = 163) | Controls (n = 1,070) | P | Men (n = 641) | Women (n = 592) | P |
|---|---|---|---|---|---|---|
| Vegetables, g/day | 127.4 (84.2-204.8) | 150.5 (95.1-225.3) | 0.06 | 143.5 (88.9-215.8) | 155.3 (96.1-229.0) | 0.12 |
| Legumes, g/day | 43.6 (24.9-71.8) | 46.3 (28.6-72.0) | 0.33 | 47.2 (30.4-75.2) | 43.0 (26.4-69.6) | 0.014 |
| Grains and cereals, g/day | 190.9 (126.7-256.3) | 171.8 (126.8-238.2) | 0.18 | 182.3 (130.9-255.3) | 168.1 (124.5-226.7) | 0.003 |
| Fruits, g/day | 218.1 (128.6-313.1) | 208.8 (128.1-320.5) | 0.89 | 201.1 (123.2-294.4) | 220.7 (137.4-333.5) | 0.004 |
| Fish, g/day | 12.5 (6.3-23.8) | 15.3 (7.0-26.2) | 0.15 | 15.2 (7.2-26.2) | 14.2 (6.3-25.7) | 0.37 |
| Red meat, g/day | 104.4 (72.6-148.0) | 107.7 (70.4-147.4) | 0.81 | 120.6 (86.5-167.0) | 91.5 (59.7-130.1) | <0.0001 |
| Dairy, g/day | 332.9 (181.4-530.7) | 336.7 (188.7-559.8) | 0.88 | 325.9 (187.6-542.9) | 347.8 (188.0-569.7) | 0.49 |
| MUFA:SFA ratio | 1.01 (0.89-1.14) | 1.04 (0.91-1.17) | 0.23 | 1.05 (0.92-1.17) | 1.03 (0.90-1.16) | 0.18 |
| (MUFA + PUFA):SFA ratio | 1.51 (1.28-1.70) | 1.57 (1.33-1.81) | 0.049 | 1.55 (1.32-1.78) | 1.57 (1.31-1.82) | 0.43 |
| Moderate alcohol intake, n (%)a | 95 (58.3) | 706 (66.0) | 0.05 | 438 (68.3) | 363 (61.3) | 0.01 |
| Total caloriesb | 2,060.1 (1,506.5-2,681.0) | 1,791.6 (1,351.2-2,329.1) | 0.0005 | 1,947.4 (1,522.1-2,507.9) | 1,656.3 (1,220.2-2,190.8) | <0.0001 |
| MeDi score 1c | 5.0 (3.0-6.0) | 5.0 (4.0-6.0) | 0.37 | 5.0 (4.0-6.0) | 5.0 (4.0-6.0) | 0.42 |
| MeDi score 2d | 5.0 (3.0-6.0) | 5.0 (4.0-6.0) | 0.27 | 5.0 (4.0-6.0) | 5.0 (3.0-6.0) | 0.46 |
Figures are medians with interquartile ranges in parentheses unless indicated otherwise.
Moderate alcohol intake was categorized as >0 and <30 g/day; intake was lower in female MCI cases [30 (45.5%)] vs. controls [333 (63.3%)]; p = 0.005.
Caloric intake was greater for male MCI cases [2,231.4 (1,731.8-2,982.1)] vs. controls [1,898.1 (1,480.2-2,445.6)]; p = 0.0001.
MeDi score 1 = Moderate alcohol and MUFA:SFA ratio.
MeDi score 2 = Moderate alcohol and (MUFA + PUFA):SFA ratio.
Table 3 presents the associations of the components of the MeDi score and the MeDi score tertiles with MCI. The odds ratio (OR) of MCI decreased significantly with higher vegetable intake (p for trend = 0.05), increasing (MUFA + PUFA):SFA ratio (p for trend = 0.01), and with moderate alcohol intake (p = 0.05). Fruit intake in the upper tertile was associated with an 11% reduced OR of MCI, and the OR of MCI decreased with increasing MeDi score, but neither the associations nor the trends were statistically significant. There was a dose-response association of increasing daily caloric intake with MCI (p for trend = 0.001). The results of the fully adjusted models were consistent with the base models suggesting no confounding by the additional variables, and the associations of vegetables and (MUFA + PUFA):SFA ratios with MCI were slightly stronger; additional adjustment for exercise did not materially change the results. In additional models, we included HbA1c, total cholesterol, and C-reactive protein since these could be potential confounders. There were no appreciable changes in the ORs for all except alcohol, although, given the increased number of variables now included in the model, the associations with vegetable intake and moderate alcohol were no longer statistically significant. The estimates of OR (95% CI) for the middle and upper tertiles compared to the lowest tertile were as follows: vegetable intake (middle tertile: OR = 0.58, 95% CI = 0.38–0.87; upper tertile: OR = 0.63, 95% CI = 0.41–0.96); moderate alcohol (OR = 1.22, 95% CI = 0.86–1.74); (MUFA + PUFA):SFA ratio (middle tertile: OR = 0.98, 95% CI = 0.66–1.46; upper tertile: OR = 0.52, 95% CI = 0.34–0.81); MeDi score 1 (middle tertile: OR = 1.07, 95% CI = 0.71–1.62; upper tertile: OR = 0.75, 95% CI = 0.47–1.20); MeDi score 2 (middle tertile: OR = 1.00, 95% CI = 0.46–1.50; upper tertile: OR = 0.72, 95% CI = 0.46–1.15). One could argue that these variables are related to diet and therefore may be in the causal pathway; including them in the model may represent overcontrolling. There was no significant interaction of the MeDi score with age, sex, years of education, and ApoE ∊4 allele.
Table 3.
Association of components of the MeDi and the MeDi score with MCI
| Variable | Controls | Cases | ORa | 95% CIa | P | pfor trend | ORb | 95% CIb | P | p for trend |
|---|---|---|---|---|---|---|---|---|---|---|
| Vegetables (without legume) | ||||||||||
| ≤ 109.6 g/day | 341 (31.9) | 70 (42.9) | 1.00 | reference | − | − | 1.00 | reference | − | − |
| 109.7-191.0 g/day | 364 (34.0) | 48 (29.4) | 0.65 | 0.45-0.94 | 0.02 | − | 0.60 | 0.40-0.88 | 0.01 | − |
| >191.0 g/day | 365 (34.1) | 45 (27.6) | 0.71 | 0.48-1.04 | 0.08 | 0.05 | 0.66 | 0.44-0.99 | 0.05 | 0.02 |
| Legumes | ||||||||||
| ≤33.5 g/day | 353 (33.0) | 58 (35.6) | 1.00 | reference | − | − | 1.00 | reference | − | − |
| 33.6-61.3 g/day | 359 (33.6) | 53 (32.5) | 0.95 | 0.65-1.39 | 0.80 | − | 0.99 | 0.67-1.48 | 0.98 | − |
| >61.3 g/day | 358 (33.5) | 52 (31.9) | 1.00 | 0.69-1.47 | 0.99 | 0.96 | 1.05 | 0.70-1.57 | 0.82 | 0.96 |
| Fruits | ||||||||||
| ≤153.4 g/day | 356 (33.3) | 55 (33.7) | 1.00 | reference | − | − | 1.00 | reference | − | − |
| 153.5-276.8 g/day | 354 (33.1) | 58 (35.6) | 1.06 | 0.73-1.54 | 0.78 | − | 1.09 | 0.74-1.63 | 0.66 | − |
| >276.8 g/day | 360 (33.6) | 50 (30.7) | 0.89 | 0.61-1.32 | 0.57 | 0.68 | 0.92 | 0.61-1.38 | 0.68 | 0.69 |
| Dairy | ||||||||||
| ≤235.2 g/day | 358 (33.5) | 53 (32.5) | 1.00 | reference | − | − | 1.00 | reference | − | − |
| 235.3-467.2 g/day | 358 (33.5) | 54 (33.1) | 1.02 | 0.70-1.50 | 0.91 | − | 0.97 | 0.65-1.46 | 0.90 | − |
| >467.2 g/day | 354 (33.1) | 56 (34.4) | 1.15 | 0.79-1.68 | 0.48 | 0.75 | 1.09 | 0.73-1.63 | 0.68 | 0.85 |
| Grains and cereals | ||||||||||
| ≤141.6 g/day | 359 (33.6) | 52 (31.9) | 1.00 | reference | − | − | 1.00 | reference | − | − |
| 141.7-212.9 g/day | 363 (33.9) | 49 (30.1) | 0.99 | 0.67-1.46 | 0.95 | − | 0.94 | 0.63-1.42 | 0.77 | − |
| >212.9 g/day | 348 (32.5) | 62 (38.0) | 1.19 | 0.82-1.72 | 0.37 | 0.56 | 1.08 | 0.73-1.61 | 0.69 | 0.78 |
| Meat | ||||||||||
| ≤83.2 g/day | 357 (33.4) | 54 (33.1) | 1.00 | reference | − | − | 1.00 | reference | − | − |
| 83.3-132.2 g/day | 353 (33.0) | 59 (36.2) | 1.15 | 0.79-1.68 | 0.46 | − | 1.16 | 0.78-1.72 | 0.46 | − |
| >132.2 g/day | 360 (33.6) | 50 (30.7) | 0.95 | 0.64-1.41 | 0.80 | 0.59 | 0.89 | 0.59-1.36 | 0.60 | 0.45 |
| Fish | ||||||||||
| ≤8.7 g/day | 353 (33.0) | 58 (35.6) | 1.00 | reference | − | − | 1.00 | reference | − | − |
| 8.8-21.2 g/day | 354 (33.1) | 58 (35.6) | 1.10 | 0.76-1.60 | 0.60 | − | 1.02 | 0.69-1.51 | 0.93 | − |
| >21.2 g/day | 363 (33.9) | 47 (28.8) | 1.07 | 0.72-1.58 | 0.75 | 0.87 | 0.99 | 0.66-1.50 | 0.98 | 0.99 |
| Alcohol | ||||||||||
| No intake or ≥30 | 364 (34.0) | 68 (41.7) | 1.00 | reference | − | − | 1.00 | reference | − | − |
| Moderate (>0 and <30) | 706 (66.0) | 95 (58.3) | 0.73 | 0.53-1.00 | 0.05 | 0.05 | 0.82 | 0.58-1.15 | 0.25 | 0.25 |
| MUFA:SFA ratio | ||||||||||
| ≤0.955 | 353 (33.0) | 58 (35.6) | 1.00 | reference | − | − | 1.00 | reference | − | − |
| 0.956-1.123 | 358 (33.5) | 54 (33.1) | 0.91 | 0.63-1.32 | 0.62 | − | 0.94 | 0.63-1.42 | 0.78 | − |
| >1.123 | 359 (33.6) | 51 (31.3) | 0.82 | 0.56-1.20 | 0.31 | 0.60 | 0.79 | 0.52-1.20 | 0.27 | 0.52 |
| (MUFA + PUFA):SFA ratio | ||||||||||
| ≤1.415 | 346 (32.3) | 65 (39.9) | 1.00 | reference | − | − | 1.00 | reference | − | − |
| 1.416-1.705 | 353 (33.0) | 59 (36.2) | 0.91 | 0.64-1.31 | 0.63 | − | 0.98 | 0.66-1.45 | 0.90 | − |
| >1.705 | 371 (34.7) | 39 (23.9) | 0.56 | 0.38-0.83 | 0.004 | 0.010 | 0.52 | 0.33-0.81 | 0.004 | 0.007 |
| Total energy | ||||||||||
| ≤l,525.9kcal | 369 (34.5) | 42 (25.8) | 1.00 | reference | − | − | 1.00 | reference | − | − |
| 1,526.0-2,142.5 kcal | 367 (34.3) | 45 (27.6) | 0.98 | 0.65-1.47 | 0.92 | − | 1.12 | 0.72-1.73 | 0.62 | − |
| >2,142.5kcal | 334 (31.2) | 76 (46.6) | 1.85 | 1.26-2.70 | 0.002 | 0.001 | 2.04 | 1.36-3.05 | 0.001 | 0.001 |
| MeDi score Ic | ||||||||||
| 0-3 | 262 (24.5) | 44 (27.0) | 1.00 | reference | − | − | 1.00 | reference | − | − |
| 4-5 | 463 (43.3) | 76 (46.6) | 1.03 | 0.71-1.50 | 0.87 | − | 0.96 | 0.65-1.43 | 0.85 | − |
| 6-9 | 345 (32.2) | 43 (26.4) | 0.86 | 0.57-1.31 | 0.49 | 0.62 | 0.80 | 0.52-1.25 | 0.33 | 0.57 |
| MeDi score 2c | ||||||||||
| 0-3 | 265 (24.8) | 47 (28.8) | 1.00 | reference | − | − | 1.00 | reference | − | − |
| 4-5 | 458 (42.8) | 73 (44.8) | 0.95 | 0.66-1.38 | 0.80 | − | 0.93 | 0.63-1.37 | 0.71 | − |
| 6-9 | 347 (32.4) | 43 (26.4) | 0.81 | 0.53-1.23 | 0.32 | 0.57 | 0.78 | 0.51-1.22 | 0.28 | 0.54 |
Figures in parentheses indicate percentages.
Odds ratio (95% confidence intervals) adjusted for age, years of education, total energy (as continuous variables), and sex. All models were adjusted with propensity weights to take into account potential nonparticipation bias. The unweighted models were essentially the same and are not presented.
Adjusted for age, years of education, total energy (continuous variables), sex, ApoE ε4 (ε4+ vs. ε4−), stroke, coronary heart disease, and depressive symptoms.
Fat intake was determined from the MUFA:SFA ratio for MeDi score 1, and from the (MUFA + PUFA):SFA ratio for MeDi score 2.
High intake of vegetables (upper tertile: OR = 0.57, 95% CI = 0.36–0.90, p for trend = 0.04), MUFA:SFA ratio (OR = 0.60, 95% CI = 0.39–0.93, p for trend = 0.03), and a higher (MUFA + PUFA):SFA ratio (OR = 0.48, 95% CI = 0.30–0.76, p for trend = 0.008) were significantly associated with a reduced OR of a-MCI but not with na-MCI (table 4).
Table 4.
Association of select components of the MeDi and the MeDi score with a-MCI and na-MCI
| Variable | Controls | Cases | ORa | 95% CIa | P | p for trend | ORb | 95% CIb | P | p for trend |
|---|---|---|---|---|---|---|---|---|---|---|
| a-MCI | ||||||||||
| Vegetables (without legumes) | ||||||||||
| ≤109.6 g/day | 341 (31.9) | 54 (44.3) | 1.00 | reference | − | − | 1.00 | reference | − | − |
| 109.7-191.0 g/day | 364 (34.0) | 39 (32.0) | 0.70 | 0.46-1.07 | 0.10 | − | 0.66 | 0.42-1.02 | 0.06 | − |
| >191.0g/day | 365 (34.1) | 29 (23.8) | 0.57 | 0.38-0.90 | 0.02 | 0.04 | 0.59 | 0.37-0.95 | 0.03 | 0.05 |
| MUFA:SFA ratio | ||||||||||
| ≤0.955 | 353 (33.0) | 50 (41.0) | 1.00 | reference | − | − | 1.00 | reference | − | − |
| 0.956-1.123 | 358 (33.5) | 35 (28.7) | 0.63 | 0.40-0.97 | 0.03 | − | 0.64 | 0.41-1.02 | 0.06 | − |
| >1.123 | 359 (33.6) | 37 (30.3) | 0.60 | 0.39-0.93 | 0.02 | 0.03 | 0.63 | 0.40-1.00 | 0.05 | 0.07 |
| (MUFA + PUFA):SFA ratio | ||||||||||
| ≤1.415 | 346 (32.3) | 51 (41.8) | 1.00 | reference | − | − | 1.00 | reference | − | − |
| 1.416-1.705 | 353 (33.0) | 42 (34.4) | 0.81 | 0.53-1.22 | 0.31 | − | 0.86 | 0.56-1.34 | 0.51 | − |
| >1.705 | 371 (34.7) | 29 (23.8) | 0.48 | 0.30-0.76 | 0.002 | 0.007 | 0.49 | 0.30-0.80 | 0.004 | 0.01 |
| MeDi score Ic | ||||||||||
| 0-3 | 262 (24.5) | 33 (27.0) | 1.00 | reference | − | − | 1.00 | reference | − | − |
| 4-5 | 463 (43.3) | 60 (49.2) | 1.07 | 0.69-1.66 | 0.75 | − | 1.01 | 0.64-1.59 | 0.98 | − |
| 6-9 | 345 (32.2) | 29 (23.8) | 0.79 | 0.48-1.30 | 0.35 | 0.38 | 0.74 | 0.44-1.25 | 0.26 | 0.39 |
| MeDi score 2c | ||||||||||
| 0-3 | 265 (24.8) | 36 (39.5) | 1.00 | reference | − | − | 1.00 | reference | − | − |
| 4-5 | 458 (42.8) | 56 (45.9) | 0.93 | 0.60-1.42 | 0.73 | − | 0.91 | 0.58-1.43 | 0.68 | − |
| 6-9 | 347 (32.4) | 30 (24.6) | 0.74 | 0.46-1.21 | 0.23 | 0.46 | 0.73 | 0.44-1.22 | 0.23 | 0.47 |
| na-MCI | ||||||||||
| Vegetables (without legumes) | ||||||||||
| ≤109.6 g/day | 341 (31.9) | 16 (39.0) | 1.00 | reference | − | − | 1.00 | reference | − | − |
| 109.7-191.0 g/day | 364 (34.0) | 9 (22.0) | 0.60 | 0.29-1.29 | 0.19 | − | 0.52 | 0.24-1.16 | 0.11 | − |
| > 191.0 g/day MUFA:SFA ratio | 365 (34.1) | 16 (39.0) | 1.41 | 0.73-2.74 | 0.31 | 0.09 | 1.18 | 0.58-2.39 | 0.64 | 0.13 |
| ≤0.955 | 353 (33.0) | 8 (19.5) | 1.00 | reference | − | − | 1.00 | reference | − | − |
| 0.956-1.123 | 358 (33.5) | 19 (46.3) | 2.57 | 1.21-5.50 | 0.01 | − | 2.75 | 1.22-6.22 | 0.01 | − |
| >1.123 | 359 (33.6) | 14 (34.1) | 1.74 | 0.78-3.87 | 0.18 | 0.05 | 1.70 | 0.71-4.08 | 0.23 | 0.04 |
| (MUFA + PUFA):SFA ratio | ||||||||||
| ≤1.415 | 346 (32.3) | 14 (34.1) | 1.00 | reference | − | − | 1.00 | reference | − | − |
| 1.416-1.705 | 353 (33.0) | 17 (41.5) | 1.37 | 0.71-2.65 | 0.35 | − | 1.57 | 0.78-3.17 | 0.21 | − |
| >1.705 | 371 (34.7) | 10 (24.4) | 0.73 | 0.34-1.54 | 0.41 | 0.22 | 0.63 | 0.27-1.47 | 0.29 | 0.07 |
| MeDi score Ic | ||||||||||
| 0-3 | 262 (24.5) | 11 (26.8) | 1.00 | reference | − | − | 1.00 | reference | − | − |
| 4-5 | 463 (43.3) | 16 (39.0) | 0.95 | 0.47-1.94 | 0.90 | − | 1.03 | 0.47-2.22 | 0.95 | − |
| 6-9 | 345 (32.2) | 14 (34.1) | 1.28 | 0.61-2.69 | 0.51 | 0.65 | 1.41 | 0.63-3.16 | 0.40 | 0.60 |
| MeDi score 2c | ||||||||||
| 0-3 | 265 (24.8) | 11 (26.8) | 1.00 | reference | − | − | 1.00 | reference | − | − |
| 4-5 | 458 (42.8) | 17 (41.5) | 1.05 | 0.52-2.12 | 0.89 | − | 1.19 | 0.55-2.54 | 0.66 | − |
| 6-9 | 347 (32.4) | 13 (31.7) | 1.21 | 0.57-2.56 | 0.63 | 0.87 | 1.37 | 0.60-3.10 | 0.45 | 0.75 |
Figures in parentheses indicate percentages.
Odds ratio (95% confidence intervals) adjusted for age, years of education, total energy (as continuous variables), and sex. All models were adjusted with propensity weights to take into account potential nonparticipation bias. There were 122 cases with a-MCI and 41 cases with na-MCI.
Adjusted for age, years of education, total energy (continuous variables), sex, ApoE ε4 (ε4+ vs. ε4−), stroke, coronary heart disease, and depressive symptoms.
Fat intake was determined from the MUFA:SFA ratio for MeDi score 1, and from the (MUFA + PUFA):SFA ratio for MeDi score 2.
Secondary Analyses
There were no significant associations of MCI with the MeDi score as quartiles (Q2: OR = 0.90, 95% CI = 0.58–1.42; Q3: OR = 1.17, 95% CI = 0.76–1.80; Q4: OR = 0.86, 95% CI = 0.57–1.31 compared to Q1; p for trend = 0.53) or as a continuous variable (OR = 0.98, 95% CI = 0.89–1.07, p = 0.63). The associations using the (MUFA + PUFA):SFA ratio were consistent with those based on the MUFA:SFA ratio (table 3), and there were no significant associations or trends when fruit and nuts or sex-specific cutpoints for alcohol intake were used in computing the MeDi score (these data are not presented).
Longitudinal Associations
Among 1,141 subjects who had MCI or were cognitively normal at baseline, and who completed ≥1 longitudinal clinical evaluation after assessment of dietary intake, there were 116 incident events (MCI, n = 93; dementia, n = 23). Due to the relatively short follow-up [median follow-up = 2.2 years (interquartile range = 1.7–2.6)], there was inadequate power to detect significant associations of the MeDi score components or the MeDi score with incident events. However, the hazard ratio (HR) for incident MCI or dementia was reduced 21% for subjects who had a MeDi score in the second tertile (HR = 0.79, 95% CI = 0.51–1.21, p = 0.28) and 25% for subjects in the upper tertile (HR = 0.75, 95% CI = 0.46–1.21, p = 0.24). The results were similar for MeDi score 2.
Discussion
In our cross-sectional findings, higher daily intake of vegetables, (MUFA + PUFA):SFA ratio, and moderate alcohol consumption were associated with a decreased OR of MCI and a-MCI (but not na-MCI). The OR of MCI decreased with higher intake of fruit and increasing MeDi score, but the trends were not statistically significant. The longitudinal findings showed a 25% reduced risk of MCI or dementia in subjects in the upper tertile of the MeDi score at baseline, but the association did not reach statistical significance.
Our findings are consistent with those of other investigators. High vegetable intake was associated with a slower rate of cognitive decline in the Chicago Health and Aging Project [32]; high intake of β-carotene, flavonoids, vitamins C and E, thiamine, and folate from dietary fruit and vegetables was associated with a lower risk of AD in the Rotterdam Study [33] and with better Mini-Mental State Exam (MMSE) scores [34]. Moderate alcohol intake has also been associated with a reduced incidence of dementia [35,36], a decreased OR of MCI [37,38], reduced progression of MCI to dementia [37], a lower risk of poor cognitive function [39], and higher MMSE scores [34]. The effects of fruits and vegetables, PUFA, MUFA, and moderate alcohol intake have been attributed to beneficial antioxidant effects on cerebrovascular disease risk and amyloid pathology. The adverse association of high caloric intake with MCI has also been observed with AD [40]. The association of dietary factors with MCI subtypes has not been evaluated. Our observation of associations of higher intake of vegetables and of higher MUFA:SFA and (MUFA + PUFA):SFA ratios with a-MCI is interesting and raises questions about the role of dietary factors in the pathogenesis of MCI and AD. However, given the cross-sectional design, the implications and relevance are not clear. We will examine these associations further in our longitudinal study of the cohort when we have a larger number of events and longer duration of follow-up.
Our preliminary longitudinal studies suggest that a high MeDi score is beneficial even in our cohort, but we may have had inadequate power to detect significant associations, possibly due to a low adherence to the MeDi. Lower adherence in our cohort is suggested by the comparison of our sample with 2 Mediterranean [8,29] and 2 US samples [9,41] where a significant association of MeDi score with cognition has been observed (table 5). Overall, daily intake of vegetables, fruit, and fish was lower, and red meat intake was higher than in 1 of the US samples. The MUFA:SFA ratio was low compared to 1 Greek sample, 1 US sample, and was also lower than a ratio of 2 for participants in the Italian Longitudinal Study of Aging [11].
Table 5.
Distribution of components of the MeDi in the current study and other studies
| Dietary variable | Median daily intake of selected foods common to the MeDi |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mayo Clinic Study of Aging |
Trichopoulou etal. [8]a |
Trichopoulou et al. [29]a |
Mitrou et al. [41]b |
Scarmeas et al. [9]c |
||||||
| men median | women median | all median | men median | women median | men median | women median | men median | women median | all median | |
| Vegetables, g/day | 143.5 | 155.3 | 148.9 | 549.9 | 499.6 | 156.8 | 183.8 | 299.8 | 317.0 | 197 |
| Legumes, g/day | 47.2 | 43.0 | 45.5 | 9.1 | 6.7 | 3.3 | 10.7 | 8.6 | 6.1 | 57 |
| Fruits, g/day | 201.1 | 220.7 | 209.6 | − | − | 176.7 | 232.5 | 286.8 | 301.0 | 472 |
| Fruits and nuts, g/day | 208.7 | 230.6 | 216.7 | 362.5 | 356.3 | − | − | 292.3 | 304.2 | − |
| Dairy, g/day | 325.9 | 347.8 | 336.6 | 196.7 | 191.1 | 285.7 | 301.1 | 186.3 | 173.1 | 182 |
| Grains and cereals, g/day | 182.3 | 168.1 | 174.4 | 177.7 | 139.7 | 212.0 | 168.4 | 415.6 | 339.4 | 184 |
| Fish, g/day | 15.2 | 14.2 | 15.0 | 23.7 | 18.8 | 32.2 | 26.9 | 76.7 | 46.3 | 20 |
| Meat, g/day | 120.6 | 91.5 | 106.7 | 120.8 | 89.8 | 111.6 | 82.2 | 335.5 | 339.4 | 85 |
| MUFA:SFA ratio | 1.05 | 1.03 | 1.04 | 1.7 | 1.7 | 0.9 | 0.9 | 1.23 | 1.22 | 0.8 |
| (MUFA + PUFA):SFA ratio | 1.55 | 1.57 | 1.56 | − | − | 1.4 | 1.4 | − | − | − |
| Moderate alcohol, %d | 68.3 | 61.3 | 65.0 | − | − | − | − | − | − | 32 |
| Total energy, kcal | 1,947.4 | 1,656.3 | 1,811.9 | 2,354.4 | 1,863.0 | 2,296.6 | 1,860.9 | 1,889 | 1,455 | 1,428 |
| MeDi score Ie | 5.0 | 5.0 | 5.0 | − | − | − | − | − | − | 4.3 |
| MeDi score 2e | 5.0 | 5.0 | 5.0 | − | − | − | − | − | − | − |
Greek populations.
US population. Estimates in the published manuscript were presented in servings per day; we used our food frequency database to generate estimates in grams per day to enable us to compare the daily intakes on the same scale.
Energy and MeDi score are the mean (demented subjects included); multiethnic US population.
Alcohol intake of >0 and <30 g/day.
Fat intake was determined from the MUFA:SFA ratio for MeDi score 1, and from the (MUFA + PUFA):SFA ratio for MeDi score 2.
Low adherence to MeDi in a community may limit the ability to detect a significant dose-response association of the MeDi score with cognition. Since the median intakes in a sample are used to determine the cutpoints for computing the MeDi score, a score in 1 community may not reflect intakes in a community with very different dietary habits [11]. Other cultural differences in the foods may also affect studies involving the MeDi score. For example, availability of fruits and vegetables year-round is different in Mediterranean regions than in a Midwestern US community.
Relatively few investigators have used the whole diet approach such as the MeDi to assess the impact of diet on cognitive function. In these studies, higher adherence to a MeDi was associated with decreasing cognitive decline assessed from the MMSE [31,42], with prevalent AD [43], a reduced risk of AD and slower cognitive decline [9], and a reduced risk of AD mortality [44], and with a borderline reduced risk of MCI incidence and MCI conversion to dementia [10]. It is evident that different measures of cognition were used, and MCI criteria were retrospectively applied in some cases. However, the widespread health benefits of the MeDi are well noted. These include beneficial effects on survival [8,29], cardiovascular risk factors and outcomes [5,6], cancer [45], and inflammation [46]. Nonetheless, additional longitudinal studies are needed to confirm the associations with MCI in a population-based setting using reliable and valid ascertainment of both dietary exposure and MCI using specified criteria for MCI at the time of evaluation as in the present study. A longer duration of follow-up in our cohort may demonstrate significant associations.
Potential limitations of our findings include the possibility of recall bias in this elderly cohort, and our failure to validate the questionnaire in our cohort. Any effect of recall bias is likely to be minimal, and reporting of dietary intake is likely to be valid for the following reasons: (a) we excluded subjects with dementia who are unlikely to report valid dietary intake; (b) participants did not know about their cognitive status, reducing the potential for biased reporting; (c) MCI cases were very mild, with a median CDR sum of boxes of only 1.0 (interquartile range = 0.5–1.5), and (d) the results remained the same after excluding subjects in the lowest 5% of the memory domain score who could have provided unreliable data (data not presented). Also, others have observed that assessment of dietary intake over a longer period (prior 1 year) may be less susceptible to bias than short-term recall [47]. The modest differences between included and excluded subjects raises the question of potential nonparticipation bias. We addressed this by assigning included subjects who had the characteristics of the excluded subjects a heavier weight in all the logistic models to account for the propensity to participate in the study. The results were similar to those that were not adjusted for propensity to participate. The cross-sectional study design prevents our ability to assess causal associations. Given the number of tests assessed in the study, there is a potential for type 2 errors; a Bonferroni correction would require a p value of ≤0.004 for statistical significance. At this p value, the associations between the upper tertile of the (MUFA + PUFA):SFA ratio and total energy intake would remain statistically significant, but the other associations would no longer be significant. The preliminary longitudinal data based on the small number of incident events suggest a benefit of a higher MeDi score for MCI or dementia. Longitudinal follow-up of the sample will enable us to obtain more reliable estimates and will increase our power to detect significant associations. The findings may be generalizable to communities with similar demographic characteristics.
Our study has several strengths. The study sample was randomly selected from the community, thus reducing the potential for referral, selection, or volunteer bias. We used a previously validated questionnaire to ascertain dietary intake of foods. In addition to a whole-diet approach, we also assessed the association of individual components of the MeDi with MCI, and with MCI subtypes, and observed differences across the subtypes. The assessment of MCI was made using information from 3 independent evaluators, and the diagnosis of MCI or normal cognition was made by consensus, at the time of the evaluation, and was based on previously specified criteria. Our findings provide insight into the dietary habits of a Midwestern US community, and suggest that in this elderly cohort, adherence to a MeDi may be low. Despite this, we demonstrated beneficial associations of certain dietary components with MCI, and our preliminary longitudinal studies suggest that a high MeDi score may be beneficial. Thus, findings from our study and other studies provide insights into the role of the MeDi and components of this diet as a potential target for intervention in clinical trials to prevent MCI, and ultimately reduce the burden of dementia.
Acknowledgements
This work was supported by NIH-NIA grants P50 AG016574 (PI: R.C.P.), U01 AG006786 (PI: R.C.P.), and K01 AG028573 (PI: R.O.R.); by NIH-NIAMS grant R01 AR030582 (PI: W.A.R.); the NIH-NIMH grant K01 MH068351 (PI: Y.E.G.), and by the Robert H. and Clarice Smith and Abigail van Buren Alzheimer's Disease Research Program.
References
- 1.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 2.Morris JC, McKeel DW, Jr, Storandt M, Rubin EH, Price JL, Grant EA, Ball MJ, Berg L. Very mild Alzheimer's disease: informant-based clinical, psychometric, and pathologic distinction from normal aging. Neurology. 1991;41:469–478. doi: 10.1212/wnl.41.4.469. [DOI] [PubMed] [Google Scholar]
- 3.Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol. 2009;66:1210–1215. doi: 10.1001/archneurol.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hooijmans CR, Kiliaan AJ. Fatty acids, lipid metabolism and Alzheimer pathology. Eur J Pharmacol. 2008;585:176–196. doi: 10.1016/j.ejphar.2007.11.081. [DOI] [PubMed] [Google Scholar]
- 5.Panagiotakos DB, Pitsavos CH, Chrysohoou C, Skoumas J, Papadimitriou L, Stefanadis C, Toutouzas PK. Status and management of hypertension in Greece: role of the adoption of a Mediterranean diet: the ATTICA Study. J Hypertens. 2003;21:1483–1489. doi: 10.1097/00004872-200308000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Psaltopoulou T, Naska A, Orfanos P, Trichopoulos D, Mountokalakis T, Trichopoulou A. Olive oil, the Mediterranean diet, and arterial blood pressure: the Greek European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Am J Clin Nutr. 2004;80:1012–1018. doi: 10.1093/ajcn/80.4.1012. [DOI] [PubMed] [Google Scholar]
- 7.Sofi F, Macchi C, Abbate R, Gensini GF, Casini A. Effectiveness of the Mediterranean diet: can it help delay or prevent Alzheimer's disease? J Alzheimers Dis. 2010 doi: 10.3233/JAD-2010-1418. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 9.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer's disease. Ann Neurol. 2006;59:912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66:216–225. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solfrizzi V, Capurso C, D'Introno A, Colacicco AM, Capurso A, Panza F. Whole-diet approach and risk of chronic disease: limits and advantages. J Am Geriatr Soc. 2006;54:1800–1802. doi: 10.1111/j.1532-5415.2006.00934.x. [DOI] [PubMed] [Google Scholar]
- 12.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Ivnik RJ, Tangalos EG, Petersen RC, Rocca WA. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 14.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 15.Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The Short Test of Mental Status. Correlations with standardized psychometric testing. Arch Neurol. 1991;48:725–728. doi: 10.1001/archneur.1991.00530190071018. [DOI] [PubMed] [Google Scholar]
- 16.Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, Kurland LT. Mayo's Older Americans Normative Studies: WAIS-R, WMS-R and AVLT norms for ages 56 through 97. Clin Neuropsychol. 1992;6:1–104. [Google Scholar]
- 17.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 18.Lucas JA, Ivnik RJ, Smith GE, Bohac DL, Tangalos EG, Graff-Radford NR, Petersen RC. Mayo's Older Americans Normative Studies: category fluency norms. J Clin Exp Neuropsychol. 1998;20:194–200. doi: 10.1076/jcen.20.2.194.1173. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler DA. Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corporation; 1987. [Google Scholar]
- 20.Ivnik RJ, Malec JF, Smith GE. Normative data for AVLT percent retention as a function of age and original learning for persons above age 54. Arch Clin Neuropsychol. 1992;7:338. [Google Scholar]
- 21.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. ed 4. Washington: American Psychiatric Association; 1994. [Google Scholar]
- 22.Block G, Coyle LM, Hartman AM, Scoppa SM. Revision of dietary analysis software for the Health Habits and History Questionnaire. Am J Epidemiol. 1994;139:1190–1196. doi: 10.1093/oxfordjournals.aje.a116965. [DOI] [PubMed] [Google Scholar]
- 23.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 24.Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, Lopez OL, DeKosky ST. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 25.Geda YE, Roberts RO, Knopman DS, Christianson TJ, Pankratz VS, Ivnik RJ, Boeve BF, Tangalos EG, Petersen RC, Rocca WA. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol. 2010;67:80–86. doi: 10.1001/archneurol.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michels KB, Bingham SA, Luben R, Welch AA, Day NE. The effect of correlated measurement error in multivariate models of diet. Am J Epidemiol. 2004;160:59–67. doi: 10.1093/aje/kwh169. [DOI] [PubMed] [Google Scholar]
- 27.Little RJA. Survey nonresponse adjustments for estimates of means. Int Stat Rev. 1986;54:139–157. [Google Scholar]
- 28.Kessler RC, Little RJ, Groves RM. Advances in strategies for minimizing and adjusting for survey nonresponse. Epidemiol Rev. 1995;17:192–204. doi: 10.1093/oxfordjournals.epirev.a036176. [DOI] [PubMed] [Google Scholar]
- 29.Trichopoulou A, Orfanos P, Norat T, Bueno-de-Mesquita B, Ocke MC, Peeters PH, van der Schouw YT, Boeing H, Hoffmann K, Boffetta P, Nagel G, Masala G, Krogh V, Panico S, Tumino R, Vineis P, Bamia C, Naska A, Benetou V, Ferrari P, Slimani N, Pera G, Martinez-Garcia C, Navarro C, Rodriguez-Barranco M, Dorronsoro M, Spencer EA, Key TJ, Bingham S, Khaw KT, Kesse E, Clavel-Chapelon F, Boutron-Ruault MC, Berglund G, Wirfalt E, Hallmans G, Johansson I, Tjonneland A, Olsen A, Overvad K, Hundborg HH, Riboli E, Trichopoulos D. Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. BMJ. 2005;330:991. doi: 10.1136/bmj.38415.644155.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solfrizzi V, Frisardi V, Capurso C, D'Introno A, Colacicco AM, Chiloiro R, Dellegrazie F, Di Palo A, Capurso A, Panza F. Whole-diet approach: working on a criterion validity for age-related cognitive decline and mild cognitive impairment. J Am Geriatr Soc. 2009;57:1944–1946. doi: 10.1111/j.1532-5415.2009.02460.x. [DOI] [PubMed] [Google Scholar]
- 31.Psaltopoulou T, Kyrozis A, Stathopoulos P, Trichopoulos D, Vassilopoulos D, Trichopoulou A. Diet, physical activity and cognitive impairment among elders: the EPIC-Greece cohort (European Prospective Investigation into Cancer and Nutrition) Public Health Nutr. 2008;11:1054–1062. doi: 10.1017/S1368980007001607. [DOI] [PubMed] [Google Scholar]
- 32.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology. 2006;67:1370–1376. doi: 10.1212/01.wnl.0000240224.38978.d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Hofman A, Witteman JC, Breteler MM. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287:3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 34.Requejo AM, Ortega RM, Robles F, Navia B, Faci M, Aparicio A. Influence of nutrition on cognitive function in a group of elderly, independently living people. Eur J Clin Nutr. 2003;57(suppl 1):S54–S57. doi: 10.1038/sj.ejcn.1601816. [DOI] [PubMed] [Google Scholar]
- 35.Ruitenberg A, van Swieten JC, Witteman JC, Mehta KM, van Duijn CM, Hofman A, Breteler MM. Alcohol consumption and risk of dementia: the Rotterdam Study. Lancet. 2002;359:281–286. doi: 10.1016/S0140-6736(02)07493-7. [DOI] [PubMed] [Google Scholar]
- 36.Truelsen T, Thudium D, Gronbaek M. Amount and type of alcohol and risk of dementia: The Copenhagen City Heart Study. Neurology. 2002;59:1313–1319. doi: 10.1212/01.wnl.0000031421.50369.e7. [DOI] [PubMed] [Google Scholar]
- 37.Solfrizzi V, D'Introno A, Colacicco AM, Capurso C, Del Parigi A, Baldassarre G, Scapicchio P, Scafato E, Amodio M, Capurso A, Panza F, Italian Longitudinal Study on Aging Working Group Alcohol consumption, mild cognitive impairment, and progression to dementia. Neurology. 2007;68:1790–1799. doi: 10.1212/01.wnl.0000262035.87304.89. [DOI] [PubMed] [Google Scholar]
- 38.Anttila T, Helkala EL, Viitanen M, Kareholt I, Fratiglioni L, Winblad B, Soininen H, Tuomilehto J, Nissinen A, Kivipelto M. Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: a prospective population based study. BMJ. 2004;329:539. doi: 10.1136/bmj.38181.418958.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galanis DJ, Joseph C, Masaki KH, Petrovitch H, Ross GW, White L. A longitudinal study of drinking and cognitive performance in elderly Japanese American men: the Honolulu-Asia Aging Study. Am J Public Health. 2000;90:1254–1259. doi: 10.2105/ajph.90.8.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luchsinger JA, Tang MX, Shea S, Mayeux R. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59:1258–1263. doi: 10.1001/archneur.59.8.1258. [DOI] [PubMed] [Google Scholar]
- 41.Mitrou PN, Kipnis V, Thiebaut AC, Reedy J, Subar AF, Wirfalt E, Flood A, Mouw T, Hollenbeck AR, Leitzmann MF, Schatzkin A. Mediterranean dietary pattern and prediction of all-cause mortality in a US population: results from the NIH-AARP Diet and Health Study. Arch Intern Med. 2007;167:2461–2468. doi: 10.1001/archinte.167.22.2461. [DOI] [PubMed] [Google Scholar]
- 42.Feart C, Samieri C, Rondeau V, Amieva H, Portet F, Dartigues JF, Scarmeas N, Barberger-Gateau P. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA. 2009;302:638–648. doi: 10.1001/jama.2009.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scarmeas N, Stern Y, Mayeux R, Luchsinger JA. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol. 2006;63:1709–1717. doi: 10.1001/archneur.63.12.noc60109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scarmeas N, Luchsinger JA, Mayeux R, Stern Y. Mediterranean diet and Alzheimer disease mortality. Neurology. 2007;69:1084–1093. doi: 10.1212/01.wnl.0000277320.50685.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trichopoulou A, Lagiou P, Kuper H, Trichopoulos D. Cancer and Mediterranean dietary traditions. Cancer Epidemiol Biomarkers Prev. 2000;9:869–873. [PubMed] [Google Scholar]
- 46.Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: the ATTICA Study. J Am Coll Cardiol. 2004;44:152–158. doi: 10.1016/j.jacc.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 47.Shahar D, Fraser D, Shai I, Vardi H. Development of a food frequency questionnaire (FFQ) for an elderly population based on a population survey. J Nutr. 2003;133:3625–3629. doi: 10.1093/jn/133.11.3625. [DOI] [PubMed] [Google Scholar]