Abstract
Background:
Recent studies have shown adjuvant therapy improves outcomes from pancreatic cancer (PC). This study investigates receipt and timing of PC treatments, and association with outcomes.
Methods:
The analysis cohort consisted of patients with newly-diagnosed PC at a single institution over 5 years. Primary Endpoints were (i) receipt of recommended therapy, and (ii) overall survival (OS).
Results:
Among 102 patients, 52 underwent resection. Out of 36 localized resected and 16 locally advanced resected (LAR) patients, 26 and 13, respectively, received adjuvant therapy. Six of the latter group received neoadjuvant therapy. Median OS for resected patients was 15.7 months (range 0.6–51.4), compared with 7.7 for unresected patients (range 0.4–32.0) (P < 0.001), and 14.0 months for patients with resection alone (range 0.6–24.4) vs. 16.1 for patients who also received adjuvant therapy (range 3.2–51.4) (P= 0.027). Out of 46 patients undergoing up-front resection, 33 had R0 surgical margins. For the six LAR patients undergoing neoadjuvant therapy, all margins were R0.
Conclusion:
After resection, a substantial proportion of patients do not receive adjuvant therapy, and have worse survival. In this study, neoadjuvant treatment increased both the proportion of patients receiving all components of recommended therapy and the R0 resection rate.
Keywords: pancreatic cancer, adjuvant therapy, neoadjuvant therapy
Introduction
Improving outcomes for patients diagnosed with pancreatic cancer continues to be a formidable challenge. In the year 2008, there will be an estimated 32 180 patients with newly diagnosed pancreatic cancer in the United States,1 where the disease ranks fourth among the leading causes of cancer-related death.2,3 Even with aggressive treatment, prognosis remains extremely poor, with less than 5% of patients surviving 5 years beyond diagnosis.2
The high rate of mortality from pancreatic cancer is multifactorial. Progression to advanced disease prior to diagnosis, biological aggressiveness of the tumours and a continuing lack of adequate systemic therapies have all been implicated as important contributing factors.4 Currently, only treatment approaches that include surgical resection offer any potential for long-term survival.3 Unfortunately, only 15% to 20% of patients present with tumours amenable to resection.5 Even in patients who do undergo resection for localized pancreatic carcinoma, the majority of patients will have relapse of disease.6
Recent studies have shown that adjuvant therapy can further improve outcomes in patients who have undergone pancreatectomy for pancreatic adenocarcinoma.7–16 While the role of adjuvant chemoradiotherapy vs. chemotherapy-alone remains controversial in light of the mixed results of the Gastrointestinal Tumor Study Group (GITSG) and European Study Group for Pancreatic Cancer (ESPAC-1) Trails,9,10,17,18 chemoradiotherapy remains highly utilized in the United States pending additional evidence.19 Unfortunately, for a variety of reasons, including comorbidities, post-operative complications, and patient choice, not all patients receive recommended adjuvant therapy. Given the known survival benefit for patients with resectable pancreatic adenocarcinoma, receipt of all recommended treatment components is extremely important. This study investigates, at the institutional level, the frequencies, timing and associated factors, for when this does or does not occur. We hypothesize that potentially modifiable factors can be identified and targeted in order to maximize the proportion of patients ultimately receiving optimized treatment. Using our institutional prospectively collected database, we examined the factors that influence the receipt of all components of therapy in patients who had surgical resection for pancreatic adenocarcinoma.
Patients and methods
Cohort selection criteria
Patients referred to a dedicated surgical oncology service for possible surgical resection of a pancreatic mass, from June 2002 through to July 2007, were identified from a prospectively maintained institutional pancreatic database. For the purposes of this study, patients with a tissue diagnosis other than pancreatic adenocarcinoma were excluded, as were those lacking follow-up data (three patients). The remaining 102 patients formed the primary cohort, representing those initially considered as potential candidates for surgical resection, pending further studies to rule out inoperable disease.
Review of patient data
Patients were categorized by stage according to the American Joint Committee on Cancer (AJCC) staging definitions for adenocarcinoma of the pancreas.20 The clinical and pathological characteristics of patients were obtained by retrospective review of electronic medical records and paper charts. The study was approved by the Institutional Review Board.
The following variables were obtained from the database: (i) patient demographic characteristics (age and gender), (ii) diagnostic and staging variables, (iii) operative procedure (pancreaticoduodenectomy or ‘Whipple procedure’, total pancreatectomy, distal pancreatectomy and proximal pancreatectomy), (iv) stage at presentation (localized, borderline resectable, locally unresectable and metastatic disease) and (v) type of combined modality therapy (neoadjuvant vs. adjuvant).
The primary endpoints for the study were percentage of patients completing components of recommended therapy and overall survival. Length of survival was calculated from the date of initial surgical diagnostic evaluation until date of death.
Clinical staging and resection criteria
Diagnostic and clinical staging evaluations for all patients included physical examination, routine laboratory testing, chest radiography and contrast-enhanced computed tomography (CT). The majority of patients also underwent endoscopic ultrasound (EUS) and had their management discussed at a weekly multi-disciplinary tumour board. After initial diagnostic work-up, patients without clearly unresectable disease also underwent staging laparoscopy. Tumour resectability was determined by the surgical oncologist, based on the following specific clinical staging criteria:21,22
Localized resectable disease: (i) no extrapancreatic disease, (ii) a patent superior mesenteric (SMV)-portal vein (PV) confluence (assuming the technical ability to resect and reconstruct this venous confluence) and (iii) a definable tissue plane between the tumour and regional arterial structures including the celiac axis, common hepatic artery and SMA.
Borderline resectable disease: (i) no extrapancreatic disease and (ii) the following possible tumour-vessel relationships: an SMV-PV confluence that can be reconstructed even if short segment venous occlusion is present, tumour abutment of the SMA of ≤180° or short segment encasement of the hepatic artery amenable to resection and reconstruction.
Locally advanced unresectable disease: (i) no extrapancreatic disease and (ii) tumour encasement of the SMA or celiac axis defined as tumour involvement of >180° of the arterial circumference.
Metastatic disease: radiographical, clinical or staging laparoscopic evidence of distant organ or peritoneal metastases.
Classification of combined modality therapy
Data regarding treatment with chemo- and/or radiotherapy were obtained from the medical records. Treating physician offices were contacted for additional information when appropriate. Adjuvant chemotherapy or chemoradiation was defined as either post-operative (standard adjuvant) or as pre-operative (neoadjuvant). Owing to our institutional standard during the time period of this study, all the patients with localized resectable disease were offered up-front surgery with standard post-operative adjuvant therapy. Neoadjuvant therapy was offered to a subset of patients with borderline resectable or locally advanced unresectable disease.
Chemoradiation regimens
Standard adjuvant therapy
All patients undergoing surgical resection were evaluated at a multi-disciplinary clinic by a medical oncologist and radiation oncologist. Although the survival benefit of adjuvant chemoradiotherapy compared with adjuvant chemotherapy alone remains controversial in light of the mixed results of important clinical trials to date (particularly ESPAC-1),9,10,17,18 the majority of institutions in the United States continue to offer combined modality adjuvant therapy pending further evidence.19 As such, both adjuvant chemotherapy and radiotherapy were considered for all resectable patients in this study.
Radiation treatments were given as external beam radiotherapy, consisting of 50.4 Gy in 28 fractions. Concomitant chemotherapy consisted of 5-flurouracil (FU), paclitaxel or capecitabine, at radio-sensitizing doses. Resected patients beyond the year 2005 who were well enough to receive systemic adjuvant chemotherapy also received gemcitabine. The majority of those patients were treated based on the RTOG 9704 regimen and received 1 month of gemcitabine followed by chemoradiotherapy followed by 3 more months of gemcitabine.
Neoadjuvant therapy
Neoadjuvant radiation treatments were also given as 50.4 Gy of external beam radiotherapy in 28 fractions. Concomitant chemotherapy consisted of 5 FU, capecitabine, infusional 5 FU and gemcitabine. One patient was treated on an in-house protocol using cetuximab together with infusional 5 FU and gemcitabine.
Clinical restaging was performed 4–6 weeks after neoadjuvant chemoradiotherapy in preparation for surgical resection. One patient received additional systemic chemotherapy with gemcitabine and erlotinib for 4 months beyond initial restaging, prior to eventual surgical resection.
Assessment of margin status
Standardized histological evaluation of the surgical specimens was performed as described by the American Joint Committee on Cancer.20 Final margins were recorded as negative (R0) or microscopically positive (R1) for tumour. There were no R2 resections for this cohort. A margin was designated positive if tumour cells were present at the inked resection margin, retroperitoneal margin, pancreatic or biliary margins. In addition, specimens were oriented by the surgeon for assessment of the portal vein groove whenever there was intra-operative evidence of possible tumour involvement at that location.
Long-term follow-up and survival
Long-term follow-up and survival data were available for all patients. Survival data from the medical records was confirmed using the social security death index. Follow-up was continued through the end-point of the study, August 2008.
Statistical analysis
SAS 9.1.3 software (SAS Institute, Cary, NC) was used for all statistical analyses of this study. Kaplan–Meier methods compared median survival.23 The log-rank test was used to assess differences between survival curves. Univariate analyses were done using χ2 tests to examine the effect of patient and disease characteristics on patient survival. Statistical significance was set at P < 0.05.
Results
Patient demographics and initial staging
Between 2002 and 2007, 102 patients were referred to our institution for surgical evaluation of a suspected pancreatic cancer. Patients ranged in age from 41 to 85 years, with a median age of 65 years. Both genders were diagnosed in similar numbers, with 54 out of 102 (53%) being males. The tumour was located in the head of the pancreas in 76 patients (75%). Of the 102 patients, 51 patients were initially clinically staged with localized disease (50%), 23 with locally advanced borderline resectable disease (22%), 15 with locally advanced unresectable disease (15%) and 13 with preliminary evidence suspicious for metastatic disease (13%). Patient characteristics for this cohort are outlined in Table 1.
Table 1.
Patient characteristics (n= 102)
| Age (years) | |
| Range | 41–85 |
| Median | 65 |
| Gender | |
| Male | 54 (52.9%) |
| Female | 48 (47.1%) |
| Location | |
| Head | 76 (74.5%) |
| Body | 7 (6.9%) |
| Tail | 11 (10.8%) |
| Multi-focal | 8 (7.8%) |
| Staging laparoscopy | |
| No | 41 (40.2%) |
| Yes | 61 (59.8%) |
| Resection | |
| No | 50 (49.0%) |
| Yes | 52 (51.0%) |
| Adjuvant therapy | |
| Standard post-operative | 33 (63.5% of resected group) |
| Neoadjuvant | 6 (11.5% of resected group) |
| Neither | 13 (25.0% of resected group) |
| Unresected | 50 |
Neoadjuvant therapy and restaging
A total of 19 patients with locally advanced borderline resectable or unresectable disease were placed on a neoadjuvant chemoradiation protocol (8 and 11 patients, respectively). Four patients with locally advanced unresectable disease were subsequently restaged to resectable after neoadjuvant therapy. One patient with borderline resectable disease at the time of starting neoadjuvant therapy had progression of disease to metastasis. Cancer staging for this cohort is shown in Table 2.
Table 2.
Tumour staging (n= 102)
| Initial clinical staging | Clinical restaging | |
|---|---|---|
| Localized | 51 | 51 |
| Borderline resectable | 23 | 26* |
| Locally unresectable | 15 | 11 |
| Metastasis | 13 | 14† |
Four patients diagnostically and/or laparoscopically staged as locally unresectable who received neoadjuvant therapy were subsequently restaged to borderline resectable.
One patient diagnostically and/or laparoscopically staged as borderline resectable who received neoadjuvant therapy was subsequently restaged to metastatic disease.
Pancreatic resection and resection margins
Out of the 102 patients diagnosed with pancreatic adenocarcinoma and evaluated for surgery, 58 patients (57%) (39 localized and 19 borderline resectable) under went surgery with curative intent. Of the remainder, 35 had locally advanced unresectable or metastatic disease, 6 were poor surgical candidates owing to co-morbid disease and 3 decided against recommended surgery. Six patients who under went surgery with curative intent (10%) were found at the time of laparotomy to have unresectable and/or metastatic disease. Pancreatic resection was performed on 52 patients (36 localized resectable and 16 borderline resectable). Resected patients underwent standard pancreaticoduodenectomy (Whipple procedure) (29), pylorus-sparing Whipple procedure (10), distal pancreatectomy (9) or total pancreatectomy (4).
Surgical resection margins were microscopically negative for tumour cells in 39 out of the 52 resections. All 36 resected patients with localized disease had traditional up-front surgery, and of those, 28 had R0 resections. Out Of 16 patients with borderline resectable disease, 11 had R0 resections. There was no significant difference in R0 rate between these two groups (Fisher's exact test P= 0.506). Of those patients with borderline resectable disease having traditional up-front surgery (10), five had R0 resections. In contrast, all six patients with locally advanced borderline resectable disease who had received neoadjuvant therapy had R0 resections (Fisher's exact test P≤ 0.093). Resected margin status information is provided in Table 3.
Table 3.
Resection margin status (n= 52)
| Characteristic | Frequency |
|---|---|
| Resection Margin | |
| R0 | 39 |
| R1 | 13 |
| Resection margin by stage and treatment | |
| Localized disease | 36 |
| R0 | 28 |
| R1 | 8 |
| Borderline resectable | 16 |
| Upfront surgery | 10 |
| R0 | 5 |
| R1 | 5 |
| Neoadjuvant therapy | 6 |
| R0 | 6 |
| R1 | 0 |
Adjuvant therapy
Of resected patients with localized disease (36), 26 received adjuvant therapy, all by a standard post-operative approach. The median time from surgical resection to initiation of standard post-operative adjuvant therapy was 79 days (range 33–175). Thirteen patients out of 16 with resected locally advanced disease received additional therapy. Nearly half of them (6 of 13) received it according to a neoadjuvant protocol. The distribution of resected patients by stage and treatment received is shown in Fig. 1.
Figure 1.
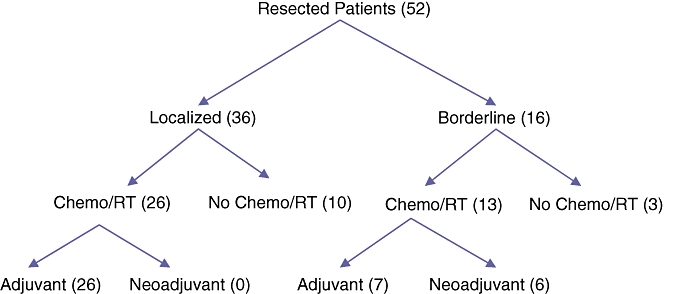
Flow diagram of resected (RT) pancreatic adenocarcinoma patients by treatment approach
Completion of therapy
Of patients with localized disease, 17 out of 36 completed scheduled adjuvant therapy; none received neoadjuvant therapy. In contrast, 13 out of 16 of the borderline group completed chemoradiation as planned. Nearly half of these (6 of 13) received up-front neoadjuvant rather than traditional post-operative therapy. Reasons for not completing recommended therapy included surgical or medical complications, disease progression, and patient preference.
Complications
Post-operative complications occurred in 27 out of 52 patients who underwent resection. Major complications included haemorrhage (2), leak or abscess (2), cardiopulmonary-related complications (2), wound infections (3), neurological complications (1) and prolonged gastric emptying (2). In addition, there were 15 other minor complications (15). Peri-operative death occurred in three patients (6%). Reported chemoradiotherapy-related toxicities included: haematological (6), constitutional (5), gastrointestinal (8) and gemcitabine-associated skin changes (3).
Survival
The median overall survival for all resected patients was 15.7 months (range 0.6–51.4) compared with 7.7 months for unresected patients (range 0.4–32.0) (log rank P < 0.001). The median survival for patients who had surgical resection alone was 14.0 months (range 0.6–24.4). In contrast, the median survival for patients who received combined modality therapy (adjuvant or neoadjuvant chemoradiation in addition to surgical resection) was 16.1 months (range 3.2–51.4) (log rank P= 0.027 vs. resection alone). Kaplan–Meier survival analysis is shown in Fig. 2.
Figure 2.
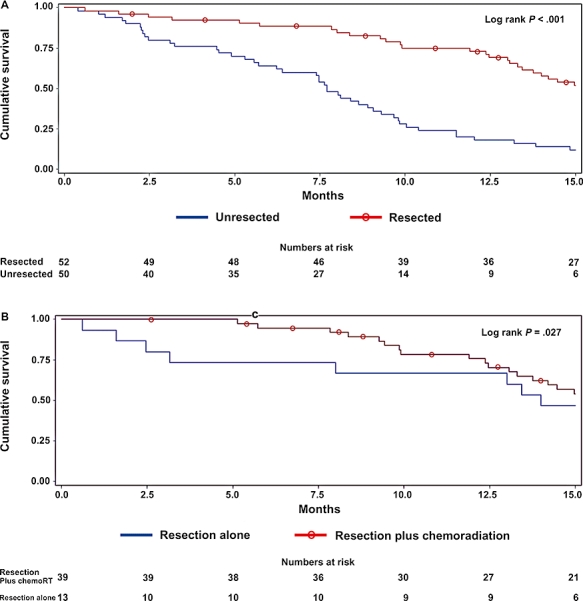
Overall Kaplan-Meier survival of patients with pancreatic adenocarcinoma reffered for surgical evaluation for (A) resected versus unresected patients and (B) resected patients with or without chemo-radiation
Discussion
The management of patients with resectable pancreatic cancer has evolved significantly over the past several years, including the evolution of what constitutes resectability to include patients with borderline disease. Surgical resection continues to be the only potential cure for patients with pancreatic adenocarcinoma,24 and while peri-operative mortality has improved dramatically in recent years,25 long-term survival after resection remains essentially unchanged.
Several recent clinical trials have indicated that adjuvant systemic chemotherapy improves outcome in patients with resected pancreatic adenocarcinoma.7,8,26 The role of adjuvant radiation remains controversial with conflicting results from three important clinical trials. However, pending further evidence, it continues to be the standard of care in the United States.16,27
We report a single-institutional experience of patients with a presumed pancreatic cancer referred for surgical evaluation. Access to patient records allowed a more in-depth review of clinical data than is typically possible with larger non-institutional database studies.
It is quite striking that fewer than three-quarters of the patients in this study with presumed localized and therefore surgically resectable disease actually underwent surgical resection (36 of 51). In addition, of these patients with localized disease that did undergo resection, 10 did not receive combined modality therapy. The survival results of this study concur with those of randomized trials indicating that those who receive combined modality therapy have a better outcome over surgical resection alone.
In contrast, in patients with borderline resectable disease, 13 of 16 resected patients received combined modality therapy. This is in part due to the fact that nearly half of these patients receive neoadjuvant (pre-operative) therapy. Despite small numbers, the outcome of the patients who received pre-operative therapy followed by surgical resection is comparable to the patients who received post-operative therapy. Considering the fact that the patients who received neoadjuvant therapy were judged to be borderline resectable and hence by definition had more advanced disease at presentation, these results are quite encouraging.
Owing to our institutional standard during the time period of the study, the majority of patients had up-front surgery. Notably, five patients who had borderline resectable disease (16) who underwent up-front surgery (10) had resulting positive margins after resection. In contrast, all six borderline resectable patients who received neoadjuvant chemoradiotherapy had R0 resections. Given the small number of patients receiving neoadjuvant therapy in this cohort, these results are supportive of continued studies to further investigate a suggested trend in cleaner resection margins with the neoadjuvant strategy, but are insufficient for definitive conclusions.
Katz et al. have recently reported the outcome from a large series of patients with borderline resectable pancreatic adenocarcinoma.28 All patients were initially treated with chemotherapy and/or chemoradiation and were subsequently restaged. The authors report very favourable outcomes in those patients with borderline resectable disease who underwent surgical resection after neoadjuvant therapy. Although much smaller in numbers, our experience with patients having borderline resectable disease receiving neoadjuvant therapy, and subsequently undergoing pancreatic resection, parallels the findings from the MD Anderson study.
As a result of the near universal recurrence of pancreatic adenocarcinoma after surgical resection, it is imperative to question all components of our current standard approach.26,29 This includes the central dogma of up-front primary surgery in localized pancreatic adenocarcinoma patients. Neoadjuvant therapy has been proposed as an alternative approach in patients with localized pancreatic adenocarcinoma.29 The safety of neoadjuvant therapy has been demonstrated in multiple, mostly single-institution, single arm phase II studies.30–32 This study supports the argument that the major advantage of pre-operative approach is to allow the patients with localized pancreatic adenocarcinoma to receive all the components of combined modality therapy. This could ultimately result in better patient selection for surgical resection and improved outcome. In addition, the neoadjuvant approach can also result in an increase of R0 resections, particularly in borderline resectable patients.
While analysing the outcome data from adjuvant and neoadjuvant trials, it is crucial to realize that not all patients with resected pancreatic adenocarcinoma will be able to receive planned adjuvant therapy. It is very unlikely that an adequately powered clinical trial will soon be able to definitively compare adjuvant and neoadjuvant approaches. We hope that our current report will fill this gap in knowledge.
This study has a number of limitations. The cohort of patients who received pre-operative therapy is small. There is also significant heterogeneity among different chemotherapy regimens. Finally the use of adjuvant therapy has evolved over the period of study.
Despite these disadvantages, this study adds additional insight to the outcome of patients with localized and locally advanced pancreatic adenocarcinoma. Neoadjuvant therapy may have a major advantage of giving the patients exposure to all components of therapy and has a potential to improve the surgical outcome. There is an urgent need for multi-institutional randomized pre-operative trials to improve the outcome of patients with resectable pancreatic adenocarcinoma.
Conflicts of interest
None declared.
References
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Evans DB, Abbruzzese JL, Willett CG. Cancer of the pancreas. In: Devita VT, Hellman S, Rosenberg SA, editors. Cancer – Principles and Practice of Oncology. Philadelphia, PA: Lippincott Williams and Wilkins; 2001. pp. 1126–1148. [Google Scholar]
- 3.deCastro SMM, Biere SSAY, LaGarde SM, Busch ORC, van Gulik TM, Gouma DJ. Validation of a nomogram for predicting survival after resection for adenocarcinoma of the pancreas. Br J Surg. 2009;96:417–423. doi: 10.1002/bjs.6548. [DOI] [PubMed] [Google Scholar]
- 4.Pisters PW, Wolff RA, Crane CH, Evans DB. Combined-modality treatment for operable pancreatic adenocarcinoma. Oncology (Williston Park) 2005;19:393–404. 409; discussion 409–410, 412–416. [PubMed] [Google Scholar]
- 5.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 6.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 7.Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 8.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 9.Kalser MH, Ellenberg SS. Pancreatic cancer: adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 10.Gastrointestinal Tumor Study Group. Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer. 1987;59:2006–2010. doi: 10.1002/1097-0142(19870615)59:12<2006::aid-cncr2820591206>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.Whittington R, Bryer MP, Haller DG, Solin LJ, Rosato EF. Adjuvant therapy of resected adenocarcinoma of the pancreas. Int J Radiat Oncol. 1991;21:1137–1143. doi: 10.1016/0360-3016(91)90268-9. [DOI] [PubMed] [Google Scholar]
- 12.Foo ML, Gunderson LL, Nagorney DM, McLlrath DC, van Heerden JA, Robinow JS, et al. Patterns of failure in grossly resected pancreatic ductal adenocarcinoma treated with adjuvant irradiation +/- 5 fluorouracil. Int J Radiat Oncol. 1993;26:483–489. doi: 10.1016/0360-3016(93)90967-z. [DOI] [PubMed] [Google Scholar]
- 13.Yeo CJ, Abrams RA, Grochow LB, Sohn TA, Ord SE, Hruban RH, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma: Postoperative adjuvant chemoradiation improves survival (a prospective, single-institution experience) Ann Surg. 1997;225:621–636. doi: 10.1097/00000658-199705000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas – 616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 15.Herman JM, Swartz MJ, Hsu CC, Winter J, Pawlik TM, Sugar E, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: Results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol. 2008;26:3503–3510. doi: 10.1200/JCO.2007.15.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corsini MM, Miller RC, Haddock MG, Donohue JH, Farnell MB, Nagorney DM, et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: the Mayo Clinic experience (1975–2005) J Clin Oncol. 2008;26:3511–3516. doi: 10.1200/JCO.2007.15.8782. [DOI] [PubMed] [Google Scholar]
- 17.Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576–1585. doi: 10.1016/s0140-6736(01)06651-x. [DOI] [PubMed] [Google Scholar]
- 18.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. correction 351 (7):726, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Crane CH, Varadhachary G, Pisters PWT, Evans DB, Wolff RA. Future chemoradiation strategies in pancreatic cancer. Semin Oncol. 2007;34:335–346. doi: 10.1053/j.seminoncol.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Greene FL American Joint Committee on Cancer. American Cancer Society. AJCC Cancer Staging Manual. 6th. New York: Springer-Verlag; 2002. [Google Scholar]
- 21.Tseng JF, Raut CP, Lee JE, Pisters PW, Vauthey JN, Abdalla EK, et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg. 2004;8:935–949. doi: 10.1016/j.gassur.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 22.Raut CP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 24.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996;223:273–279. doi: 10.1097/00000658-199603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McPhee JT, Hill JS, Whalen GF, Zayaruzny M, Litwin DE, Sullivan ME, et al. Perioperative mortality for pancreatectomy: a national perspective. Ann Surg. 2007;246:246–253. doi: 10.1097/01.sla.0000259993.17350.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duffy A, O'Reilly EM. What is the optimal treatment of localized pancreatic adenocarcinoma? Oncology (Williston Park) 2008;22:1283–1291. discussion 1294, 1296–1298. [PubMed] [Google Scholar]
- 27.Gutt R, Liauw SL, Weichselbaum RR. Adjuvant radiotherapy for resected pancreatic cancer: a lack of benefit or a lack of adequate trials? Nat Clin Pract Gastroenterol Hepatol. 2009;6:38–46. doi: 10.1038/ncpgasthep1301. [DOI] [PubMed] [Google Scholar]
- 28.Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833–846. doi: 10.1016/j.jamcollsurg.2007.12.020. discussion 846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans DB for the Multidisciplinary Pancreatic Cancer Study Group. Resectable pancreatic cancer: the role for neoadjuvant/preoperative therapy. HPB (Oxford) 2006;8:365–368. doi: 10.1080/13651820600804005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 31.Varadhachary GR, Wolff RA, Crane CH, Sun CC, Lee JE, Pisters PW, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3487–3495. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 32.Heinrich S, Schafer M, Weber A, Hany TF, Bhure U, Pestalozzi BC, et al. Neoadjuvant chemotherapy generates a significant tumor response in resectable pancreatic cancer without increasing morbidity: results of a prospective phase II trial. Ann Surg. 2008;248:1014–1022. doi: 10.1097/SLA.0b013e318190a6da. [DOI] [PubMed] [Google Scholar]


