Abstract
One of the most striking outcomes of coevolution between species is egg mimicry by brood parasitic birds, resulting from rejection behavior by discriminating host parents. Yet, how exactly does a host detect a parasitic egg? Brood parasitism and egg rejection behavior provide a model system for exploring the relative importance of different visual cues used in a behavioral task. Although hosts are discriminating, we do not know exactly what cues they use, and to answer this it is crucial to account for the receiver's visual perception. Color, luminance (“perceived lightness”) and pattern information have never been simultaneously quantified and experimentally tested through a bird's eye. The cuckoo finch Anomalospiza imberbis and its hosts show spectacular polymorphisms in egg appearance, providing a good opportunity for investigating visual discrimination owing to the large range of patterns and colors involved. Here we combine field experiments in Africa with modeling of avian color vision and pattern discrimination to identify the specific visual cues used by hosts in making rejection decisions. We found that disparity between host and foreign eggs in both color and several aspects of pattern (dispersion, principal marking size, and variability in marking size) were important predictors of rejection, especially color. These cues correspond exactly to the principal differences between host and parasitic eggs, showing that hosts use the most reliable available cues in making rejection decisions, and select for parasitic eggs that are increasingly mimetic in a range of visual attributes.
Keywords: brood parasitism, coevolution, egg color, egg pattern, vision
Reciprocal selection and arms races among species are widespread in nature, and a key force in evolution (1). The remarkable mimicry of host eggs by coevolved brood parasites is well established to result from selection on parasitic eggs through visual discrimination by hosts (2). In rejecting a parasitic egg, a host parent must successfully detect and distinguish it from the eggs in its own clutch. Birds’ eggs, like most natural objects, comprise a variety of visual information, including color and various features of pattern. However, which of these features are used by an animal to identify and discriminate between objects? Egg discrimination and subsequent rejection behavior by hosts provides a system for investigating the relative importance of different visual cues that might be used by a receiver in making a behavioral decision. Although there is a long history of experiments investigating the features that hosts use to detect and reject a parasitic egg in their nest (2–4), until recently these have been constrained by lack of quantification methods with respect to visual signals (5). Most assessments of egg appearance have relied on human assessment, although avian vision differs from human vision in multiple respects (6). Overall, few studies have investigated exactly which components of a visual signal are used by a receiver and their relative importance, especially in the context of the receiver's visual system.
In the light of recent advances in analyzing color differences as seen by nonhuman animals (6, 7), three studies have used advanced perceptual models of avian discrimination to investigate cuckoo and host egg coloration (8–10). These confirmed that quantification of visual signals used by hosts to detect parasitism provides much greater insights when analyzed with models of bird vision than does subjective human assessment (5). However, although valuable, these studies were not directly linked to rejection experiments (8), involved species that were rarely parasitized and artificial eggs that were not a close match to host egg colors (9), or involved systems in which host rejection of foreign eggs does not occur (10).
Although such perceptual models of color discrimination can accurately predict various types of behavior (7), color is just one attribute of a visual signal. The two-dimensional arrangement of a visual signal, its pattern, is also crucial (6, 11), and pattern appears to be an important aspect of egg mimicry in many brood parasites (3, 4, 12). In general, objective analysis of pattern in studies of animal coloration in general is still rare although not nonexistent (13), and in the context of brood parasitism there is only one objective study to date. This study assessed common cuckoo Cuculus canorus mimicry of host eggs using digital image analysis, and found that the level of pattern mimicry in different host-specific races relates strongly to previously established rates of egg rejection of nonmimetic eggs in the corresponding host (14). No study to date has objectively quantified pattern mimicry in egg rejection experiments.
We experimentally investigated egg rejection and mimicry in the cuckoo finch Anomalospiza imberbis and its most common host, the tawny-flanked prinia Prinia subflava. In this system, both parties are highly polymorphic with respect to egg color and pattern, and parasites can be excellent mimics of hosts (Fig. 1), suggesting that they have been locked in a long period of coevolution. The great diversity in egg appearance makes this an ideal system to determine the relative importance of different visual cues in host decision making.
Fig. 1.
Host eggs (Left) and parasitic eggs (Right), illustrating variation in color and pattern. Egg color variation is continuous in avian color space, but to human eyes eggs can be broadly categorized as (from top to bottom) olive, blue, white, or red. In this image, pairs of eggs have been matched according to these categories and do not necessarily come from the same parasitized clutches.
In this study, we first compared the visual attributes of real parasitic and host eggs, using image analysis of pattern information (11, 14) and models of avian color and luminance discrimination that quantify the perceived differences between objects (7); luminance refers to achromatic information, or how light or dark something appears. Second, we carried out field experiments in Zambia to identify the best avian-perceived predictors of host acceptance or rejection of foreign eggs, which would provide strong evidence that they are used as cues by hosts. We used conspecific eggs as surrogate experimental parasitic eggs, exploiting the extreme natural variation in host egg appearance to present hosts with eggs differing from their own to varying degrees.
Quantifying and Comparing Host and Parasite Egg Appearance
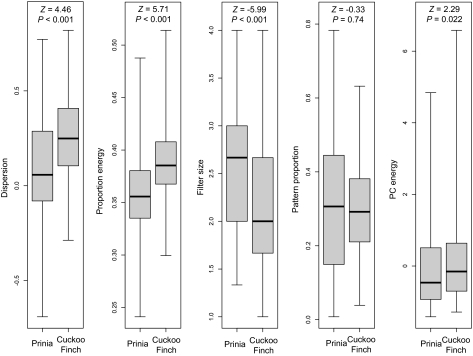
We compared the natural variation in host and parasitic eggs to identify which pattern traits differ principally between the two, versus those traits that are most accurately mimetic. We compared our quantified pattern attributes (Methods) for real host and parasitic eggs (n = 224 and 85 respectively), using one egg per clutch (host or parasite) to avoid pseudoreplication. The distributions overlapped extensively for all five pattern variables, confirming that parasitic eggs are a qualitatively good match to host eggs (Fig. 2). Average pattern proportion (the proportion of the egg surface covered with markings; Methods) did not differ between host and parasite, whereas PC energy (a synthetic measure of marking contrast and variability) was significantly higher on average in parasites, but highly variable in both parties (Fig. 2). Three pattern variables differed highly significantly between host and parasite: proportion energy (the relative importance of the dominant marking size) and pattern dispersion (the degree to which markings were concentrated toward the thick end of the egg) were both higher in parasites than hosts, whereas filter size (an inverse of measure of predominant marking size) was lower (Fig. 2). This implies that parasitic egg patterns were on average composed of larger markings than those of their hosts, tended to be more dominated by a single marking size, and were typically more concentrated at one end of the egg than those of their hosts. These differences are intuitively visible to a human eye in the sample eggs shown in Fig. 1.
Fig. 2.
Pattern differences between host eggs (n = 224) and parasitic eggs (n = 85). Whiskers indicate ranges, and statistical values refer to Wilcoxon rank sum tests. Cuckoo finch eggs had markings more concentrated toward the wide end of the egg (Dispersion), more variation in egg marking size (Proportion energy), and larger markings on average (Filter size, an inverse measure of marking size) than did host eggs.
Next, we compared the possible ranges of color and luminance contrasts between host and parasitic eggs, and among host eggs. We aimed to assess how different host and parasitic eggs can be if parasitic eggs are laid at random in host nests (Methods). We were interested in among-host differences as a reflection of the range of possible cues arising when using conspecific eggs as surrogate parasitic eggs in our rejection experiments (described below). To do this we calculated discrimination values [just noticeable differences (jnds), reflecting the perceived degree of difference through a bird’s eyes; Methods] between 5,000 randomly chosen pairs of host and parasite eggs, and 5,000 randomly chosen pairs of host eggs, thus generating two distributions of possible contrast values per trait. These distributions were remarkably similar for each trait (host versus parasite, color: mean jnd = 6.09 ±3.33, range = 0.15–18.53; luminance: mean jnd = 6.45 ±4.90, range = 0.00–28.14; host versus host, color: mean jnd = 6.82 ±3.76, range = 0.05–18.90; luminance: mean jnd = 7.02 ±5.03, range = 0.00–24.65). Conspecific eggs were therefore good surrogates for real parasitic eggs in the rejection experiments, presenting hosts with biologically realistic color and luminance discrimination tasks. We sought to challenge hosts with difficult rejection decisions, and thus the actual ranges of color and luminance contrasts for eggs involved in rejection experiments were slightly lower (color: mean jnd = 4.71 ±2.72, range = 0.13–15.50; luminance: mean jnd = 4.63 ±3.25, range = 0.15–13.99) than those between randomly contrasted host eggs (described above).
Egg Rejection Experiments: What Visual Cues Are Used by Hosts?
In all, 63 eggs were rejected and 62 eggs were accepted in our egg rejection experiments (Methods; n = 125 trials). We used these to quantify the relative importance of color, luminance, and our five pattern variables as predictors of rejection behavior (Methods details how contrasts between host and experimental eggs were calculated). These potential cues can be regarded as independent of one another because bivariate analyses showed only weak correlations among the seven potential cues, unlikely to introduce collinearity problems (tolerance >0.8). We then modeled predictors of host rejection using logistic regression. In doing so we tested whether the following extraneous variables influenced rejection behavior: difference in egg length and breadth between host and experimental eggs, clutch size (range 2–4, mean 3.1), state of incubation, whether laying was still in progress, and time of day of experimental parasitism; none of these explained any significant variation.
The final model contained four predictor variables, showing that differences in color, pattern dispersion, marking size, and the degree of dominance of the main marking size predicted rejection decisions (Table 1). Altogether, these explained 31.9% of the variance in rejection rate (15). Color and pattern each accounted for about half of the variance explained (Table 1). The difference in pattern dispersion (the difference between the narrow and wide regions of the egg in the proportion of pattern coverage) was the most important pattern variable, accounting for nearly a quarter of the overall variance explained (Table 1). No variance was explained by discrimination values for luminance, or by contrasts for PC energy and pattern proportion.
Table 1.
Predictors of egg rejection in experimentally parasitized nests
| Predictor | Slope ± SE | Z | P | I% |
| Color | 0.369 ± 0.095 | 3.86 | <0.001 | 0.491 |
| Pattern dispersion | 2.536 ± 0.873 | 2.90 | 0.004 | 0.231 |
| Pattern proportion energy | 15.916 ± 6.964 | 2.29 | 0.022 | 0.157 |
| Pattern filter size | 0.926 ± 0.437 | 2.12 | 0.034 | 0.122 |
I% refers to the proportion of the overall variance explained by the model (31.9%) accounted for by each variable independently (43).
Discussion
Many visual signals are composed of multiple cues, including both color and pattern. We found clear evidence that host parents integrated several such cues in deciding whether to accept or reject a potentially parasitic egg in their nest. This experiment is unique in its simultaneous consideration of pattern, color, and luminance cues in this type of behavioral task. Contrast in color (as perceived through a bird's eyes) was the single most important cue, whereas luminance contrast was not used. This is consistent with current understanding of discrimination behavior in animals, which suggests that color is usually the principal cue used in high light levels (7, 16). Theory predicts that discrimination values (jnd) of less than 1 mean that an observer is incapable of discriminating between two objects, whereas values greater than 3 should usually be discriminable (7, 17). However, some cuckoo finch hosts rejected eggs with jnd values of less than 1 (jnd range for rejected eggs: 0.7–15.5), and accepted eggs with jnd values considerably greater than 3 (jnd range for accepted eggs: 0.12–9.6), emphasizing that they use several sources of information in making rejection decisions, including pattern (discussed below). Prinias’ rejection decisions might also be influenced by color categorization (grouping similar colors, such as blue, red, UV, etc, irrespective of their absolute differences); although largely unstudied, this is known to occur in birds (18). To humans, the continuous variation in egg color falls broadly into four categories (Fig. 1).
Unlike color, differences between eggs in background luminance per se did not influence rejection behavior, and previous work suggests that luminance becomes more important at light levels lower than those in this study (8, 10, 19). However, luminance differences are key in defining patterns (16, 20), and three features of eggshell patterning combined were equally as important as color in predicting rejection decisions (Table 1): differences between host and parasite eggs in (i) pattern dispersion, the extent to which markings were concentrated at one pole of the egg (3); (ii) marking size, the predominant size of egg markings; and (iii) proportion energy, the contribution of the main marking size to the overall pattern. These traits also appear to be important to common cuckoo hosts, demonstrated by a recent comparative analysis relating pattern mimicry to the propensity of different host species to reject eggs (14). Most importantly, the pattern cues used in prinia rejection decisions are precisely those that differ most consistently between real parasitic and host eggs across the population (Fig. 2). Thus, prinias’ decision making is most sensitive to visual cues providing reliable information on egg identity independent of an individual's own phenotype. This should select for increasingly mimetic parasites, but in practice host polymorphisms make it very difficult to achieve perfect mimicry. Negative frequency–dependent (apostatic) selection should act on both host egg color and pattern variation, potentially presenting cuckoo finches with a constantly moving multidimensional target. We suggest that the lack of correlation among different pattern variables could itself be a product of selection favoring egg patterns with maximal information content.
Despite this well-tuned discrimination, hosts ignore one apparently ideal pattern cue: all host eggs possess “scribbled” fine lines (Fig. 1) that parasites never reproduce. This should be a fail-safe cue, acting as a “signature” that parasites cannot forge, yet unscribbled parasitic eggs are regularly accepted. Why this is so remains a conundrum, just as the absence of antiparasite defenses in some apparently long-term hosts of cowbirds and cuckoos also still defy adaptive explanation [reviewed in (21)].
Brood parasitism has long been a model system for investigating coevolution, and several classic studies have investigated egg rejection behavior and mimicry using subjective human assessment (2, 3). An interesting question is how valid the findings of these past studies are, given that the potential pitfalls of using humans to discriminate or rank animal colors and patterns are now well understood (22). Not only might human perception be error prone or inaccurate in assessing signals aimed at other animals, but humans also differ among themselves in how they rank colors, and may be strongly affected by other factors such as ambient light conditions, especially in the field (23, 24). Models of receiver visual perception can greatly improve studies of visual signals, and highlight the limitations of using human assessment; for example, a high proportion of birds may look sexually dimorphic to other birds, but monomorphic to humans (25). These differences in assessment are rarely only the result of birds having UV vision, because “hidden” dimorphism is also common in the human visible part of the spectrum. Color aside, human assessment does not objectively distinguish different components of pattern, which are now known to be multiple and uncorrelated in hosts of common cuckoos (14) as well as cuckoo finches (this study). Overall, the validity of human assessment in past work may relate to the host's ability to detect foreign eggs and hence the refinement of parasitic mimicry. Further work would be very valuable to determine the level of error potentially introduced by human assessment in the context of egg rejection, as already carried out for avian plumage color (25).
Color and pattern measures explained about a third of the variation in rejection behavior, which is large compared with preceding investigations of egg rejection (3). However, the large proportion remaining unexplained corroborates previous studies in emphasizing the importance of additional factors independent of mimicry in rejection behavior. Responses of common cuckoo hosts to foreign eggs are highly sensitive to perceived risk of parasitism, including risk perception socially transmitted from conspecifics (26, 27). Learning also plays a powerful role in antiparasite defenses, including individual learning of own egg appearance (26). Such effects may be more pronounced in prinias, which are relatively long-lived [survival rate = 0.60 (28)]. Learning may also help to explain why color but not luminance predicted rejection, as color contrast in birds is also more readily memorized than luminance (29).
Irrespective of these additional factors, visual discrimination is a key factor in predicting the rejection of parasitic eggs by hosts, demonstrating the selective mechanisms shaping the remarkable mimetic polymorphisms in this system. More broadly, our study shows that considering visual signals as a whole, together with the receiver's visual perception, can reveal how receivers make a behavioral decision based on integrating multiple visual cues. In the context of coevolutionary arms races, our findings raise the suggestion that multiple visual cues may respond to reciprocal selection, and that their interaction can itself comprise an adaptive defense.
Methods
Study System and Site.
Prinias and cuckoo finches were studied within an area of ca. 800 ha on and around Musumanene Farm (16°47′S, 26°54′E) in the Choma District of southern Zambia, during January to March 2007–2009. The habitat is a mixture of woodland, grassland and old agricultural fields, where prinias are abundant. They build loosely woven, oval-shaped nests with a side entrance, stitched among leaves of low herbaceous plants in exposed sunny positions. Cuckoo finches remove one or more host eggs when laying, and parasitized nests contain either one or two parasitic eggs laid by the same female, with or without host eggs. We distinguished parasitic from host eggs by the absence of fine lines on parasitic eggs (Discussion). Cuckoo finches lay eggs haphazardly with respect to host egg morph: 57% (21 of 37 naturally parasitized nests in which host eggs were still present) of laying attempts found were in a host clutch differing in color morph to the parasitic egg (morphs categorized by human eyes as in Fig. 1, because reflectance spectra were unavailable in most cases). This is unavoidably a subjective measure and an underestimate, as hosts probably rejected additional mismatched eggs before nests were discovered, but serves to show that parasites appear not to be able to target host color morphs that match their own. The parasitism rate at our site is at least 19%, again an underestimate because of host rejection. Cuckoo finch hatchlings typically outcompete any host young that hatch, leading to strong fitness costs of parasitism (30). Nest videos and observations confirmed that prinias ejected both cuckoo finch eggs and experimental conspecific eggs by puncture-ejection.
Quantifying Eggshell Pattern.
We modified an approach developed in Stoddard and Stevens (14) to quantify egg patterns from digital photographs. First, we rescaled each egg image to 50 pixels/mm using egg measurements taken in the field, because photographs were taken at slightly variable distances. We calibrated our images to linearize the relationship between the image value recorded and radiance, and converted each egg image to reflectance values by equalization with respect to a gray standard (11). We only used the image from the camera's green (mediumwave) sensor as this corresponds most closely to an avian luminance channel (31), and small markings and pattern information are principally encoded by achromatic information (16, 20).
Following image calibration, we used a self-written program in MATLAB (Mathworks) and its Image Processing toolbox to obtain several measures of pattern from identically sized samples of the wide, middle, and narrow regions of the egg (each ∼20% of the total egg area). First, we used a “granularity” approach similar to that recently used to analyze cuttlefish camouflage and avian egg markings (13, 14, 32). Here, each image of an egg is filtered into a set of new images using fast Fourier transformation, followed by applying seven octave-wide, isotropic band-pass filters (13). Each new image contains pattern information at different spatial scales, with smaller filter sizes capturing larger, low spatial frequency markings, and larger filter sizes capturing smaller, high spatial frequency markings. Although not precisely equating to a real visual system, this method resembles how visual information is encoded at different spatial frequencies during early visual processing (33, 34). Each of these different images or “granularity bands” (13) contains information about the relative contribution of different marking sizes to the overall pattern. From these we measured several different aspects of pattern [as in (14)]. For each granularity band (one to seven), we calculated the overall pattern “energy” as the sum of the squared pixel values in each image divided by the number of pixels in the image (14, 32), with these seven values producing a “granularity spectrum” (32). The maximum energy value in the spectrum corresponds to the filter size containing the highest energy, and thus the predominant marking size. The proportion of the total energy contained in this filter size with the highest energy (proportion energy) provides a measure of how important the main marking size is to the overall egg pattern; higher values indicate that this marking size dominates. The total energy of the spectrum (total energy) corresponds to the overall amplitude, and provides a measure of pattern contrast (32). The standard deviation of the energy values (across all seven scales) is a measure of how much variation in marking size exists. Low values indicate a relatively even contribution of different marking sizes, whereas high values indicate that one or a few marking sizes dominate. Total energy and standard deviation energy were highly positively correlated, so we calculated a synthetic measure as their first principal component (explaining >98.7% of the variance; eigenvalues >1.98). All variables above were significantly repeatable among egg regions, and we therefore analyzed averages per egg.
Finally, we calculated the proportion of each egg that was covered with markings. We first thresholded each image into a binary format, with markings encoded by a 1.00 and the ground color encoded by a zero (14), and then calculated the proportion of the total pixel values that corresponded to a marking. From this we determined what proportion of the egg (on average across all three regions) was covered with markings (average pattern proportion), and the difference between the narrow wide regions of the egg in the proportion of pattern coverage (pattern dispersion). For each measure, we took the absolute difference between the foreign egg and mean of host eggs in the clutch as an index of contrast between host and experimental eggs.
Modeling Avian Color Perception.
Reflectance spectra of freshly collected eggs were taken indoors with an Ocean Optics USB2000 spectrophotometer, with a PX-2 pulsed xenon light source and an R400-7-UV/VIS reflectance probe (all Ocean Optics), standardized using a Spectralon 99% white reflectance standard (Labsphere). We held each egg at a constant angle (45°) distance (5 mm) from the probe tip using an attached slanted plastic sleeve. We analyzed the mean of five measurements of each egg's ground color (i.e., avoiding pattern markings), taken throughout the egg. Irradiance (“ambient” light) within nests was measured in the field (during sunny weather between 1100 and 1400 hours) using a cosine corrected probe (Ocean Optics). Five measurements were taken at different angles within each of five nests, and the mean was analyzed.
Because spectral sensitivity data are unavailable for the prinia hosts used in this study, we calculated predicted photon catches for both a blue tit's Cyanistes caeruleus single and double cones (35) and a zebra finch's Taeniopygia guttata single cones (double cone data for this species were unavailable) (36). These species are not closely related and inhabit different light environments, so the differences between them assess the level of error associated with not having spectral sensitivity data for our study species. Current evidence indicates that most higher passerines differ relatively little in their spectral sensitivity (37), and as expected there was little difference between the photon catch values obtained using these two species [less than a 0.05 (±0.01 SD) average difference for each single cone type]. Therefore, here we only report further modeling with respect to the better-studied blue tit system. Following calculation of photon catches, we used a log form of a model of visual discrimination that accurately predicts discrimination behavior in observers (7). We used a version of the model based on color differences, using the single cones (7), and luminance (achromatic) differences, using the double cones (17). For color discrimination modeling, we used retinal single cone proportions of the blue tit (35). The output is in terms of jnds; jnd values of less than 1.00 mean that two objects should not be discriminable; values between 1.00 and 3.00 should be difficult to discriminate except under optimal viewing conditions; and larger values allow increasingly easy discrimination (17).
Egg Rejection Experiments.
Each trial involved a different host female. Using conspecific eggs as experimental parasitic eggs avoids pitfalls of artificially constructed eggs (38). Although, in principle, using artificially marked or modified eggs could allow varying egg phenotypes in just one or a few variables of interest, this is not possible in our system. First, host rejection behavior is so refined that producing artificially marked eggs that are not all rejected with ease is exceptionally difficult. Second, artificially marked eggs would need to use paints or dyes calibrated to look accurate to the bird rather than the human visual system, including UV information. Although research into antipredator coloration has created artificial stimuli that resemble natural backgrounds, these have either been necessarily simplified, such as lacking in color (39), or simply calibrated broadly to encompass a natural range of non–UV-reflecting colors (40), neither of which would be appropriate for the present questions.
We carried out experiments during the host's laying period, when possible, but also during other stages of incubation. Stage of incubation was scored on a scale of 0–6 (41) and assessed by shining light through the eggshell, or using known laying or hatching dates. We mimicked cuckoo finch laying behavior by removing one host egg when placing an experimental egg. All eggs were measured with digital calipers and photographed in RAW format alongside a 17% neutral gray card (Kodak) using a Fuji Finepix S7000 digital camera. Reflectance spectra of the removed host egg was subsequently measured indoors (discussed above). We regarded the color of the removed egg as representative of the host clutch, because repeatability of photon catches among prinia eggs within clutches was very high (repeatability > 0.93, F61,81 > 21.82, P < 0.001).
Experimental clutches were visited frequently (daily when possible) to determine the outcome. Experimental eggs that disappeared while the rest of the clutch remained in the nest were considered rejected, as predators remove the entire clutch. Experimental eggs that remained for 3 days were considered accepted. This threshold was justified, given that of 44 host nests containing an experimental egg on day 3 and subsequently revisited, in only one case had rejection subsequently occurred (on day 4). Although high predation implies overestimated rejection rates as a proportion of all experiments, because acceptance requires nest survival to day 3, this does not confound assessment of traits predicting rejection.
Statistical Analyses.
We used logistic regression models implemented in R (42) to analyze the predictors of egg rejection, with binomial error structure, logit link function, and model simplification via changes in AIC. We used the R package hier.part to partition variance explained (43).
Acknowledgments
In Zambia we thank Ian and Emma Bruce-Miller, Mary Counsell, Bruce and Julian Danckwerts, and John and Royce Colebrook-Robjent for their hospitality; the Zambia Wildlife Authority for permits; and Lazaro Hamusikili, Kiverness Moto, Colllins Moya, Refi, Collins, Averd and Stanley Munkombwe, Moses Siyabwaba, and Dunne Siyapolo for finding nests. In Cambridge we thank Nick Davies, Rebecca Kilner, and Justin Welbergen. C.N.S. was funded by a Royal Society Dorothy Hodgkin Research Fellowship, Sidney Sussex College and Newnham College, Cambridge, and by the DST/NRF Centre of Excellence at the Percy FitzPatrick Institute, University of Cape Town. M.S. was funded by a Biotechnology and Biological Sciences Research Council David Phillips Research Fellowship (BB/G022887/1) and Girton College, Cambridge.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Dawkins R, Krebs JR. Arms races between and within species. Proc R Soc Lond B Biol Sci. 1979;205:489–511. doi: 10.1098/rspb.1979.0081. [DOI] [PubMed] [Google Scholar]
- 2.Brooke M de L, Davies NB. Egg mimicry by cuckoos Cuculus canorus in relation to discrimination by hosts. Nature. 1988;335:630–632. [Google Scholar]
- 3.Lahti DC, Lahti AR. How precise is egg discrimination in weaverbirds? Anim Behav. 2002;63:1135–1142. [Google Scholar]
- 4.Rothstein SI. Mechanisms of avian egg recognition: Which egg parameters elicit responses by rejecter species? Behav Ecol Sociobiol. 1982;11:229–239. [Google Scholar]
- 5.Safran RJ, Vitousek MN. Evolutionary biology: Arms races in the eye of the beholder. Curr Biol. 2008;18:734–736. doi: 10.1016/j.cub.2008.07.045. [DOI] [PubMed] [Google Scholar]
- 6.Endler JA, Mielke PWJ. Comparing color patterns as birds see them. Biol J Linn Soc. 2005;86:405–431. [Google Scholar]
- 7.Vorobyev M, Osorio D. Receptor noise as a determinant of colour thresholds. Proc R Soc Lond B Biol Sci. 1998;265:351–358. doi: 10.1098/rspb.1998.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avilés JM. Egg colour mimicry in the common cuckoo Cuculus canorus as revealed by modelling host retinal function. Proc R Soc Lond B Biol Sci. 2008;275:2345–2352. doi: 10.1098/rspb.2008.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassey P, Honza M, Grim T, Hauber ME. The modelling of avian visual perception predicts behavioural rejection responses to foreign egg colours. Biol Lett. 2008;4:515–517. doi: 10.1098/rsbl.2008.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langmore NE, Stevens M, Maurer G, Kilner RM. Are dark cuckoo eggs cryptic in host nests? Anim Behav. 2009;78:461–468. [Google Scholar]
- 11.Stevens M, Párraga A, Cuthill IC, Partridge JC, Troscianko T. Using digital photography to study animal coloration. Biol J Linn Soc. 2007;90:211–237. [Google Scholar]
- 12.Davies NB, Brooke M de L. Cuckoos versus reed warblers: Adaptations and counteradaptations. Anim Behav. 1988;36:262–284. [Google Scholar]
- 13.Barbosa A, et al. Cuttlefish camouflage: The effects of substrate contrast and size in evoking uniform, mottle or disruptive body patterns. Vision Res. 2008;48:1242–1253. doi: 10.1016/j.visres.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Stoddard MC, Stevens M. Pattern mimicry of host eggs by the common cuckoo, as seen through a bird's eye. Proc R Soc Lond B Biol Sci. 2010;277:1387–1393. doi: 10.1098/rspb.2009.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faraway JJ. Extending the Linear Model with R. Boca Raton, FL: Chapman & Hall/CRC; 2005. [Google Scholar]
- 16.Osorio D, Vorobyev M. Photoreceptor spectral sensitivities in terrestrial animals: Adaptations for luminance and colour vision. Proc R Soc Lond B Biol Sci. 2005;272:1745–1752. doi: 10.1098/rspb.2005.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siddiqi A, Cronin TW, Loew ER, Vorobyev M, Summers K. Interspecific and intraspecific views of color signals in the strawberry poison frog Dendrobates pumilio. J Exp Biol. 2004;207:2471–2485. doi: 10.1242/jeb.01047. [DOI] [PubMed] [Google Scholar]
- 18.Ham AD, Osorio D. Colour preference and colour vision in poultry chicks. Proc R Soc Lond B Biol Sci. 2007;274:1941–1948. doi: 10.1098/rspb.2007.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lind O, Kelber A. Avian colour vision: Effects of variation in receptor sensitivity and noise data on model predictions as compared to behavioural results. Vision Res. 2009;49:1939–1947. doi: 10.1016/j.visres.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Jones CD, Osorio D. Discrimination of oriented visual textures by poultry chicks. Vision Res. 2004;44:83–88. doi: 10.1016/j.visres.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Davies NB. Cuckoos, Cowbirds and Other Cheats. London: Poyser; 2000. [Google Scholar]
- 22.Bennett ATD, Cuthill IC, Norris KJ. Sexual selection and the mismeasure of color. Am Nat. 1994;144:848–860. [Google Scholar]
- 23.Endler JA. On the measurement and classification of colour in studies of animal colour patterns. Biol J Linn Soc. 1990;41:315–352. [Google Scholar]
- 24.Stevens M, Cuthill IC. The unsuitability of html-based colour charts for estimating animal colours—a comment on Berggren & Merilä. Front Zool. 2005;2:1–14. doi: 10.1186/1742-9994-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eaton MD. Human vision fails to distinguish widespread sexual dichromatism among sexually “monochromatic” birds. Proc Natl Acad Sci USA. 2005;102:10942–10946. doi: 10.1073/pnas.0501891102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lotem A, Nakamura H, Zahavi A. Constraints on egg discrimination and cuckoo-host co-evolution. Anim Behav. 1995;49:1185–1209. [Google Scholar]
- 27.Davies NB, Welbergen JA. Social transmission of a host defense against cuckoo parasitism. Science. 2009;324:1318–1320. doi: 10.1126/science.1172227. [DOI] [PubMed] [Google Scholar]
- 28.Peach WJ, Hanmer DB, Oatley TB. Do Southern African songbirds live longer than their European counterparts? Oikos. 2001;93:235–249. [Google Scholar]
- 29.Osorio D, Jones CD, Vorobyev M. Accurate memory for colour but not pattern contrast in chicks. Curr Biol. 1999;9:199–202. doi: 10.1016/s0960-9822(99)80089-x. [DOI] [PubMed] [Google Scholar]
- 30.Vernon CJ. The breeding of the Cuckoo Weaver Anomalospiza imberbis in southern Rhodesia. Ostrich. 1964;35:260–263. [Google Scholar]
- 31.Stevens M, Cuthill IC. Disruptive coloration, crypsis and edge detection in early visual processing. Proc R Soc Lond B Biol Sci. 2006;273:2141–2147. doi: 10.1098/rspb.2006.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiao C-C, Chubb C, Buresch KC, Siemann L, Hanlon RT. The scaling effects of substrate texture on camouflage patterning in cuttlefish. Vision Res. 2009;49:1647–1656. doi: 10.1016/j.visres.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Campbell FW, Robson JG. Applications of Fourier analysis to the visibility of gratings. J Physiol. 1968;197:551–556. doi: 10.1113/jphysiol.1968.sp008574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Godfrey D, Lythgoe JN, Rumball DA. Zebra stripes and tiger stripes: The spatial frequency distribution of the pattern compared to that of the background is significant in display and crypsis. Biol J Linn Soc. 1987;32:427–433. [Google Scholar]
- 35.Hart NS, Partridge JC, Cuthill IC, Bennett ATD. Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine: The blue tit (Parus caeruleus L.) and the blackbird (Turdus merula L.) J Comp Physiol [A] 2000;186:375–387. doi: 10.1007/s003590050437. [DOI] [PubMed] [Google Scholar]
- 36.Hart NS, Partridge JC, Bennett ATD, Cuthill IC. Visual pigments, cone oil droplets and ocular media in four species of estrildid finch. J Comp Physiol [A] 2000;186:681–694. doi: 10.1007/s003590000121. [DOI] [PubMed] [Google Scholar]
- 37.Ödeen A, Håstad O. Complex distribution of avian color vision systems revealed by sequencing the SWS1 opsin from total DNA. Mol Biol Evol. 2003;20:855–861. doi: 10.1093/molbev/msg108. [DOI] [PubMed] [Google Scholar]
- 38.Martín-Vivaldi M, Soler M, Møller AP. Unrealistically high costs of rejecting artificial model eggs in cuckoo Cuculus canorus hosts. J Avian Biol. 2002;33:295–301. [Google Scholar]
- 39.Stevens M, Winney IS, Cantor A, Graham J. Object outline and surface disruption in animal camouflage. Proc R Soc Lond B Biol Sci. 2009;276:781–786. doi: 10.1098/rspb.2008.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuthill IC, et al. Disruptive coloration and background pattern matching. Nature. 2005;434:72–74. doi: 10.1038/nature03312. [DOI] [PubMed] [Google Scholar]
- 41.Spottiswoode CN, Colebrook-Robjent JFR. Eggshell puncturing by the brood parasitic Greater Honeyguide and potential host counteradaptations. Behav Ecol. 2007;18:792–799. [Google Scholar]
- 42.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 43.Chevan A, Sutherland M. Hierarchical partitioning. Am Stat. 1991;45:90–96. [Google Scholar]