The current epidemic of obesity and diabetes in the face of a surfeit of calories contrasts the natural selection to survive famine that confronted our ancestors. The report in PNAS by Zhao et al. (1) shows that ghrelin is an important hormone in this process. By studying mice under severe caloric restriction, they show that knockout (KO) of the enzyme ghrelin O-acyltransferase (GOAT), necessary to convert ghrelin to its active form, manifests the first clear phenotype seen in KO of ghrelin activity; ghrelin is necessary for triggering the growth-hormone (GH) response to nutritional deprivation that prevents hypoglycemia and death.
The road to ghrelin's discovery started with the observation that morphine stimulates GH secretion, isolation of the enkephalins, and development of enkephalin analogs that selectively stimulate secretion of GH [GH-secretagogues (GHSs)] and orally active GHS. One such GHS, MK-0677, was used to expression clone the GHS receptor (GHS-R) (2). Using cell lines expressing this receptor, Kojima et al. (3) isolated and characterized its endogenous ligand (ghrelin) from rat stomach extracts. They discovered that the third amino acid of ghrelin had an unexpectedly large mass; it was a serine residue modified by the attachment of octanoate, a medium-chain fatty acid. Removing the octanoate blocked ghrelin's ability to bind to or activate its receptor. This acylation of a peptide with a medium-chain fatty acid is unique to ghrelin.
Although ghrelin was named for its GH-releasing activity, its pleiotropic effects, including increased appetite, altered gastrointestinal motility, and regulated lipid and glucose metabolism, cardiac function, blood pressure, immune function, cell proliferation, sleep, anxiety, and even memory, soon became apparent (4). However, KO of ghrelin or GHS-R or the combined KO of both receptor and ligand had no distinctive phenotype (5). The reason that no phenotype was observed with these KO animals is likely because previous studies did not employ the prolonged (10 days) severe nutritional restriction (40% of normal levels) that is used in the study of Zhao et al. (1). Ghrelin's potent orexigenic properties led a number of groups to suggest that ghrelin may be important in the pathogenesis of obesity, type 2 diabetes, and Prader–Willi syndrome (6, 7).
Recently, two groups independently identified GOAT, which had been previously known as an orphan member of a family of membrane-bound O-acyltransferase enzymes (MBOATs) (8, 9). GOAT is expressed in the ghrelin-producing cells of the stomach and small intestine. GOAT is required for the attachment of octanoate to preproghrelin and has no other known activity (8, 9).
The report by Zhao et al. (1) combines molecular biological approaches with an integrative biological experimental paradigm. They study a GOAT KO mouse that, therefore, lacks acyl-ghrelin and expose it to prolonged nutritional restriction (40% of normal levels). They show that ghrelin is important for the maintenance of the blood–glucose levels needed for survival during prolonged nutrient restriction. The depletion of fat reserves after more than 3 days of nutritional restriction with feeding one time per 24 h results in debilitating hypoglycemia at the end of the day in the GOAT KO mice but not wild-type mice. Thus a clear metabolic phenotype has been demonstrated in the GOAT KO mouse.
Thus, ghrelin is critical in the counterregulatory ensemble of factors that maintains blood glucose (Fig. 1). This repertoire includes catecholamines, glucagon, glucocorticoids, and GH. Earlier studies suggested that ghrelin might increase insulin resistance and impair insulin secretion (10). Zhao et al. (1) define a physiological role for ghrelin; it orchestrates the enhanced GH secretion induced by prolonged nutritional restriction. It must be emphasized that these mice were calorie restricted but not fasted. This is important, because the lipid group that is attached to preproghrelin by GOAT is likely derived from free fatty acids (FFA) in the lumen of the gut rather than circulation (11). Indeed, with prolonged fasting (>37.5 h) in humans, ghrelin levels are suppressed, whereas desacyl-ghrelin is tonically secreted (12). If the FFA required for activation of ghrelin must come from the gut, then ghrelin will be suppressed in wild-type mice during long-term fasting; this contrasts the increased levels described by Zhao et al. (1) during nutritional restriction.
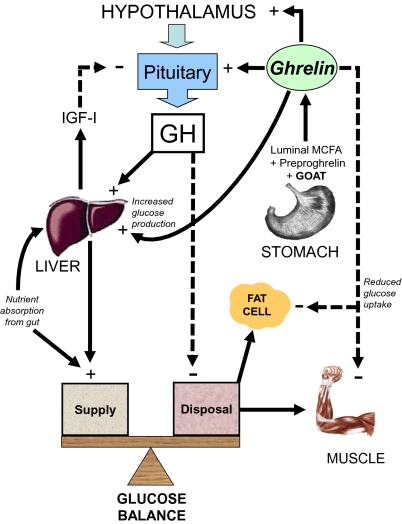
Fig. 1.
Regulation of glucose balance by GH and ghrelin. Glucose supply is controlled by absorption from the gut as well as hepatic glucose production and glucose disposal rate. Circulating ghrelin levels are regulated through acylation of preproghrelin by GOAT and depend on the presence of luminal medium-chain fatty acids (MCFA). Ghrelin increases GH release. GH release from the pituitary is inhibited by IGF-I negative feedback. Ghrelin increases hepatic glucose production and decreases glucose uptake in skeletal muscle and fat cells. GH increases hepatic glucose production by inhibiting insulin action. GH decreases glucose disposal to skeletal muscle and possibly, fat tissue indirectly through lipolysis and increase in FFA. During conditions of prolonged nutritional restriction when fat depots and hepatic glycogen content are depleted, GH levels are elevated by decreased IGF-I negative feedback and increased ghrelin. Low portal insulin levels reduce hepatic GH receptor expression and reduce IGF-I secretion despite high GH levels. In addition, the low insulin levels lead to high ghrelin secretion, because insulin normally inhibits ghrelin release.
The evidence presented suggests that GH is pivotal in preserving normal blood–glucose levels. This is supported by the lower levels of GH observed in the GOAT KO mice and the rescue of blood glucose by infusion of GH or ghrelin. This emphasizes the physiological importance of direct effects of GH in the severely nutritionally restricted state, a time when the levels of insulin-like growth factor I (IGF-I) are low. GH levels are raised in starved infants (at a time when glucose may be low) with profoundly low IGF-I levels, and this reverses with refeeding. Thus, they are at least partially GH-resistant (13).
Under conditions of nutritional restriction, GH levels are high, leading to mobilization of fat to provide the required calories. However, after prolonged nutritional restriction, as in this study, the fat depots are depleted, and this is shown by both body-composition analysis and reduced levels of circulating FFA. In addition, the hepatic glycogen content is depleted in both wild-type and KO mice at the end of the study. Thus, the mechanism by which GH maintains blood glucose must be caused by reducing the energy expenditure or glucose uptake in the peripheral tissues or increased gluconeogenesis (Fig. 1).
GH is considered to exert diabetogenic effects by increasing insulin resistance in peripheral tissues. Keller and Zapf (14) showed that, in the absence of GH, glucose transport in fat cells is maximal and cannot be further increased by insulin. However, when GH is present, glucose transport is inhibited, and insulin is able to reverse this inhibition. The traditional concept that GH antagonizes insulin action may need to be reversed and substituted by insulin antagonizing the GH-mediated tonic inhibition of glucose transport. This framework fits with the experimental data that show that GH secretion is enhanced in the GOAT KO mouse in the nutritionally restricted state but not to the level attained in the wild-type mouse under the same conditions. If glucose transport is inhibited in a dose-dependent manner, the tonic inhibition of glucose transport in fat, and possibly muscle, would be greater in the wild-type mouse, thereby protecting the mouse from neuroglycopenia. Rabinowitz et al. (15) showed in humans that GH increases lipolysis and release of FFA from fat cells, and it inhibits glucose uptake in both muscle and fat; they posited that this process prevents neuroglycopenia in times of famine. This is what Zhou et al. (1) show, and additionally, they note that this is a ghrelin-dependent process. Another critical step in this cascade is portal insulin concentration regulation of the expression of GH-R to determine the GH sensitivity of the liver and its ability to produce IGF-I; IGF-I feeds back to inhibit GH secretion.
Ghrelin is critical in the counterregulatory ensemble of factors that maintains blood glucose.
In the presence of low portal insulin, as in calorie-restricted states, liver GH receptors are reduced, thus reducing IGF-I levels. Subsequent long-loop IGF-1 feedback to the pituitary is diminished resulting in elevated GH secretion (16).
This model of GH tonically suppressing glucose transport into fat cells also explains why GH-deficient animals and humans are obese yet sensitive to insulin. GH is the partitioning hormone that directs the use and storage of nutrients, and ghrelin plays a role in its regulation. A similar association between GH and ghrelin has been shown in clinical studies under fed conditions (17) and with the administration of GHS-R antagonists (18).
Ghrelin has direct, GH-independent effects on glucose metabolism, such as increasing hepatic glucose production and decreasing the glucose disposal rate (10, 19). Zhou et al. (1) do not specifically address the question as to what extent ghrelin and GH independently modulate glucose levels during caloric restriction. GH continues to exert its effects under conditions of significant loss of body fat mass and low circulating FFA levels. Although the traditional concept in which GH acts on glucose metabolism and increases insulin resistance favors a role for FFA (20), the data presented in this paper strongly support direct effects of GH on glucose homeostasis, independent of insulin, IGF-I, or FFA.
GH plays a fundamental role in maintaining metabolic homeostasis and body composition. The critical role of ghrelin in regulating these processes is highlighted in the study of Zhao et al. (1). The brain has an obligatory requirement to metabolize glucose to meet its energy requirements. The body's response to hypoglycemia is to mount a stress response, including the release of corticotropin releasing hormone and GH releasing hormone from the hypothalamus to stimulate corticotropin and GH that act to raise blood glucose. This study (1) suggests that the ghrelin-producing cell senses glucose to regulate ghrelin release. Ghrelin is essential for maintaining the level of GH required in prevention of neuroglycopenia and thus, preservation of life.
Footnotes
The authors declare no conflict of interest.
See companion article on page 7467 in issue 16 of volume 107.
References
- 1.Zhao T-J, et al. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA. 2010;107:7467–7472. doi: 10.1073/pnas.1002271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howard AD, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- 3.Kojima M, Hosoda H, Matsuo H, Kangawa K. Ghrelin: Discovery of the natural endogenous ligand for the growth hormone secretagogue receptor. Trends Endocrinol Metab. 2001;12:118–122. doi: 10.1016/s1043-2760(00)00362-3. [DOI] [PubMed] [Google Scholar]
- 4.Korbonits M, Goldstone AP, Gueorguiev M, Grossman AB. Ghrelin—a hormone with multiple functions. Front Neuroendocrinol. 2004;25:27–68. doi: 10.1016/j.yfrne.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol. 2003;23:7973–7981. doi: 10.1128/MCB.23.22.7973-7981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 7.Cummings DE, et al. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med. 2002;8:643–644. doi: 10.1038/nm0702-643. [DOI] [PubMed] [Google Scholar]
- 8.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez JA, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA. 2008;105:6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Y, Asnicar M, Saha PK, Chan L, Smith RG. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab. 2006;3:379–386. doi: 10.1016/j.cmet.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Kirchner H, et al. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med. 2009;15:741–745. doi: 10.1038/nm.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, et al. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab. 2008;93:1980–1987. doi: 10.1210/jc.2007-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soliman AT, et al. Serum insulin-like growth factors I and II concentrations and growth hormone and insulin responses to arginine infusion in children with protein-energy malnutrition before and after nutritional rehabilitation. Pediatr Res. 1986;20:1122–1130. doi: 10.1203/00006450-198611000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Keller S, Zapf J. Effect of insulin on glucose transporter distribution in white fat cells from hypophysectomized rats. FEBS Lett. 1989;259:189–193. doi: 10.1016/0014-5793(89)81525-x. [DOI] [PubMed] [Google Scholar]
- 15.Rabinowitz D, Klassen GA, Zierler KL. Effect of human growth hormone on muscle and adipose tissue metabolism in the forearm of man. J Clin Invest. 1965;44:51–61. doi: 10.1172/JCI105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunger D, Yuen K, Salgin B. Growth hormone effects on glucose metabolism. Horm Res Paediatr. 2007;67:37–42. [Google Scholar]
- 17.Nass R, et al. Evidence for acyl-ghrelin modulation of growth hormone release in the fed state. J Clin Endocrinol Metab. 2008;93:1988–1994. doi: 10.1210/jc.2007-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zizzari P, et al. Endogenous ghrelin regulates episodic growth hormone (GH) secretion by amplifying GH Pulse amplitude: Evidence from antagonism of the GH secretagogue-R1a receptor. Endocrinology. 2005;146:3836–3842. doi: 10.1210/en.2005-0212. [DOI] [PubMed] [Google Scholar]
- 19.Vestergaard ET, et al. Ghrelin infusion in humans induces acute insulin resistance and lipolysis independent of growth hormone signaling. Diabetes. 2008;57:3205–3210. doi: 10.2337/db08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Møller N, Jørgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30:152–177. doi: 10.1210/er.2008-0027. [DOI] [PubMed] [Google Scholar]