Abstract
Background:
There is no prospective randomized data comparing laparoscopic to open hepatectomy. This study compared short- and long-term outcomes in patients undergoing hepatectomy for colorectal metastases (CRM), who were suitable for either laparoscopic or open surgery.
Methods:
Data were prospectively collected from consecutive patients undergoing hepatic resection of CRM at a single centre (1987–2007). Patients who were suitable for laparoscopic resection (Group 1) were compared with patients whose tumour characteristics would best be considered for open resection (Group 2).
Results:
Out of 1152 hepatectomies, 266 (23.1%) were deemed suitable for a laparoscopic approach. The median (IQR) number of metastases was greater in Group 2 [2(1–20) vs. 1(1–10), P < 0.001], as was the mean (SD) tumour size [5.3(3.6) cm vs. 3.3(1.2) cm, P < 0.001]. The median (IQR) operation time [210 (70) min vs. 240 (90) min, P < 0.001] and blood loss [270 (265) ml vs. 355 (320) ml, P < 0.001] were less in Group 1. There was no difference in length of stay, morbidity or mortality. Patients in Group 2 had a higher R1 resection rate (14.9%) compared with Group 1 (4.5%, P < 0.001) and lower 5-year survival (37.8% vs. 44.2%, P= 0.005).
Discussion:
Current criteria for laparoscopic hepatectomy selects patients who have more straight-forward surgery, with less risk of an involved resection margin and better long-term survival, compared with patients unsuited to a laparoscopic approach. Clearly defined criteria for laparoscopic hepatectomy are essential to allow meaningful analysis of outcomes and the results of unrandomized series of laparoscopic hepatectomies must be interpreted with caution.
Keywords: hepatectomy, metastatic colorectal cancer, laparoscopy, outcome
Introduction
Liver resection, in combination with neoadjuvant or adjuvant chemotherapy, is firmly established as the standard of care for patients with colorectal metastases, with variable 5-year survival rates ranging from 25 to 44%.1–5 Advances in anaesthetic and surgical techniques have improved the short-term outcomes from open liver resection, with in-hospital mortality less than 4% in modern case series.3,5 Liver resection has associated peri-operative morbidity, including wound complications, hepatic insufficiency, bile leak, bleeding, renal failure and cardio-respiratory compromise and complications can occur in up to 30% of cases.5,6
Minimally invasive surgery has claimed its rite of passage in many intra-abdominal procedures, with laparoscopic cholecystectomy, fundoplication, splenectomy and colonic resection the preferred standard of care. However, the place for laparoscopic liver surgery remains controversial. The first anatomical laparoscopic liver resection was a left lateral sectionectomy, reported in 1996 by Azagra.7 With advances in equipment and techniques, hepatic surgeons are rising to the challenge of laparoscopic hepatectomy. As with laparoscopic colorectal surgery and laparoscopic cholecystectomy, there are potential short-term advantages to laparoscopic hepatectomy in terms of reduced post-operative wound complications and pain, better cosmesis, shorter in-hospital stay and early return to work.8,9 However, any laparoscopic operation must have an equivalent morbidity and mortality to the open approach before it is accepted as a viable alternative. Furthermore, if the resection is for malignancy, oncological clearance and long-term survival must be comparable to the open approach.
While there is no published randomized controlled trial of laparoscopic vs. open hepatectomy, recent small case-controlled studies suggest that a laparoscopic approach has superior short-term outcomes to the open approach.10–15 These findings are confirmed in a meta-analysis,9 which concluded that laparoscopic liver resection was safe and feasible when performed by experienced surgeons in selected patients. However, it did acknowledge a significant bias towards the laparoscopic approach in the included studies. Thus these results must be interpreted with caution. While the criteria for laparoscopic resection will vary depending on the institution and surgeon's experience, patient comorbidity, previous surgery and preference, tumour size and location are the major influences on the practicality of a laparoscopic operation. Cases suitable for a laparoscopic resection have tended to be those with smaller metastases,16 more favourably situated within the liver, not involving the hepatic veins/ caval confluence or hilum.17,18 There is consensus that patients with metastases of diameter greater than 6 cm, or metastases sited near the hilum or vena cava, in segments 1, 4a, 7 and 8, or those invading adjacent extra-hepatic structures, are more suitable for open surgery.19,20
The aim of the present study was to compare short- and long-term outcomes in patients undergoing liver resection for colorectal metastases who were potentially suitable for a laparoscopic hepatectomy, compared with those for whom an open resection would be more appropriate. Specifically, we analysed the outcomes of a large cohort of patients who underwent open liver resection for colorectal metastases and compared a subset of that group who were potentially suitable for a laparoscopic approach, to those patients in whom resection was deemed unsuitable for a minimal invasive approach by current criteria.
Patients and methods
Study subjects
This was a study of consecutive patients undergoing open hepatic resection for colorectal liver metastases at a single tertiary referral centre between 1987 and 2007, prior to the introduction of laparoscopic liver resection in this unit. Patient inclusion criteria were age older than 18 years and a confirmed primary colorectal cancer with at least one resectable hepatic colorectal metastasis. Patients with and without neoadjuvant chemotherapy prior to liver resection were included in the study. Patients with extra-hepatic disease or who were undergoing a repeat hepatic resection were excluded from the analysis.
Data collection
Patient data were collected prospectively on a database (Access®; Microsoft Corporation, Redmond, Washington, USA) comprising 268 data fields. This was compiled contemporaneously using standardized proformas and encompassed patient symptoms, pre-operative assessment, surgical treatment, post-operative course, histopathology and long-term outcomes.
Data definitions
Resectable disease was defined as the ability to completely remove all liver metastases, regardless of size, number, distribution or width of resection margin, while preserving a sufficient volume of functioning hepatic parenchyma [usually 25–30% of functioning liver volume as estimated by computed tomography (CT) or magnetic resonance imaging (MRI)], taking into account the portal venous and hepatic arterial inflow, hepatic venous outflow and biliary drainage. These criteria have remained unchanged throughout the study period. The surgical and anaesthetic techniques used have been previously reported by this unit.2,21,22 Peri-operative mortality was defined as a death during the same hospital admission or within 90 days of the date of the operation. Post-operative complications were classified as minor, relevant (delayed patient discharge) or major (complications requiring urgent medical or surgical intervention).
The nomenclature and extent of hepatic resection were recorded according to the Brisbane 2000 Terminology of Liver Anatomy and Resections.23 Major liver resection was defined as the resection of three or more hepatic segments (hemihepatectomy and extended hemihepatectomy). A minor resection was defined as the resection of fewer than three segments, including wedge resections.
Study design
All patients included in the study were divided into two groups according to their suitability for laparoscopic (Group 1) or open (Group 2) hepatectomy. Patients potentially suitable for a laparoscopic hepatectomy were those with metastases less than 6 cm in maximum diameter, located in segments 2, 3, 4b, 5, 6, 7 or 8, who underwent an anatomical or non-anatomical resection of disease. Patients suitable for isolated resection of segments 4a, 7 or 8 were excluded from Group 1. In addition, patients whose metastases were 6 cm or greater in diameter, or close/involving the hepatic venous/caval confluence, the hilum, or in the caudate (segment 1) were deemed unsuitable for a potentially laparoscopic resection (Group 2). Similarly, patients who had bilobar disease, requiring an extended resection, or major plus minor resection(s) were excluded from the potentially laparoscopic group and entered into Group 2. Finally, patients who had tumours invading adjacent structures such as the diaphragm or chest wall and patients requiring additional procedures not felt to be suitable for a minimally invasive approach were excluded from Group 1. All patients who underwent synchronous bowel and liver resection were deemed suitable for a minimally invasive combined approach (Group 1). These criteria were derived from literature review16–20 and the consensus of expert opinion [Professor O James Garden, Professor Robert Padbury, Mr Nick O’Rourke and the senior author, Mr Myrddin (Merv) Rees]. The authors accept that this retrospective creation of two groups from a database is somewhat artificial and does not necessarily reflect the current reality of selection for a laparoscopic approach by increasingly experienced teams.
Study end-points
The short-term outcome measures included intra-operative parameters such as operation time (taken from induction of anaesthesia to the patient leaving the operating room at the end of surgery), intra-operative blood loss, the requirement for blood transfusion and post-operative morbidity and mortality, including the percentage of patients with involved (R1) resection margins on histological analysis. The long-term outcome measured was cancer-specific survival. Follow-up of patients was performed either in Basingstoke, or for patients residing at a distance from the hospital by the local referring clinician (surgeon or oncologist) in postal or telephone correspondence. There was a dedicated data collator responsible for completion of the entire follow-up data set.
Statistical analysis
Data were entered on a Statistical Package for the Social Sciences (version 14) software package (SPSS Inc., Chicago, IL, USA). Data were analysed using t-tests, chi-squared and Fisher's exact test and Kaplan–Meier survival curves, where appropriate. A P-value of less than 0.05 was considered to indicate a statistically significant difference between groups.
Results
Patient and primary tumour details
Figure 1 shows that 1152 liver resections were performed for colorectal metastases during the study period. Based on the type of liver resection, there were 576 (50%) patients that were deemed suitable for a minimally invasive approach. By excluding patients with tumours 6 cm or greater in diameter, 370 patients remained. Using stepwise exclusion for tumours close to or involving the hilum or inferior vena cava (IVC), involvement of surrounding structures and additional procedures not suitable for a laparoscopic approach, respectively, we identified 266 (23.1%) patients as potentially suitable for a laparoscopic hepatectomy (Group 1). The 886 excluded patients comprised Group 2. The patient demographics in Groups 1 and 2 are detailed in Table 1 and shows that there was no difference between age, gender, Duke's stage or tumour differentiation between the groups. However, patients in Group 2 were more likely to have an elevated carcinoembryonic antigen (CEA) prior to their liver resection.
Figure 1.
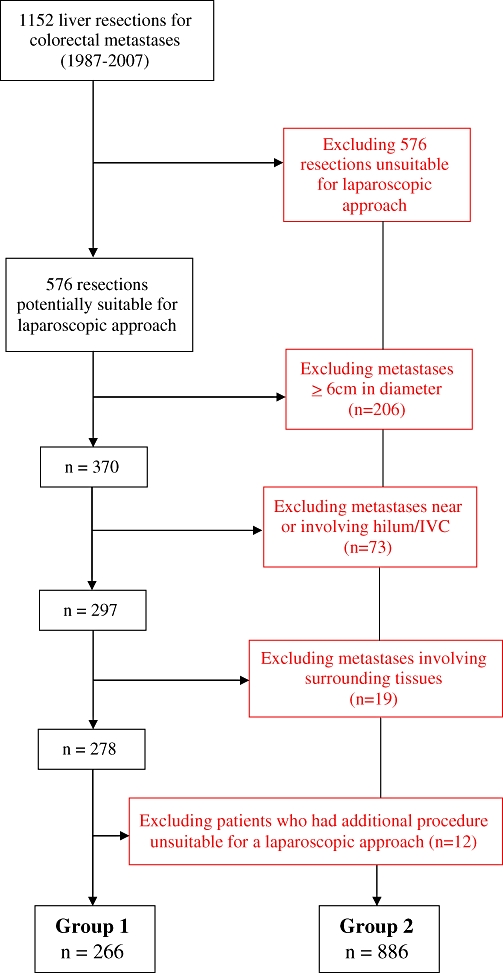
Flow chart to show derivation of study groups
Table 1.
Patient demographic characteristics and operative details of patients undergoing hepatic resections for colorectal liver metastases with a curative intent. Patients are grouped according to whether they are considered suitable (Group 1) or not suitable (Group 2) for laparoscopic resection
| Risk factors | Group 1 n= 266 | Group 2 n= 886 | P-value |
|---|---|---|---|
| Age mean (SD) | 61.9 (10.4) | 62.3 (10.1) | 0.622 |
| Gender | 0.390 | ||
| Female | 105 (39.5) | 324 (36.6) | |
| Male | 161 (60.5) | 562 (63.4) | |
| Primary tumour LN status | 0.359 | ||
| Negative | 107 (40.4) | 326 (37.2) | |
| Positive | 158 (59.6) | 549 (62.7) | |
| Primary tumour differentiation | 0.237 | ||
| Well | 33 (12.5) | 101 (11.5) | |
| Moderate | 205 (77.4) | 713 (81.3) | |
| Poor | 27 (10.2) | 63 (7.2) | |
| Primary tumour type | 0.745 | ||
| Adenocarcinoma | 259 (97.7) | 854 (97.4) | |
| Mucinous (>50%) | 6 (2.3) | 23 (2.6) | |
| Neo-adjuvant chemotherapy | 0.97 | ||
| No | 201 (75.6) | 667 (75.5) | |
| Yes | 65 (24.4) | 217 (24.5) | |
| CEA level | <0.001 | ||
| <6 ng/ml | 95 (35.7) | 255 (28.8) | |
| 6–60 ng/ml | 72 (27.1) | 249 (28.1) | |
| >60 ng/ml | 22 (8.3) | 182 (20.5) | |
| Not measured | 77 (28.9) | 200 (22.6) | |
| Number of hepatic metastases | |||
| Median (range) | 1 (1–10) | 2 (1–20) | <0.001 |
| 1–3 | 251 (94.4) | 715 (80.7) | |
| >3 | 15 (5.6) | 171 (19.3) | <0.001 |
| Distribution of liver metastases | |||
| Right lobe | 201 (75.6) | 407 (45.9) | |
| Left lobe | 56 (21.8) | 149 (16.8) | |
| Bilobar | 7 (2.6) | 315 (35.6) | <0.001 |
| Hilar / caudate / gall-bladder | 0 (0) | 294 (33.2) | – |
| Local involvement | |||
| No infiltration | 75 (9.0) | 836 (91.8) | |
| Diaphragmatic involvement | 3 (5.0) | 60 (6.6) | 0.299 |
| Other organs | 2 (13.3) | 15 (1.6) | 0.563 |
| Tumour diameter; mean (SD) | 3.3 (1.2) | 5.3 (3.6) | <0.001 |
| Type of liver resection | |||
| Right hepatectomy | 137 (51.5) | 178 (20.1) | |
| Right hepatectomy + NA | 0 | 77 (8.7) | |
| Left hepatectomy | 28 (10.5) | 69 (7.8) | |
| Left hepatectomy + NA | 0 | 69 (7.8) | |
| Extended right hepatectomy | 0 | 130 (14.7) | |
| Extended left hepatectomy | 0 | 30 (3.4) | |
| Left lateral sectionectomy | 20 (7.5) | 48 (5.4) | |
| Left lateral sectionectomy + NA | 1 (0.4) | 42 (4.7) | |
| Bisegmentectomy ± NA | 21 (7.9) | 123 (13.9) | |
| Segmentectomy ± NA | 39 (14.7) | 85 (9.6) | |
| NA (single or multiple) | 20 (7.5) | 35 (3.9) |
Numbers in parentheses represent percentages unless otherwise stated.
CEA, carcinoembryonic antigen; NA, non-anatomical wedge excision.
Details of hepatic metastases
Table 1 shows that the median (interquartile range) number of liver metastases was 2 (1–20) in the patients unsuitable for a laparoscopic resection, significantly higher than in those potentially suitable for a laparoscopic approach [1(1–10), P < 0.001]. Furthermore, the metastases in the patients in this group (Group 2) were of larger maximum diameter (5.3 cm vs. 3.3 cm in Group 1, P < 0.001) and were more likely to involve both sides of the liver (35.5%), compared with Group 1 (2.6%, P < 0.001) (Table 1).
Immediate intra-operative outcomes
The liver resections were significantly quicker, with less associated blood loss in the patients potentially suited for a laparoscopic approach (Group 1) (Table 2). The median blood loss in this group was 270 ml, significantly lower than that in Group 2 (355 ml, P < 0.001). In Groups 1 and 2, the median fall in haemoglobin was 1.3 and 1.4 g/dl, respectively, confirming no occult haemorrhage in either group. There was no difference in the number of intra-operative adverse surgical events between the two groups (Table 2).
Table 2.
Intra-operative, post-operative outcomes and long-term survival of patients undergoing hepatic resections for colorectal liver metastases with a curative intent. Patients are grouped according to whether they are considered suitable (Group 1) or not suitable (Group 2) for laparoscopic resection
| Risk factors | Group 1 n= 266 | Group 2 n= 886 | P-value |
|---|---|---|---|
| Operative time; median (IQR) in min | 210 (70) | 240 (90) | <0.001 |
| Blood loss; median (IQR) in ml | 270 (265) | 355 (320) | <0.001 |
| Change in Hb; mean (SD) in units | 1.3 (1.2) | 1.4 (1.3) | 0.395 |
| Length of hospital stay: mean (SD) | 10.0 (6.5) | 10.5 (8.4) | 0.338 |
| Intra-operative adverse events | 3 (1.1) | 19 (2.1) | 0.288 |
| Post-operative adverse events | |||
| All complications | 56 (21.1) | 227 (25.6) | 0.144 |
| Slight | 30 (11.3) | 80 (9.0) | |
| Relevant | 18 (6.8) | 108 (12.2) | |
| Life threatening | 8 (3.0) | 39 (4.4) | 0.044 |
| Surgical complications | 5 (1.9) | 31 (3.5) | 0.183 |
| Medical complications | 53 (19.9) | 208 (23.5) | 0.225 |
| Post-operative mortality | |||
| 30-day | 3 (1.1) | 12 (1.4) | 0.533 |
| 90-day | 4 (1.5) | 15 (1.7) | 0.544 |
| Resection margin | |||
| Not involved (R0) | 252 (95.5) | 751 (85.1) | |
| Involved (R1) | 12 (4.5) | 131 (14.9) | <0.001 |
| Cancer specific survival; in years | |||
| 5-year (SE) | 44.2 (3.8) | 37.8 (2.1) | 0.005a |
| 7-year (SE) | 36.9 (3.9) | 32.1 (2.2) | 0.004a |
| Median (SE) | 4.5 (0.3) | 3.5 (1.6) | 0.005a |
Log-rank test.
Short-term post-operative outcomes
The short-term outcomes are detailed in Table 2. The mean length of stay was 10 days in both groups. There was no difference in overall morbidity between the groups (24.8% vs. 25.6% P= NS), but significantly more relevant and life-threatening complications in the patients unsuited to a laparoscopic approach (P= 0.044). The 30- and 90-day mortality were similar in Group 1 (1.1% and 1.5%) compared with Group 2 (1.4% and 1.7%, P= 0.53 and 0.54, respectively). From the histological analysis, there were significantly more patients with an involved (R1) resection margin in Group 2 (14.9%) compared with Group 1 (4.5%, P < 0.001) (Table 2).
Cancer-specific survival
The associated cancer-specific 5 and 7-year survival and median cancer-specific survival for both groups is shown in Table 2. The 3, 5 and 7-year cancer-specific survival for patients in Group 1 was 69.7%, 44.2% and 36.9%, respectively, significantly better than those patients in Group 2 (59.1%, 37.8% and 32.1%, P = 0.005, 0.005 and 0.004, respectively). Figure 2 shows a Kaplan–Meier plot of the cancer-specific survival in both groups, with the median survival being 4.5 years in Group 1, compared with 3.5 years in Group 2 (Log-rank test, chi-square = 7.873, 1 d.f., P= 0.005).
Figure 2.
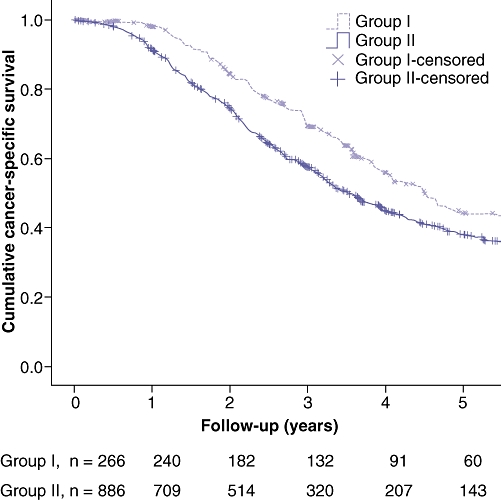
Cancer-specific survival in patients undergoing hepatic resection for colorectal liver metastases with a curative intent grouped according to whether these are considered suitable (Group 1) or not suitable (Group 2) for laparoscopic resection. Log-rank: chi-square = 7.873, 1 d.f., P= 0.005
Discussion
The role of laparoscopic hepatectomy in the treatment of colorectal metastases has yet to be defined. In a recent large case series of 590 minimally invasive hepatic procedures, only 40 were for colorectal liver metastases, the majority being for hepatocellular carcinoma (n= 210) or benign tumours (n= 176).24 In the Brisbane series of 84 laparoscopic hepatectomies, 33 (39%) were for malignancy, with 22 being performed for colorectal metastases.25 Whilst there is currently no prospective randomized controlled trial of laparoscopic vs. open hepatectomy, some recent case-controlled studies have attempted to compare the two approaches.10–15
However, laparoscopic hepatectomy is still evolving and many surgeons remain on a learning curve for this operation. Daniel Cherqui recently published his experience of 166 laparoscopic hepatectomies, performed between 1996 and 2007. He had a significantly lower blood loss and conversion rate in the second half of his series.26 As with any procedure, case selection is the key and in the absence of randomized trials, this current study asks whether the results of hepatectomy potentially suitable for a laparoscopic approach are comparable to those where an open hepatectomy would be more appropriate. From a large prospective cohort of open liver resections performed in a single centre, those cases potentially suitable for a laparoscopic approach were identified and their short-and long-term outcomes compared with those cases where a laparoscopic approach was not feasible. Cases suitable for a laparoscopic approach were by definition those with smaller tumours, more favourably sited within the liver. In addition, this current study showed that these patients potentially suited for a laparoscopic approach had fewer liver metastases, with a lower pre-operative CEA, suggesting a lower tumour burden compared with the potentially open group. While there was no difference in Duke's stage between the groups, patients unsuitable for a laparoscopic approach (Group 2) were more likely to have had bilobar disease. However, there was a similar percentage of major resections in both groups (Table 1) and indeed 62% of patients in the potentially laparoscopic group underwent a major resection, which is in contrast to most published series of laparoscopic liver resections. The study showed that surgery took significantly longer in Group 2, with more blood loss, although the median blood transfusion in both groups was zero. This is likely to be related to the greater burden of metastatic disease in this group and the more unfavourable tumour location. Furthermore, in patients with a complex distribution of liver metastases, who may have received neoadjuvant chemotherapy, a parenchymal-sparing approach to liver resection should be adopted, to avoid post-operative hepatic insufficiency.27 This is more challenging laparoscopically. A number of authors have tried to address this potential bias regarding case selection with case-controlled studies, and have shown reduced blood loss in patients undergoing laparoscopic liver resection, compared with open.10–15 These data support the findings of this current study and thereby confirm that case-mix bias must be taken into consideration when comparing the results of laparoscopic and open liver resections.
This study found no difference in the length of hospital stay between the two groups, with a mean stay of 10 days in each. This is in the absence of an enhanced recovery programme, with some patients living at a considerable distance (200 miles) from the hospital. The median stay for all patients undergoing liver resection in our centre in the past year (n= 175) is six days (unpublished data) and with the adoption of an enhanced-recovery programme, we are aiming to discharge most patients in 3–4 days after a major hepatectomy. In Daniel Cherqui's series of 37 laparoscopic left lateral sectionectomies, performed between 1997 and 2005, the mean length of stay was 7.8 days,28 although this was reduced to 4.3 days in the second half of his series. In a Norwegian series of 53 laparoscopic hepatic resections, 42 of which were for colorectal metastases, the median hospital stay was 3.5 days.29 In a case-controlled study of 20 cases of laparoscopic liver resection compared with 20 open liver resections, Troisi and co-workers showed that the laparoscopic group had a shorter mean hospital stay (7.1 days) compared with the open group (10.45 days, P= 0.008).30 However, in this series, there was a 10% conversion rate to open for uncontrollable haemorrhage, with one patient requiring haemodialysis. Moreover, there was a 25% incidence of incisional hernias recorded in the open group, which is extremely high and compares unfavourably to that in our own experience, where it is extremely rare. In this cohort, only two patients (0.17%) have undergone a surgical repair of an incisional hernia, both at the time of a repeat liver resection. The suggestion that a laparoscopic approach is associated with a shorter length of stay is supported by three further small case-controlled studies, which show a reduction in hospital stay of 1,10 212 and 311 days, respectively, using a laparoscopic approach.
Our study showed no difference in overall morbidity or mortality between patients potentially suitable for a laparoscopic approach, compared with those best suited for an open approach. This finding is confirmed by two published meta-analyses on laparoscopic vs. open liver resection8,9 and likely to be a testament to improved peri-operative care worldwide. The involved (R1) resection margin rate, however, was significantly higher in the group unsuited for a laparoscopic approach (14.9% vs. 4.5%, P < 0.001). This is likely to be related to case-selection, as our Unit has previously shown that a non-anatomical or extended resection, >3 hepatic metastases involving >50% of the liver and bilobar disease are all independent predictors of a positive resection margin.31 These predictive factors not only reflect tumour load, but also the proximity of metastases to key portal or venous structures. The relationship between narrow or involved margins and extensive disease was first demonstrated by Elias in 199832 and subsequently confirmed by Pawlik.33 Patients with more metastases, bilobar disease or those requiring an extended resection are more likely to have a narrow resection margin because of the complexity of achieving a radical clearance32 and these patients are not suitable for a laparoscopic approach by current criteria. In a case series of 53 laparoscopic liver resections, 42 of which were for colorectal metastases, despite the high proportion of non-anatomic resections (n = 45), which DeMatteo has shown are associated with a higher R1 margin rate,34 the R1 rate was only 6%,29 comparable to the findings in Group 1 of this study.
The 5-year cancer-specific survival for patients potentially suitable for a laparoscopic liver resection was 44.2%, significantly better than those patients unsuitable (37.8%, P = 0.005). This is likely to be explained by the greater tumour burden in this latter group (increased tumour size/ number and higher pre-operative CEA) and their higher involved resection margin rate, as these factors are well-established predictors of a poor long-tem outcome.3,5,6 There are two published studies to date that looked at long-term survival from laparoscopic liver resection for colorectal metastases. The Brisbane group reported a 75% 2-year survival,25 with Gayet's group reporting a 3-year overall and disease-free survival of 87% and 51%, respectively, in 41 patients undergoing laparoscopic liver resection for colorectal metastases.35 These survival figures are comparable with the 3-year disease-free survival of 69.7% reported here in Group 1. Thus, with the current paucity of prospective randomized or case-matched data, the long-term outcome of laparoscopic compared with open liver resection for colorectal metastases is as yet unclear.
In conclusion, this study shows that in patients with colorectal liver metastases, the current criteria for laparoscopic hepatectomy has selected a group of patients who have more straight-forward surgery, removing a lower tumour burden, with less risk of an involved resection margin and better long-term survival, compared with patients unsuited to a laparoscopic approach. Clearly defined criteria for laparoscopic liver resection are important to allow risk-stratified comparison of outcomes in patients undergoing liver resection for colorectal metastases.
Acknowledgments
The authors would like to acknowledge the following hepatobiliary surgeons for their expert advice and input with the conception and direction of this paper:
Professor O. James Garden, Regius Professor of Surgery, Royal Infirmary of Edinburgh, Scotland, UK, past President of the Association of Upper Gastrointestinal Surgeons (AUGIS) and current council member of the International Hepato-pancreatico-biliary Association (IHPBA).
Nick O’Rourke, Consultant Hepatobiliary Surgeon at the Royal Brisbane and Women's Hospital, Brisbane Australia and President-elect of the Australian and New Zealand Hepato-pancreatico-biliary Association (ANZHPBA).
Professor Rob Padbury, Professor of Hepatobiliary and Transplant Surgery, Flinders Hospital, Adelaide, Australia and current President of the ANZHPBA.
Conflicts of interest
None declared.
References
- 1.Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343:1405–1410. doi: 10.1016/s0140-6736(94)92529-1. [DOI] [PubMed] [Google Scholar]
- 2.Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal cancer: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–1246. doi: 10.1002/bjs.1800771115. [DOI] [PubMed] [Google Scholar]
- 3.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adam R, Huguet E, Azoulay D, Castaing D, Kunstlinger F, Levi F, et al. Hepatic resection after down-staging of unresectable hepatic colorectal metastases. Surg Oncol Clin N Am. 2003;12:211–220. doi: 10.1016/s1055-3207(02)00085-6. [DOI] [PubMed] [Google Scholar]
- 5.Rees M, Tekkis PP, Welsh FKS, O’Rourke TR, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 6.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 7.Azagra JS, Goergen M, Gilbart E, Jacobs D. Laparoscopic anatomical (hepatic) left lateral segmentectomy-technical aspects. Surg Endosc. 1996;10:758–761. doi: 10.1007/BF00193052. [DOI] [PubMed] [Google Scholar]
- 8.Laurence JM, Lam VW, Langcake ME, Hollands MJ, Crawford MD, Pleass HC. Laparoscopic hepatectomy, a systematic review. ANZ J Surg. 2007;77:948–953. doi: 10.1111/j.1445-2197.2007.04288.x. [DOI] [PubMed] [Google Scholar]
- 9.Simillis C, Constantinides VA, Tekkis PP, Darzi A, Lovegrove R, Jiao L, et al. Laparoscopic versus open hepatic resections for benign and malignant neoplasms -a meta-analysis. Surgery. 2007;141:203–211. doi: 10.1016/j.surg.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 10.Aldrighetti L, Pulitano C, Catena M, Arru M, Guzzetti E, Casati M, et al. A prospective evaluation of laparoscopic versus open left lateral hepatic sectionectomy. J Gastrointest Surg. 2008;12:457–462. doi: 10.1007/s11605-007-0244-6. [DOI] [PubMed] [Google Scholar]
- 11.Lee KF, Cheung YS, Chong CN, Tsang YY, Ng WW, Ling E, et al. Laparoscopic versus open hepatectomy for liver tumours: a case control study. Hong Kong Med J. 2007;13:442–448. [PubMed] [Google Scholar]
- 12.Morino M, Morra I, Rosso E, Miglietta C, Garrone C. Laparoscopic vs open hepatic resection: a comparative study. Surg Endosc. 2003;17:1914–1918. doi: 10.1007/s00464-003-9070-4. [DOI] [PubMed] [Google Scholar]
- 13.Lesurtel M, Cherqui D, Laurent A, Tayar C, Fagniez PL. Laparoscopic versus open left lateral hepatic lobectomy: a case-control study. J Am Coll Surg. 2003;196:236–242. doi: 10.1016/S1072-7515(02)01622-8. [DOI] [PubMed] [Google Scholar]
- 14.Polignano FM, Quyn AJ, de Figueiredo RS, Henderson NA, Kulli C, Tait IS. Laparoscopic versus open liver segmentectomy: prospective, case-matched, intention-to-treat analysis of clinical outcomes and cost effectiveness. Surg Endosc. 2008;22:2564–2570. doi: 10.1007/s00464-008-0110-y. [DOI] [PubMed] [Google Scholar]
- 15.Dagher I, Di Giuro G, Dubrez J, Lainas P, Smadja C, Franco D. Laparoscopic versus open right hepatectomy; a comparative study. Am J Surg. 2009;198:173–177. doi: 10.1016/j.amjsurg.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Hironori K, Masaru T, Yuichiro O, Akira T, Toshio K, Tetsuya M, et al. Laparoscopy-assisted hepatectomy for giant hepatocellular carcinoma. Surg Laparosc Endosc Percutan Tech. 2008;18:127–131. doi: 10.1097/SLE.0b013e318158237b. [DOI] [PubMed] [Google Scholar]
- 17.Mala T, Edwin B. Role and limitations of laparoscopic liver resection of colorectal metastases. Dig Dis. 2005;23:142–150. doi: 10.1159/000088596. [DOI] [PubMed] [Google Scholar]
- 18.Gigot JF, Glineur D, Santiago Azagra J, Goergen M, Ceuterick M, Morino M, et al. Laparoscopic liver resection for malignant liver tumors: preliminary results of a multicenter European study. Ann Surg. 2002;236:90–97. doi: 10.1097/00000658-200207000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gagner M, Rogula T, Selzer D. Laparoscopic liver resection: benefits and controversies. Surg Clin North Am. 2004;84:451–462. doi: 10.1016/j.suc.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Tang CN, Tsui KK, Ha JP, Yang GP, Li MK. A single-centre experience of 40 laparoscopic liver resections. Hong Kong Med J. 2006;12:419–425. [PubMed] [Google Scholar]
- 21.Rees M, Plant G, Wells J, Bygrave S. One hundred and fifty hepatic resections: the evolution of technique towards bloodless surgery. Br J Surg. 1996;83:1526–1529. doi: 10.1002/bjs.1800831110. [DOI] [PubMed] [Google Scholar]
- 22.Basu S, Tamijmarane A, Bulters D, Wells J, John T, Rees M. An alternative method of wound pain control following hepatic resection: a preliminary study. HPB. 2004;6:186–189. doi: 10.1080/13651820410030844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strasberg SM, Belghiti J, Clavien PA, Gadzijev E, Garden OJ, Lau WY, et al. Terminology of liver anatomy and resections. HPB. 2000;2:333–339. [Google Scholar]
- 24.Buell JF, Thomas MT, Rudich S, Marvin M, Nagubandi R, Ravindra KV, et al. Experience with more than 500 minimally invasive hepatic procedures. Ann Surg. 2008;248:475–486. doi: 10.1097/SLA.0b013e318185e647. [DOI] [PubMed] [Google Scholar]
- 25.O’Rourke N, Shaw I, Nathanson L, Martin I, Fielding G. Laparoscopic resection of hepatic colorectal metastases. HPB. 2004;6:230–235. doi: 10.1080/13651820410023978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bryant R, Laurent A, Tayar C, Cherqui D. Laparoscopic liver resection – understanding its role in current practice: the Henri Mondor Hospital experience. Ann Surg. 2009;250:103–111. doi: 10.1097/SLA.0b013e3181ad6660. [DOI] [PubMed] [Google Scholar]
- 27.Zorzi D, Laurent A, Pawlik TM, Lauwers GY, Vauthey JN, Abdalla EK. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274–286. doi: 10.1002/bjs.5719. [DOI] [PubMed] [Google Scholar]
- 28.Chang S, Laurent A, Tayar C, Karoui M, Cherqui D. Laparoscopy as a routine approach for left lateral sectionectomy. Br J Surg. 2007;94:58–63. doi: 10.1002/bjs.5562. [DOI] [PubMed] [Google Scholar]
- 29.Mala T, Edwin B, Rosseland AR, Gladhaug I, Fosse E, Mathisen O. Laparoscopic liver resection: experience of 53 procedures at a single center. J Hepatobiliary Pancreat Surg. 2005;12:298–303. doi: 10.1007/s00534-005-0974-3. [DOI] [PubMed] [Google Scholar]
- 30.Troisi R, Montalti R, Smeets P, Van Huysse J, Van Vlierberghe H, Colle I, et al. The value of laparoscopic liver surgery for solid benign hepatic tumors. Surg Endosc. 2008;22:38–44. doi: 10.1007/s00464-007-9527-y. [DOI] [PubMed] [Google Scholar]
- 31.Welsh FK, Tekkis PP, O’Rourke T, John TG, Rees M. Quantification of risk of a positive (R1) resection margin following hepatic resection for metastatic colorectal cancer: an aid to clinical decision-making. Surg Oncol. 2008;17:3–13. doi: 10.1016/j.suronc.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Elias D, Cavalcanti A, Sabourin JC, Pignon JP, Ducreux M, Lasser P. Results of 136 curative hepatectomies with a safety margin of less than 10mm for colorectal metastases. J Surg Oncol. 1998;69:88–93. doi: 10.1002/(sici)1096-9098(199810)69:2<88::aid-jso8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 33.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeMatteo RP, Palese C, Jarnagin WR, Sun RL, Blumgart LH, Fong Y. Anatomic segmental hepatic resection is superior to wedge resection as an oncologic operation for colorectal liver metastases. J Gastrointest Surg. 2000;4:178–184. doi: 10.1016/s1091-255x(00)80054-2. [DOI] [PubMed] [Google Scholar]
- 35.Vibert E, Perniceni T, Levard H, Denet C, Shahri NK, Gayet B. Laparoscopic liver resection. Br J Surg. 2006;93:67–72. doi: 10.1002/bjs.5150. [DOI] [PubMed] [Google Scholar]


