Abstract
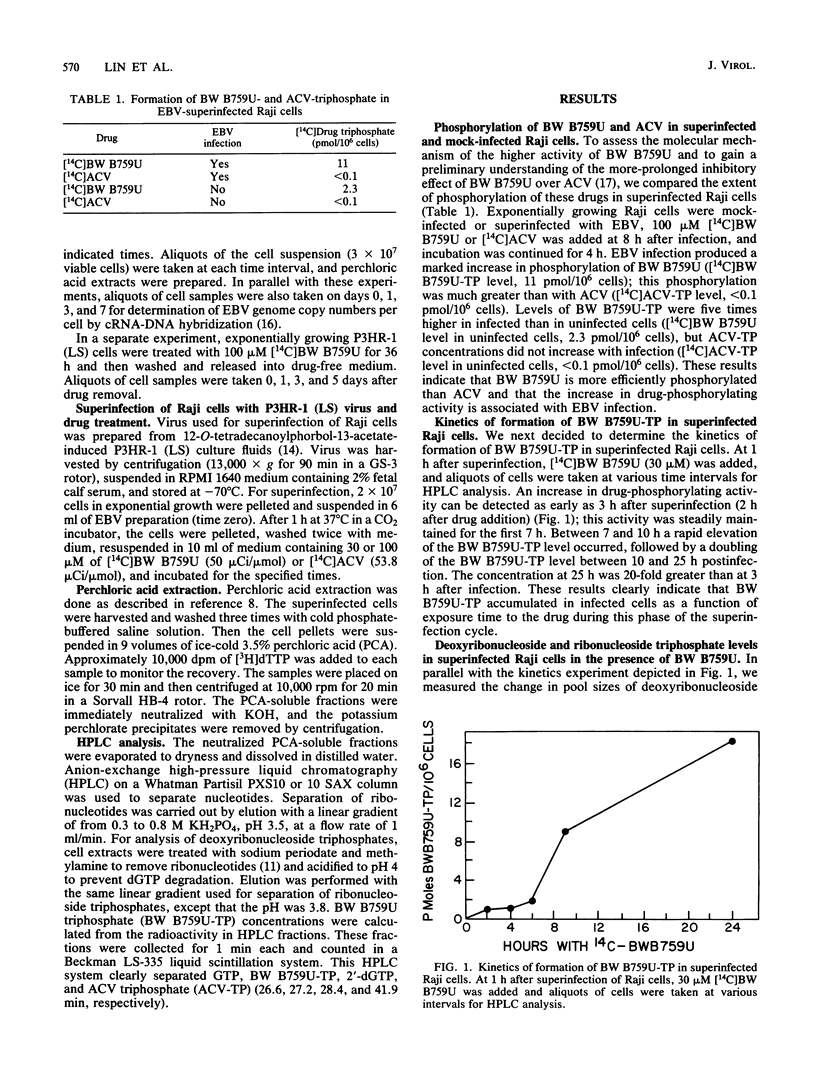
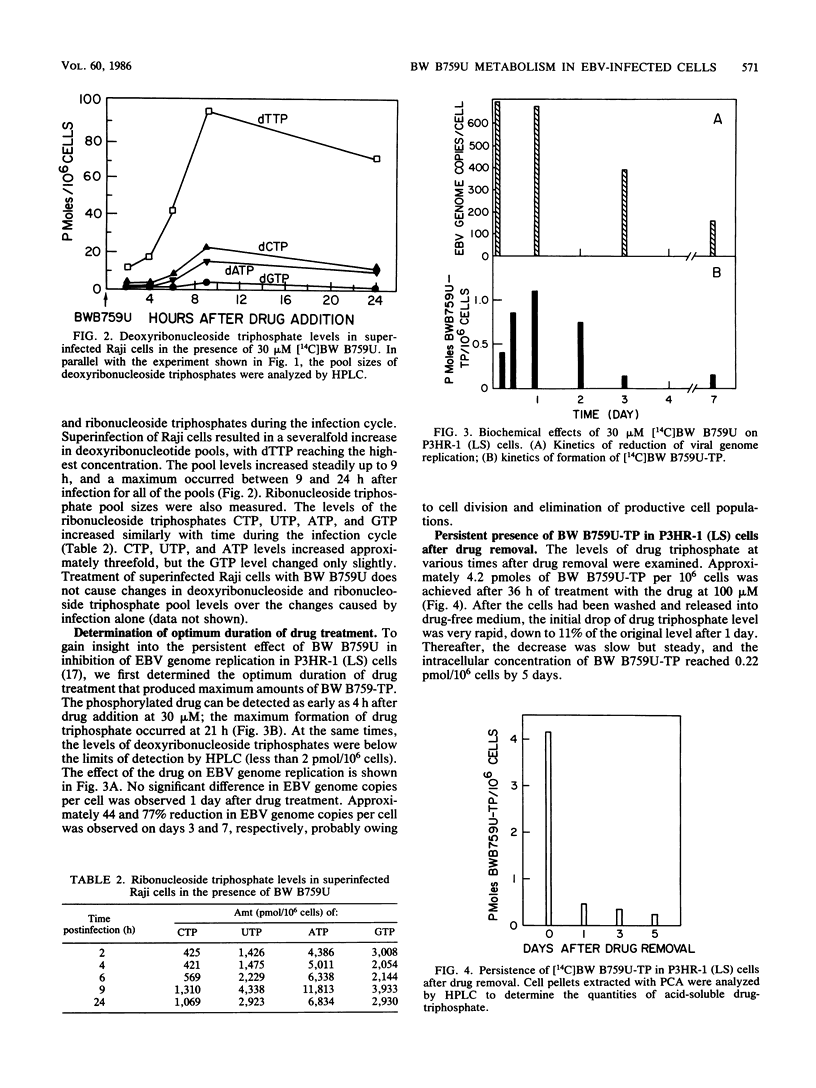
9-([2-Hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine (BW B759U) is more potent and has a more prolonged inhibitory effect against Epstein-Barr virus (EBV) in vitro than does acyclovir (ACV). To assess the mechanism of this difference, we first compared the extent of phosphorylation of the two drugs in superinfected Raji cells. BW B759U is phosphorylated to levels 100-fold higher than is ACV. In addition, lower levels of phosphorylation of BW B759U and ACV were observed in uninfected Raji cells. Studies on the kinetics of formation of BW B759U triphosphate in superinfected Raji cells indicated that drug-phosphorylating activity was detected as early as 3 h after superinfection; this activity was steadily maintained for the first 7 h, followed by a burst of activity between 7 and 10 h and a doubling of phosphorylation between 10 and 25 h. During the superinfection cycle, the pool sizes of deoxyribonucleoside and ribonucleoside triphosphates were increased and reached their maxima at 10 h after infection. The maximal amount of triphosphorylated drug in a virus producer cell, P3HR-1 (LS), was obtained at 21 h after drug treatment. During long-term drug treatment, approximately 44 and 77% reduction in EBV genome copies per cell was observed on days 3 and 7, respectively. In a separate experiment, after treatment of P3HR-1 (LS) cells with BW B759U for 36 h, 4.2 pmol of BW B759U triphosphate per 10(6) cells was achieved. After the cells were released into drug-free medium, drug triphosphate was rapidly decreased to 11% of the original level in 1 day. Thereafter, the decrease was slow but steady, down to 0.22 pmol/10(6) P3HR-1 cells by 5 days. We calculated that 0.22 pmol of BW B759U triphosphate per 10(6) cells represents a cellular concentration of 0.22 microM, which is theoretically enough to inhibit EBV replication. This is based upon a comparison with the 50% effective dose of BW B759U (0.05 microM) for inhibition of genome replication and a Ki of 0.08 microM for BW B759U triphosphate inhibition of EBV DNA polymerase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton W. T., Karkas J. D., Field A. K., Tolman R. L. Activation by thymidine kinase and potent antiherpetic activity of 2'-nor-2'-deoxyguanosine (2'NDG). Biochem Biophys Res Commun. 1982 Oct 29;108(4):1716–1721. doi: 10.1016/s0006-291x(82)80109-5. [DOI] [PubMed] [Google Scholar]
- Biron K. K., Stanat S. C., Sorrell J. B., Fyfe J. A., Keller P. M., Lambe C. U., Nelson D. J. Metabolic activation of the nucleoside analog 9-[( 2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine in human diploid fibroblasts infected with human cytomegalovirus. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2473–2477. doi: 10.1073/pnas.82.8.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. T., Estes J. E., Huang E. S., Pagano J. S. Epstein-Barr virus-associated thymidine kinase. J Virol. 1978 Apr;26(1):203–208. doi: 10.1128/jvi.26.1.203-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Grill S. P., Dutschman G. E., Nakayama K., Bastow K. F. Metabolism of 9-(1,3-dihydroxy-2-propoxymethyl)guanine, a new anti-herpes virus compound, in herpes simplex virus-infected cells. J Biol Chem. 1983 Oct 25;258(20):12460–12464. [PubMed] [Google Scholar]
- Cheng Y. C., Huang E. S., Lin J. C., Mar E. C., Pagano J. S., Dutschman G. E., Grill S. P. Unique spectrum of activity of 9-[(1,3-dihydroxy-2-propoxy)methyl]-guanine against herpesviruses in vitro and its mode of action against herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1983 May;80(9):2767–2770. doi: 10.1073/pnas.80.9.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou J. F., Cheng Y. C. Interaction of Epstein-Barr virus DNA polymerase and 5'-triphosphates of several antiviral nucleoside analogs. Antimicrob Agents Chemother. 1985 Mar;27(3):416–418. doi: 10.1128/aac.27.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A. K., Pagano J. S. Phosphorylation of acyclovir in vitro in activated Burkitt somatic cell hybrids. Antimicrob Agents Chemother. 1983 Jul;24(1):10–14. doi: 10.1128/aac.24.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. K., Davies M. E., DeWitt C., Perry H. C., Liou R., Germershausen J., Karkas J. D., Ashton W. T., Johnston D. B., Tolman R. L. 9-([2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine: a selective inhibitor of herpes group virus replication. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4139–4143. doi: 10.1073/pnas.80.13.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank K. B., Chiou J. F., Cheng Y. C. Interaction of herpes simplex virus-induced DNA polymerase with 9-(1,3-dihydroxy-2-propoxymethyl)guanine triphosphate. J Biol Chem. 1984 Feb 10;259(3):1566–1569. [PubMed] [Google Scholar]
- Garrett C., Santi D. V. A rapid and sensitive high pressure liquid chromatography assay for deoxyribonucleoside triphosphates in cell extracts. Anal Biochem. 1979 Nov 1;99(2):268–273. doi: 10.1016/s0003-2697(79)80005-6. [DOI] [PubMed] [Google Scholar]
- Germershausen J., Bostedor R., Field A. K., Perry H., Liou R., Bull H., Tolman R. L., Karkas J. D. A comparison of the antiviral agents 2'-nor-2'-deoxyguanosine and acyclovir: uptake and phosphorylation in tissue culture and kinetics of in vitro inhibition of viral and cellular DNA polymerases by their respective triphosphates. Biochem Biophys Res Commun. 1983 Oct 31;116(2):360–367. doi: 10.1016/0006-291x(83)90530-2. [DOI] [PubMed] [Google Scholar]
- Lin J. C., Shaw J. E., Smith M. C., Pagano J. S. Effect of 12-O-tetradecanoyl-phorbol-13-acetate on the replication of Epstein-Barr virus. I. Characterization of viral DNA. Virology. 1979 Nov;99(1):183–187. doi: 10.1016/0042-6822(79)90052-7. [DOI] [PubMed] [Google Scholar]
- Lin J. C., Smith M. C., Pagano J. S. Activation of latent Epstein-Barr virus genomes: selective stimulation of synthesis of chromosomal proteins by a tumor promoter. J Virol. 1983 Mar;45(3):985–991. doi: 10.1128/jvi.45.3.985-991.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. C., Smith M. C., Pagano J. S. Comparative efficacy and selectivity of some nucleoside analogs against Epstein-Barr virus. Antimicrob Agents Chemother. 1985 Jun;27(6):971–973. doi: 10.1128/aac.27.6.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. C., Smith M. C., Pagano J. S. Effects of 12-O-tetradecanoyl-phorbol-13-acetate on cell proliferation and Epstein-Barr virus DNA replication. Virology. 1982 Feb;117(1):186–194. doi: 10.1016/0042-6822(82)90518-9. [DOI] [PubMed] [Google Scholar]
- Lin J. C., Smith M. C., Pagano J. S. Prolonged inhibitory effect of 9-(1,3-dihydroxy-2-propoxymethyl)guanine against replication of Epstein-Barr virus. J Virol. 1984 Apr;50(1):50–55. doi: 10.1128/jvi.50.1.50-55.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar E. C., Cheng Y. C., Huang E. S. Effect of 9-(1,3-dihydroxy-2-propoxymethyl)guanine on human cytomegalovirus replication in vitro. Antimicrob Agents Chemother. 1983 Oct;24(4):518–521. doi: 10.1128/aac.24.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooka T., Calender A., de Turenne M., Daillie J. Effect of arabinofuranosylthymine on the replication of Epstein-Barr virus and relationship with a new induced thymidine kinase activity. J Virol. 1983 Apr;46(1):187–195. doi: 10.1128/jvi.46.1.187-195.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano J. S., Datta A. K. Perspectives on interactions of acyclovir with Epstein-Barr and other herpes viruses. Am J Med. 1982 Jul 20;73(1A):18–26. doi: 10.1016/0002-9343(82)90057-2. [DOI] [PubMed] [Google Scholar]
- Smee D. F., Boehme R., Chernow M., Binko B. P., Matthews T. R. Intracellular metabolism and enzymatic phosphorylation of 9-(1,3-dihydroxy-2-propoxymethyl)guanine and acyclovir in herpes simplex virus-infected and uninfected cells. Biochem Pharmacol. 1985 Apr 1;34(7):1049–1056. doi: 10.1016/0006-2952(85)90608-2. [DOI] [PubMed] [Google Scholar]
- Smee D. F., Martin J. C., Verheyden J. P., Matthews T. R. Anti-herpesvirus activity of the acyclic nucleoside 9-(1,3-dihydroxy-2-propoxymethyl)guanine. Antimicrob Agents Chemother. 1983 May;23(5):676–682. doi: 10.1128/aac.23.5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair M. H., Miller W. H., Miller R. L., Lambe C. U., Furman P. A. Inhibition of cellular alpha DNA polymerase and herpes simplex virus-induced DNA polymerases by the triphosphate of BW759U. Antimicrob Agents Chemother. 1984 Feb;25(2):191–194. doi: 10.1128/aac.25.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe T., Clough W. Epstein-Barr virus induces a unique pyrimidine deoxynucleoside kinase activity in superinfected and virus-producer B cell lines. Biochemistry. 1985 Apr 9;24(8):2027–2033. doi: 10.1021/bi00329a034. [DOI] [PubMed] [Google Scholar]



