Abstract
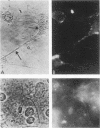
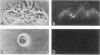
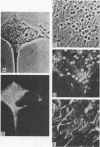
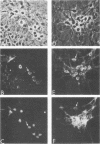
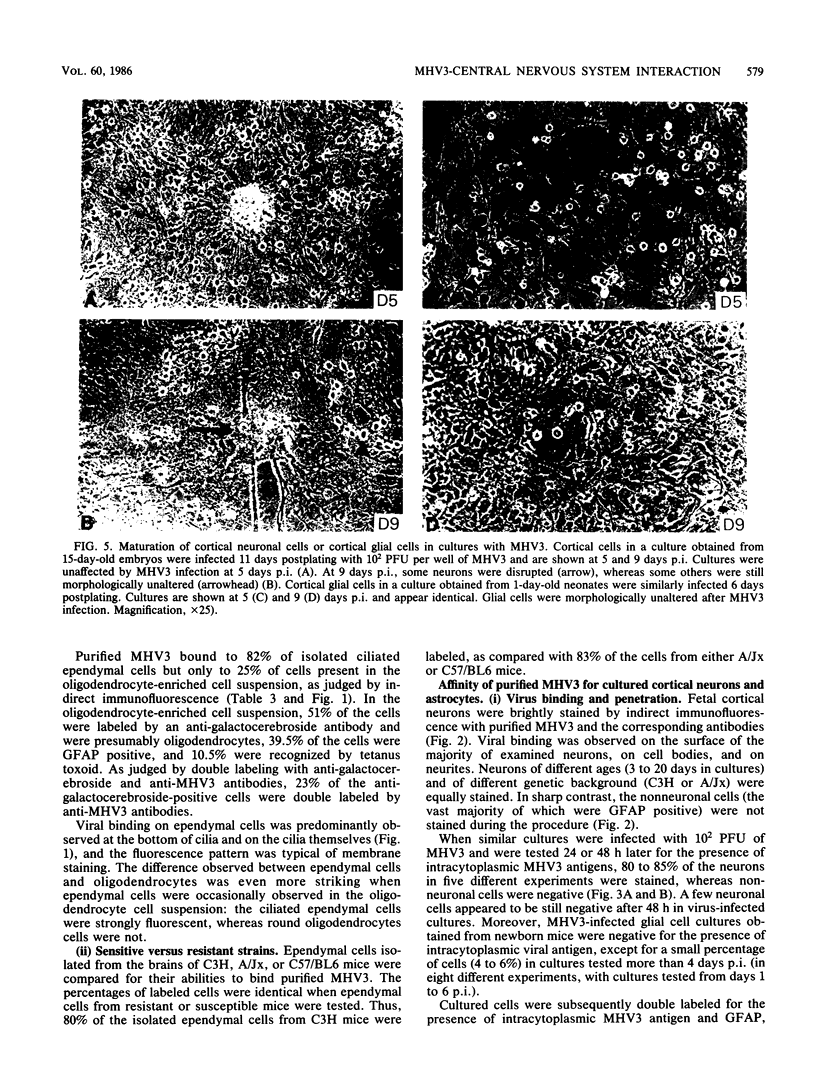
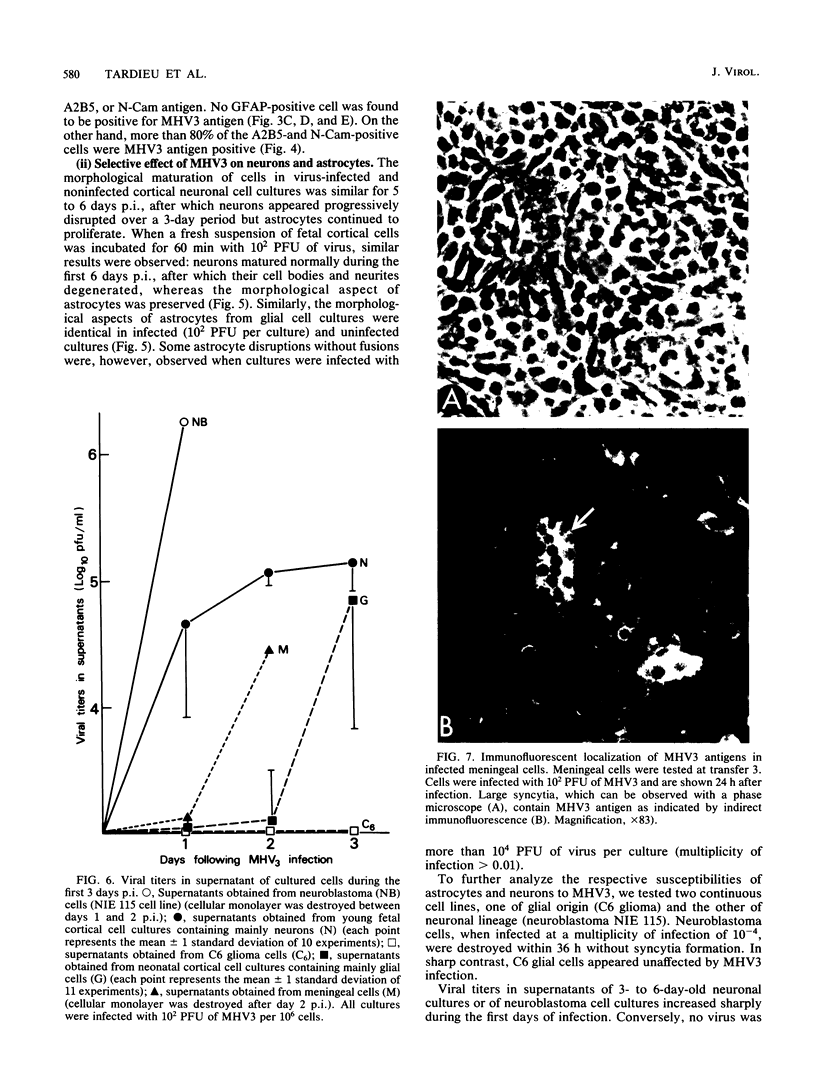
The ability of a neurotropic virus, mouse hepatitis virus type 3 (MHV3), to invade the central nervous system (CNS) and to recognize cells selectively within the brain was investigated in vivo and in vitro. In vivo, MHV3 induced in C3H mice a genetically controlled infection of meningeal cells, ependymal cells, and neurons. In vitro, purified MHV3 bound to the surface of isolated ependymal cells and cultured cortical neurons but not to oligodendrocytes or cultured astrocytes. MHV3 replicated within cultured cortical neurons and neuroblastoma cells (NIE 115); infected cultured neurons nonetheless survived and matured normally for a 7-day period postinfection. On the other hand, MHV3 had a low affinity for cortical glial cells or glioma cells (C6 line), both of which appear to be morphologically unaltered by viral infection. Finally, MHV3 infected and disrupted cultured meningeal cells. This suggests that differences in the affinity of cells for MHV3 are determinants of the selective vulnerability of cellular subpopulations within the CNS. In vivo, a higher titer of virus was needed for CNS penetration in the genetically resistant (A/Jx) mice than in the susceptible (C57/BL6) mouse strain. However, in spite of viral invasion, no neuropathological lesions developed. In vitro viral binding to adult ependymal cells of susceptible and resistant strains of mice was identical. Genetic resistance to MHV3-CNS infection appeared to be mediated both by a peripheral mechanism limiting viral penetration into the CNS and by intra-CNS mechanisms, presumably at a stage after viral attachment to target cells.
Full text
PDF
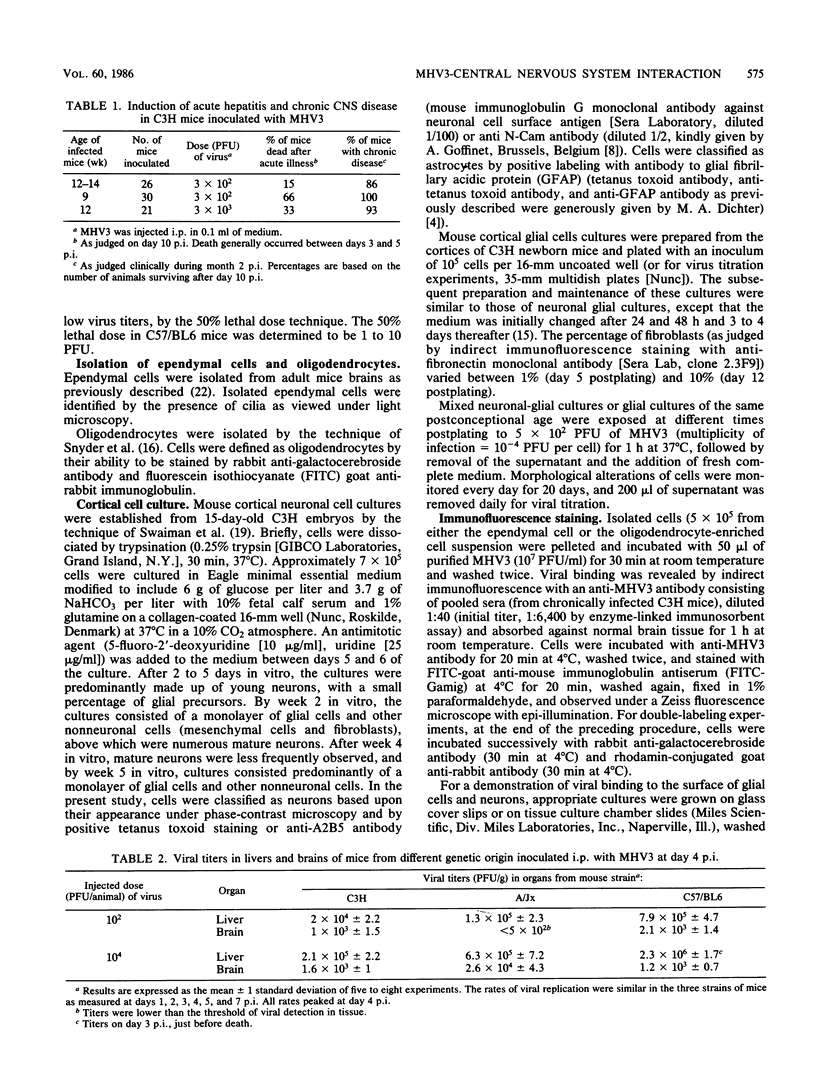

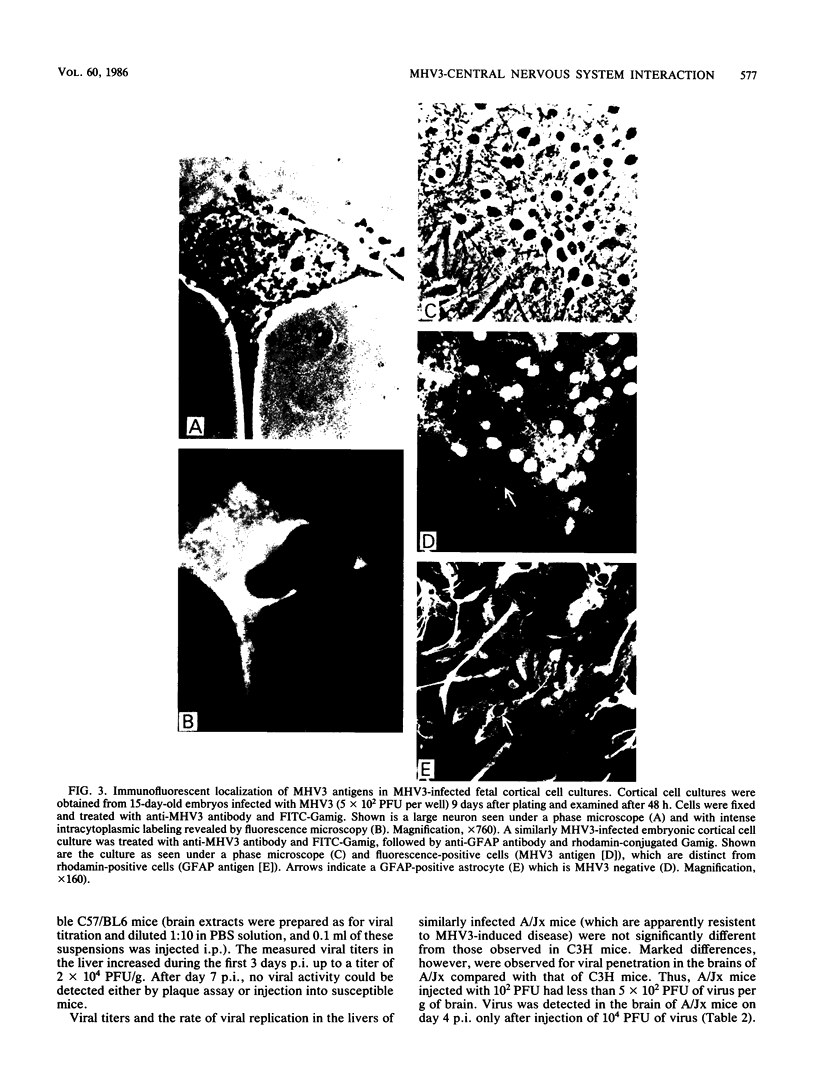
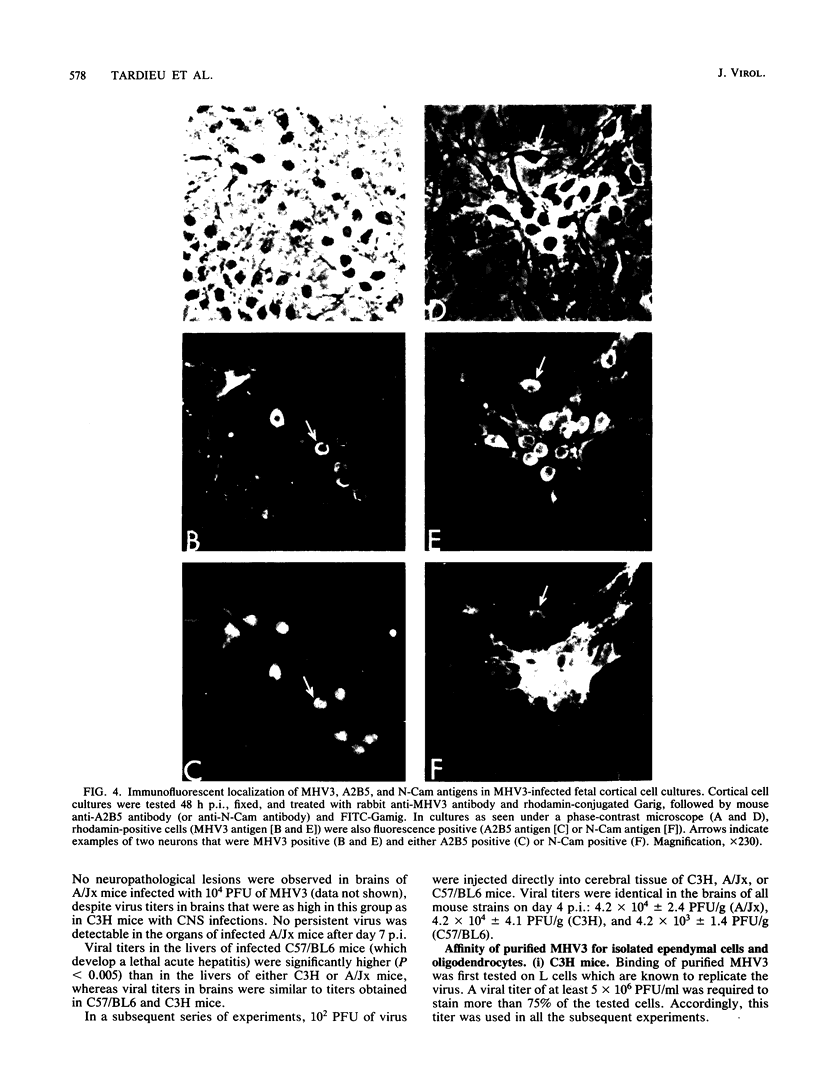


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheiter H., Baechi T., Haller O. Adult mouse hepatocytes in primary monolayer culture express genetic resistance to mouse hepatitis virus type 3. J Immunol. 1982 Sep;129(3):1275–1281. [PubMed] [Google Scholar]
- Dichter M. A., Weiner H. L. Infection of neuronal cell cultures with reovirus mimics in vitro patterns of neurotropism. Ann Neurol. 1984 Nov;16(5):603–610. doi: 10.1002/ana.410160512. [DOI] [PubMed] [Google Scholar]
- Dubois-Dalcq M. E., Doller E. W., Haspel M. V., Holmes K. V. Cell tropism and expression of mouse hepatitis viruses (MHV) in mouse spinal cord cultures. Virology. 1982 Jun;119(2):317–331. doi: 10.1016/0042-6822(82)90092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M., Rentier B., Hooghe-Peters E., Haspel M. V., Knobler R. L., Holmes K. Acute and persistent viral infections of differentiated nerve cells. Rev Infect Dis. 1982 Sep-Oct;4(5):999–1014. doi: 10.1093/clinids/4.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figà-Talamanca L., Gualandi C., Di Meo L., Di Battista G., Neri G., Lo Russo F. Hyperthermia after discontinuance of levodopa and bromocriptine therapy: impaired dopamine receptors a possible cause. Neurology. 1985 Feb;35(2):258–261. doi: 10.1212/wnl.35.2.258. [DOI] [PubMed] [Google Scholar]
- Fleming J. O., Stohlman S. A., Harmon R. C., Lai M. M., Frelinger J. A., Weiner L. P. Antigenic relationships of murine coronaviruses: analysis using monoclonal antibodies to JHM (MHV-4) virus. Virology. 1983 Dec;131(2):296–307. doi: 10.1016/0042-6822(83)90498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goridis C., Deagostini-Bazin H., Hirn M., Hirsch M. R., Rougon G., Sadoul R., Langley O. K., Gombos G., Finne J. Neural surface antigens during nervous system development. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):527–537. doi: 10.1101/sqb.1983.048.01.057. [DOI] [PubMed] [Google Scholar]
- Haspel M. V., Lampert P. W., Oldstone M. B. Temperature-sensitive mutants of mouse hepatitis virus produce a high incidence of demyelination. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4033–4036. doi: 10.1073/pnas.75.8.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. T. Selective vulnerability of neural cells to viral infections. Brain. 1980 Sep;103(3):447–472. doi: 10.1093/brain/103.3.447. [DOI] [PubMed] [Google Scholar]
- Knobler R. L., Haspel M. V., Oldstone M. B. Mouse hepatitis virus type 4 (JHM strains). induced fatal central nervous system disease. I. genetic control and murine neuron as the susceptible site of disease. J Exp Med. 1981 Apr 1;153(4):832–843. doi: 10.1084/jem.153.4.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi E., Gilden D. H., Wroblewska Z., Rorke L. B., Weiss S. R. Experimental demyelination produced by the A59 strain of mouse hepatitis virus. Neurology. 1984 May;34(5):597–603. doi: 10.1212/wnl.34.5.597. [DOI] [PubMed] [Google Scholar]
- Le Prevost C., Levy-Leblond E., Virelizier J. L., Dupuy J. M. Immunopathology of mouse hepatitis virus type 3 infection. Role of humoral and cell-mediated immunity in resistance mechanisms. J Immunol. 1975 Jan;114(1 Pt 1):221–225. [PubMed] [Google Scholar]
- McCarthy K. D., Harden T. K. Identification of two benzodiazepine binding sites on cells cultured from rat cerebral cortex. J Pharmacol Exp Ther. 1981 Jan;216(1):183–191. [PubMed] [Google Scholar]
- Prévost C. L., Virelizier J. L., Dupuy J. M. Immunopathology of mouse hepatitis virus type 3 infection. III. Clinical and virologic observation of a persistent viral infection. J Immunol. 1975 Sep;115(3):640–643. [PubMed] [Google Scholar]
- Snyder D. S., Raine C. S., Farooq M., Norton W. T. The bulk isolation of oligodendroglia from whole rat forebrain: a new procedure using physiologic media. J Neurochem. 1980 Jun;34(6):1614–1621. doi: 10.1111/j.1471-4159.1980.tb11252.x. [DOI] [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V., Behnke J. Isolation of coronavirus envelope glycoproteins and interaction with the viral nucleocapsid. J Virol. 1980 Jan;33(1):449–462. doi: 10.1128/jvi.33.1.449-462.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Takemoto K. K. Enhanced growth of a murine coronavirus in transformed mouse cells. Infect Immun. 1972 Oct;6(4):501–507. doi: 10.1128/iai.6.4.501-507.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaiman K. F., Neale E. A., Fitzgerald S. C., Nelson P. G. A method for large-scale production of mouse brain cortical cultures. Brain Res. 1982 Mar;255(3):361–369. doi: 10.1016/0165-3806(82)90004-9. [DOI] [PubMed] [Google Scholar]
- Tardieu M., Epstein R. L., Weiner H. L. Interaction of viruses with cell surface receptors. Int Rev Cytol. 1982;80:27–61. doi: 10.1016/S0074-7696(08)60366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu M., Goffinet A., Harmant-van Rijckevorsel G., Lyon G. Ependymitis, leukoencephalitis, hydrocephalus, and thrombotic vasculitis following chronic infection by mouse hepatitis virus 3 (MHV 3). Acta Neuropathol. 1982;58(3):168–176. doi: 10.1007/BF00690797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu M., Weiner H. L. Viral receptors on isolated murine and human ependymal cells. Science. 1982 Jan 22;215(4531):419–421. doi: 10.1126/science.6276976. [DOI] [PubMed] [Google Scholar]
- Virelizier J. L., Dayan A. D., Allison A. C. Neuropathological effects of persistent infection of mice by mouse hepatitis virus. Infect Immun. 1975 Nov;12(5):1127–1140. doi: 10.1128/iai.12.5.1127-1140.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Drayna D., Averill D. R., Jr, Fields B. N. Molecular basis of reovirus virulence: role of the S1 gene. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5744–5748. doi: 10.1073/pnas.74.12.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]