Abstract
The inflammatory response in amyotrophic lateral sclerosis (ALS) is well documented but the underlying cellular mechanisms have not been fully elucidated. We report that microglia isolated from the mutant human SOD1 G93A transgenic mouse model of ALS, have an increased response to the inflammatory stimulus, lipopolysaccharide. Cell surface area and F4/80 surface marker, both indicators of cell activation, are increased relative to transgenic wild-type human SOD1 microglia (SOD1 WT). Monocyte chemoattractant protein-1 (MCP-1), known to be increased in ALS, is produced at 3 fold higher levels by SOD1 G93A than by SOD1 WT microglia, under activating conditions. This novel finding implicates ALS microglia as a source of the increased MCP-1 levels detected in ALS patients and the ALS mouse model.
Keywords: microglia, amyotrophic lateral sclerosis, monocyte chemoattractant protein 1
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder in which there is injury and cell death of upper and lower motor neurons, causing progressive and eventually fatal failure of the neuromuscular system. Pathological analysis demonstrates that the neurodegenerative process is accompanied by microglial activation and astrogliosis. While the aetiology of the disease is yet to be fully elucidated, studies on animals models of ALS as well as on human tissue samples have shown that several factors contribute to motor neuron injury: these include inflammatory mediators and interactions between motor neurons and glial cells [1]. Also, there is a tendency in ALS for the disease to start focally and to spread progressively to contiguous groups of motor neurons [2]. In an animal model of the disease, transgenic mice expressing human mutant Cu/Zn superoxide dismutase 1 (SOD1), non-neuronal, microglial and astrocytic cells make a clear contribution to the propagation of motor neuron injury[3-5].
Microglia, as the main immunocompetent cell type of the CNS, have the potential to secrete most of the inflammatory mediators that could contribute to disease progression. Some of these have been shown to be elevated in the CSF and CNS tissues of ALS patients. Thus, the CSF of ALS patients has been reported to contain increased levels of monocyte chemoattractant protein-1 (MCP-1), prostaglandin (PG) E2, and tumor necrosis factor (TNF)-α, when compared to samples from controls [6-9]. These and other mediators were also elevated in the CNS of ALS animal models compared to controls (reviewed in Sargsyan et al, 2005 [1]). Microglia expressing mutant human SOD1, isolated from the SOD1 G93A transgenic model of ALS and activated with lipopolysaccharide (LPS), were shown to secrete greater amounts of TNF-α, superoxide and nitric oxide when compared to control microglia [5,10,11]. However, a systematic study investigating how the overexpression of mutant SOD1 in microglia may affect their cellular and secretory properties under inflammatory conditions has not been conducted and the source of MCP-1 in ALS has not been identified. Here, we examined some of the properties of microglia isolated from the SOD1 G93A murine model of ALS and control animals transgenic for wild-type human SOD1. Morphological properties (cell surface area, index of ramification, activation marker F4/80 expression), indicative of the activation status of microglia [12], and the secretion of cytokines, chemokines and PGE2, were measured under both resting and inflammatory conditions induced by treatment with lipopolysaccharide (LPS). This study demonstrates that SOD1 G93A microglia have altered morphological properties and elevated production of MCP-1, a chemokine that is consistently and strongly associated with ALS.
Methods
Cell culture preparations
All animals were handled in accordance with the guidelines of the Animals (Scientific Procedures) Act 1986. The cortices of neonatal (1-2 days old) human mutant SOD1 G93A transgenic (SOD1 G93A) mice ((B6SJLTg(SOD1*G93A)1Gur/J purchased from Jackson Laboratories), human wild type SOD1 transgenic (SOD1 WT) mice, and their non-transgenic littermates, NTG (G93A) and NTG (WT), respectively, were stripped of meninges, washed and triturated in Hank’s balanced salt solution (HBSS) with Ca2+/Mg2+ containing 0.04 % trypsin (Sigma), 0.1 mg/ml collagenase (Calbiochem) and 0.05 mg/ml DNaseI (Sigma). After trituration, the enzymatic process was stopped by addition of an equal volume of complete medium (Dulbecco’s modified Eagle’s medium (DMEM, Cambrex), 10 % heat inactivated fetal calf serum (FCS, BioSera), 100 units/ml penicillin and 100 μg/ml streptomycin (Gibco)) and the cells plated on poly-L-lysine (Sigma) coated coverslips at 60 000 cells/cm2. For the purification of microglia, the confluent cultures were subjected to mild trypsinisation [13], which resulted in microglial preparations of >90 % purity and 30 000 microglia/coverslip. Across the genotypes there was no significant difference between the mean cell numbers per coverslip during resting or activated states, as assessed by cell counts on representive coverslips from 6 independent SOD1 G93A and 3 independent SOD1 WT cultures. Resting and activation/inflammatory conditions were achieved by incubating microglia in complete medium with vehicle (HBSS) or with 1 μg/ml LPS for 24 hours, respectively.
Immunocytochemistry
The cells were washed with phosphate-buffered saline (PBS), fixed with 4 % paraformaldehyde for 15 min and permeabilised with 0.1 % Triton X-100. Non-specific binding was blocked with 5 % FCS in PBS for 30 min. The cells were incubated with a rat anti-mouse F4/80 (Serotec) and isotype control (Serotec) primary antibodies in blocking buffer at room temperature for 1 h. After washing in PBS, the cells were incubated with a goat anti-rat FITC secondary antibody (Serotec) in blocking buffer at room temperature for 1 h and nuclei stained with 0.2 μg/ml Hoechst 33342 (Intergen) solution for 1 min. The coverslips were mounted after additional washes in PBS.
Microscopy and image analyses
Stained cells were viewed under an Axiovert 200 microscope (Carl Zeiss) fitted with a digital camera (Hamamatsu), and pictures generated in Openlab 3.7.1 software. Cell surface area, index of ramification (IR), and F4/80 expression were quantified using ImageJ software (NIH, USA). The IR values were calculated as described previously [14].
Measurement of secreted inflammatory mediators
Supernatants from the resting and activated cells were collected and centrifuged to remove cells, then tested for the presence of analytes (interleukin (IL)-3, -4, -5, -6, -10, -12p70, -13, keratinocyte chemokine (KC), interferon (IFN)-γ, MCP-1, and TNF-α) using a cytometric bead array (Becton Dickinson) assay, as per the manufacturer’s protocol. PGE2 was measured with an ELISA kit (R & D systems) according to the manufacturer’s protocol.
Statistical analyses
Data were analysed using GraphPad Prism 5.0 software. Single comparison of MCP-1 production by activated NTG (G93A) and SOD1 G93A cells was conducted using the Mann-Whitney test. All other data sets were analysed by multiple comparisons using one way ANOVA with Tukey’s post test when data fitted a Gaussian distribution or Kruskal-Wallis test with Dunn’s post test when the data did not fit a Gaussian distribution.
Results
1) Morphological activation parameters
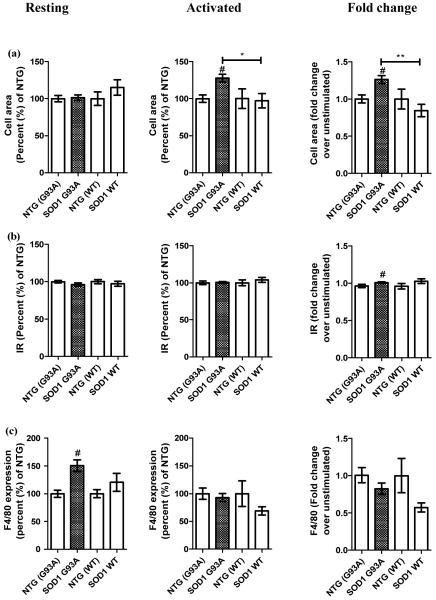
To gauge the activation status of resting microglial cells and the degree of activation by an inflammatory stimulus, the morphological properties and F4/80 expression of SOD1 G93A and SOD1 WT microglia were compared to microglia from respective non-transgenic (NTG) littermate controls. Resting SOD1 G93A microglia had similar surface areas to the resting control microglia, indicating that SOD1 G93A microglia under resting conditions have a morphology associated with the physiological resting phenotype. However, the cell surface area increased significantly in SOD1 G93A microglia on activation with LPS (Figure 1a, mean ± standard error of the mean (SEM): 128 ± 5 % for SOD1 G93A versus 100 ± 5 % for NTG (G93A), 100 ± 13 % NTG (WT), and 97 ± 10 % for SOD1 WT, p<0.05). The fold change value, which measures the magnitude of the change from the resting to the activated states, showed that while SOD1 G93A microglia increased the cell area 1.26 fold, the cell area of SOD1 WT microglia reduced 0.84 fold (Figure 1a, mean ± SEM: 1.26 ± 0.05 fold for SOD1 G93A versus 1 ± 0.05 fold for NTG (G93A), 1 ± 0.1 fold for NTG (WT), and 0.84 ± 0.08 fold for SOD1 WT, p<0.01). This indicates that SOD1 G93A and SOD1 WT microglia have divergent responses to the activation stimulus. The average index of ramification, which reports on the degree of microglial amoeboid morphology known to be associated with activation, did not differ between SOD1 G93A and SOD1 WT microglia under resting or activated states (Figure 1b), although there was a slight increase in the fold change in IR between SOD1 G93A and NTG (G93A) microglia (Figure 1b, mean ± SEM: 1 ± 0.01 fold for SOD1 G93A versus 0.96 ± 0.02 fold for NTG (G93A), p<0.05) that was not seen in WT SOD1 cells.
Figure 1.
SOD1 G93A microglia showed altered cellular properties when compared to NTG (G93A), NTG (WT) and SOD1 WT control microglia. (a) – cell area, (b) – index of ramification (IR), and (c) – F4/80 expression; the data represent mean ± SEM shown in percentage (%) relative to the average value for the respective NTG cells set at 100 %. The data were obtained from five (NTG (G93A) and SOD1 G93A) and three (NTG (WT) and SOD1 WT) independent experiments. The resting and activated state values as well as the fold change values between the two states per genotype are shown. # p<0.05 between SOD1 G93A and NTG (G93A); * p<0.05 and ** p<0.01 between SOD1 G93A and SOD1 WT as measured by Kruskal-Wallis test with Dunn’s post test.
The activation marker F4/80 was expressed at significantly higher levels on resting SOD1 G93A cells when compared to resting NTG (G93A) microglia (Figure 1c, mean ± SEM: 150.4 ± 10 % for SOD1 G93A versus 100 ± 7 % for NTG (G93A), p<0.05), again a difference not seen in SOD1 WT cells. However, expression levels increased equally in microglia of both genotypes after activation, suggesting that the presence of SOD1 G93A can increase F4/80 expression in the absence of external stimuli.
2) Secretion of inflammatory mediators
To investigate the levels of inflammatory molecules secreted by SOD1 G93A microglia under resting and activating conditions, the concentrations of various cytokines and chemokines and PGE2, were measured in the conditioned media of the SOD1 G93A and the NTG (G93A) cells using a cytometric bead array assay using identical numbers of cells for each condition and genotype. We measured the levels of molecules that are associated with microglial activation (e.g. MCP-1, TNF-α) and those that are not (e.g. IFN-γ) because it is unknown how the overexpression of the SOD1 G93A in microglia can affect its secretory phenotype. The concentrations of the analytes, including TNF-α, were not significantly different between the SOD1 G93A and NTG (G93A) microglial conditioned media (Table 1) except for MCP-1. SOD1 G93A microglia secreted significantly higher levels of MCP-1 than did activated NTG (G93A) cells (Table 1, mean ± SEM: 17020 ± 3222 pg/ml for SOD1 G93A versus 7350 ± 2383 pg/ml for NTG (G93A), p<0.05). A more detailed investigation of MCP-1 production showed that activated SOD1 G93A microglia produced significantly greater levels of MCP-1 than did NTG (G93A), SOD1 WT and NTG (WT) microglia, with the fold change values showing over 3 fold greater production of MCP-1 by activated SOD1 G93A microglia than by control microglia (Figure 2, mean ± SEM: 27 ± 5 fold for SOD1 G93A versus 8 ± 3 fold for NTG (G93A), 3 ± 0.3 fold for NTG (WT) and 3 ± 0.4 for SOD1 WT, p<0.05).
Table 1.
Cytokine, chemokine and PGE2 production by resting and activated NTG (G93A) and SOD1 G93A microglia. Analyte concentrations (pg/ml) in resting and activated microglia-conditioned media were measured by cytometric bead array assay or ELISA (PGE2). Data are means ± SEM for three independent experiments
| Analyte | Resting | Activated | ||
|---|---|---|---|---|
| NTG (G93A) | SOD1 G93A | NTG (G93A) | SOD1 G93A | |
| IL-3 | 1.260±0.520 | 2.370±0.780 | 15.54±6.290 | 20.34±8.230 |
| IL-4 | ND | ND | ND | 6.590±2.970 |
| IL-5 | ND | ND | 24.03±12.04 | 40.10±17.17 |
| IL-6 | 58.50±20.51 | 41.87±13.27 | 66110±24890 | 85120±28620 |
| IL-10 | 30.95±14.52 | 46.59±15.76 | 265.0±74.93 | 489.0±115.2 |
| IL-12p70 | ND | 17.49±5.570 | 136.9±50.36 | 188.0±81.06 |
| IL-13 | 5.320±1.850 | 8.570±2.720 | 51.22±21.85 | 65.62±27.39 |
| KC | 2391±749.7 | 1821±672.0 | 27826±4831 | 25837±2750 |
| IFN-γ | ND | ND | 9.690±3.690 | 13.09±4.990 |
| MCP-1 | 888.4±186.8 | 640.4±143.2 | 7350±2383 | 17020±3222* |
| TNF-α | 73.89±19.98 | 78.84±24.40 | 46400±20110 | 17295±2700 |
| PGE2 | 234.4±87.27 | 216.2±79.26 | 3450±292.7 | 3350±282.3 |
ND = not detected
p<0.05.
Figure 2.
Activated SOD1 G93A microglia produced significantly more MCP-1 than did NTG (G93A), NTG (WT) and SOD1 WT control microglia. The data represent mean ± SEM from conditioned media of 5 NTG (G93A), 7 TG G93A, 3 NTG (WT) and 3 SOD1 WT microglial cultures that did not differ in cell numbers under resting and activated states (see Materials and Methods). The resting and activated state values as well as the fold change values between the two states per genotype are shown. # p<0.05 between SOD1 G93A and NTG (G93A) as measured by Kruskal-Wallis test with Dunn’s post test; * p<0.05 and ** p<0.01 between SOD1 G93A and SOD1 WT as measured by one way ANOVA with Tukey’s post test.
Discussion
Activated microglia have potentially neurotoxic properties and may have a deleterious role in neurodegenerative diseases such as ALS. Here, we report that SOD1 G93A microglia show altered morphological properties, possibly indicating an increased potential for activation, in both the resting and activated states. In the presence of G93A mutant SOD1, F4/80 is elevated even in the resting state. This macrophage cell surface marker has been used widely to distinguish cells of myeloid lineage and murine F4/80 is known to be upregulated during microglial activation [15,16]. The function of F4/80 in mice is yet unknown, but its human homolog EMR1 and other members of the EMR family were shown to bridge the functions of innate and adaptive immune systems [17]. Thus, the elevated F4/80 expression on the resting state SOD1 G93A microglia may make them more responsive to the immunologic changes when compared to the resting control cells, resulting in earlier or excessive inflammatory response.
Microglial morphology may, to some degree, indicate an activation status and so the increase in cell surface area in activated SOD1 G93A microglia could represent an enhanced inflammatory response to a stimulus [12]. This may also be reflected in the greater shift towards amoeboid morphology in these cells. Taken together, these data suggest that mutant but not wild-type SOD1 may induce a pro-inflammatory shift in the properties of microglia.
We have also demonstrated that activated SOD1 G93A microglia have elevated MCP-1 production when compared to control cells. The activation was induced by incubating the cells for 24 h with 1 μg/ml LPS. We applied this concentration of LPS to replicate the inflammatory conditions used in other studies, which have previously attempted to investigate alterations in the properties of microglia induced by the presence of mutant SOD1 [5,10,11]. Using our microglial cultures obtained from neonatal animals, we were unable to detect increased production of TNF-α by TG G93A microglial cells, despite re-testing the conditioned media using TNF-α ELISA kits (data not shown). This finding corresponds with previously published results of Weydt and colleagues (2004) [10] who demonstrated that TG G93A microglia isolated form adult, but not neonatal, animals showed increased production of TNF-α when compared to that of NTG (G93A) microglia.
The findings of the current report indicate that activated SOD1 G93A microglia are a possible source of elevated MCP-1 levels in ALS. Overexpression of MCP-1 in the mouse CNS causes an encephalopathy and chronic activation of microglia [20]. MCP-1 increases the recruitment of leukocytes to sites of CNS injury [21] and may alter permeability of the blood-brain barrier [22]. MCP-1 can also be secreted by neurons and astrocytes, and its receptor, CCR2, is constitutively expressed on neurons and astrocytes [23]. These findings suggest that MCP-1 exerts autocrine and paracrine effects on most cell types in the CNS.
MCP-1 was shown to be neuroprotective by upregulating the expression of neurotrophic molecules, such as bFGF, in astrocytes, and astrocytic MCP-1 was directly neuroprotective in excitotoxicity [24]. However, under physiologic conditions MCP-1 has also been shown to induce neuronal cell death via excitotoxicity and oxidative stress mediated by microglial overstimulation and unregulated inflammatory reactivity [25]. Thus, we speculate that the increased SOD1 G93A microglial inflammatory phenotype, along with increased MCP-1, described in this study may result in a pro-inflammatory microglial response to activation by damaged motor neurons, which could propagate motor neuronal injury and death in ALS. MCP-1 is thus a candidate inflammatory mediator which could play a key role in the propagation of neuronal damage in ALS.
Conclusion
In the present study we examined morphological and secretory properties of SOD1 G93A microglia under resting and activating conditions. Activated SOD1 G93A microglia have a marked increase in cell surface area when compared to the control cells, indicative of a greater level of activation by LPS. We also identified that activated SOD1 G93A microglia produced much greater levels of MCP-1 than did the activated control cells, an increased production of over 3 fold. This is of particular interest as MCP-1 is the only chemokine consistently reported to be elevated in the CNS and CSF of ALS patients. The known properties of MCP-1 mean that this molecule may represent a key mediator in the propagation of motor neuron injury and disease progression and an attractive target for anti-inflammatory therapeutic approaches.
Acknowledgements
We are thankful the Wellcome Trust for funding the current study. SAS was supported by a Wellcome Trust Prize PhD studentship.
Source of support: this study was supported by the Wellcome Trust, grant number 073083/Z/03/Z
Abbreviations
- ALS
Amyotrophoic lateral sclerosis
- SOD1
superoxide dismutase 1
- WT
wild-type
- MCP-1
monocyte chemoattractant protein-1
- CSF
cerebrospinal fluid
- PG
prostaglandin
- TNF
tumor necrosis factor
- LPS
lipopolysaccharide
- NTG
non-transgenic
- DMEM
Dulbecco’s modified Eagle’s medium
- FCS
fetal calf serum
- HBSS
Hank’s buffered salts solution
- PBS
phosphate buffered saline
- IL
interleukin
- IR
index of ramification
- KC
keratinocyte chemokine
- IFN
interferon
Footnotes
Disclosure: the authors declare that they have no conflict of interests
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sargsyan S, Monk P, Shaw P. Microglia as potential contributors to motor neuron injury in amyotrophic lateral sclerosis. Glia. 2005;51:241–253. doi: 10.1002/glia.20210. [DOI] [PubMed] [Google Scholar]
- [2].Brooks B. Emerging directions in ALS therapeutics: palliative therapies at the advent of the twenty-first century. Clin Neurosci. 3:386–392. [PubMed] [Google Scholar]
- [3].Boillée S, Yamanaka K, Lobsiger C, Copeland N, Jenkins N, Kassiotis G, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- [4].Yamanaka K, Boillee S, Roberts E, Garcia M, McAlonis-Downes M, Mikse O, et al. Mutant SOD1 in cell types other than motor neurons and oligodendrocytes accelerates onset of disease in ALS mice. Proc Natl Acad Sci U S A. 2008;105:7594–7599. doi: 10.1073/pnas.0802556105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Beers D, Henkel J, Xiao Q, Zhao W, Wang J, Yen A, et al. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2006;103:16021–16026. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Poloni M, Facchetti D, Mai R, Micheli A, Agnoletti L, Francolini G, et al. Circulating levels of tumour necrosis factor-alpha and its soluble receptors are increased in the blood of patients with amyotrophic lateral sclerosis. Neurosci Lett. 2000;287:211–214. doi: 10.1016/s0304-3940(00)01177-0. [DOI] [PubMed] [Google Scholar]
- [7].Wilms H, Sievers J, Dengler R, Bufler J, Deuschl G, Lucius R. Intrathecal synthesis of monocyte chemoattractant protein-1 (MCP-1) in amyotrophic lateral sclerosis: further evidence for microglial activation in neurodegeneration. J Neuroimmunol. 2003;144:139–142. doi: 10.1016/j.jneuroim.2003.08.042. [DOI] [PubMed] [Google Scholar]
- [8].Baron P, Bussini S, Cardin V, Corbo M, Conti G, Galimberti D, et al. Production of monocyte chemoattractant protein-1 in amyotrophic lateral sclerosis. Muscle Nerve. 2005;32:541–544. doi: 10.1002/mus.20376. [DOI] [PubMed] [Google Scholar]
- [9].Nagata T, Nagano I, Shiote M, Narai H, Murakami T, Hayashi T, et al. Elevation of MCP-1 and MCP-1/VEGF ratio in cerebrospinal fluid of amyotrophic lateral sclerosis patients. Neurol Res. 2007;29:772–776. doi: 10.1179/016164107X229795. [DOI] [PubMed] [Google Scholar]
- [10].Weydt P, Yuen E, Ransom B, Möller T. Increased cytotoxic potential of microglia from ALS-transgenic mice. Glia. 2004;48:179–182. doi: 10.1002/glia.20062. [DOI] [PubMed] [Google Scholar]
- [11].Xiao Q, Zhao W, Beers D, Yen A, Xie W, Henkel J, et al. Mutant SOD1(G93A) microglia are more neurotoxic relative to wild-type microglia. J Neurochem. 2007;102:2008–2019. doi: 10.1111/j.1471-4159.2007.04677.x. [DOI] [PubMed] [Google Scholar]
- [12].abd-el-Basset E, Fedoroff S. Effect of bacterial wall lipopolysaccharide (LPS) on morphology, motility, and cytoskeletal organization of microglia in cultures. J Neurosci Res. 1995;41:222–237. doi: 10.1002/jnr.490410210. [DOI] [PubMed] [Google Scholar]
- [13].Saura J, Tusell J, Serratosa J. High-yield isolation of murine microglia by mild trypsinization. Glia. 2003;44:183–189. doi: 10.1002/glia.10274. [DOI] [PubMed] [Google Scholar]
- [14].Eder C, Schilling T, Heinemann U, Haas D, Hailer N, Nitsch R. Morphological, immunophenotypical and electrophysiological properties of resting microglia in vitro. Eur J Neurosci. 1999;11:4251–4261. doi: 10.1046/j.1460-9568.1999.00852.x. [DOI] [PubMed] [Google Scholar]
- [15].Kauppinen TM, Higashi Y, Suh SW, Escartin C, Nagasawa K, Swanson RA. Zinc triggers microglial activation. J Neurosci. 2008;28:5827–5835. doi: 10.1523/JNEUROSCI.1236-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen LC, Smith A, Ben Y, Zukic B, Ignacio S, Moore D, et al. Temporal gene expression patterns in G93A/SOD1 mouse. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5:164–171. doi: 10.1080/14660820410017091. [DOI] [PubMed] [Google Scholar]
- [17].Hamann J, Koning N, Pouwels W, Ulfman L, van Eijk M, Stacey M, et al. EMR1, the human homolog of F4/80, is an eosinophil-specific receptor. Eur J Immunol. 2007;37:2797–2802. doi: 10.1002/eji.200737553. [DOI] [PubMed] [Google Scholar]
- [18].Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- [19].Davalos D, Grutzendler J, Yang G, Kim J, Zuo Y, Jung S, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- [20].Huang D, Wujek J, Kidd G, He T, Cardona A, Sasse M, et al. Chronic expression of monocyte chemoattractant protein-1 in the central nervous system causes delayed encephalopathy and impaired microglial function in mice. FASEB J. 2005;19:761–772. doi: 10.1096/fj.04-3104com. [DOI] [PubMed] [Google Scholar]
- [21].Mahad DJ, Ransohoff RM. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) Seminars in immunology. 2003;15:23–32. doi: 10.1016/s1044-5323(02)00125-2. [DOI] [PubMed] [Google Scholar]
- [22].Stamatovic SM, Keep RF, Kunkel SL, Andjelkovic AV. Potential role of MCP-1 in endothelial cell tight junction ‘opening’: signaling via Rho and Rho kinase. J Cell Sci. 2003;116:4615–4628. doi: 10.1242/jcs.00755. [DOI] [PubMed] [Google Scholar]
- [23].de Haas AH, van Weering HR, de Jong EK, Boddeke HW, Biber KP. Neuronal chemokines: versatile messengers in central nervous system cell interaction. Mol Neurobiol. 2007;36:137–151. doi: 10.1007/s12035-007-0036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kalehua A, Nagel J, Whelchel L, Gides J, Pyle R, Smith R, et al. Monocyte chemoattractant protein-1 and macrophage inflammatory protein-2 are involved in both excitotoxin-induced neurodegeneration and regeneration. Exp Cell Res. 2004;297:197–211. doi: 10.1016/j.yexcr.2004.02.031. [DOI] [PubMed] [Google Scholar]
- [25].Sheehan J, Zhou C, Gravanis I, Rogove A, Wu Y, Bogenhagen D, et al. Proteolytic activation of monocyte chemoattractant protein-1 by plasmin underlies excitotoxic neurodegeneration in mice. J Neurosci. 2007;27:1738–1745. doi: 10.1523/JNEUROSCI.4987-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]