Abstract
Evolutionary loss of traits can result from negative selection on a specific phenotype, or if the trait is selectively neutral, because the phenotype associated with the trait has become redundant. Even essential traits may be lost, however, if the resulting phenotypic deficiencies can be compensated for by the environment or a symbiotic partner. Here we demonstrate that loss of an essential me-tabolic trait in parasitic wasps has evolved through environmental compensation. We tested 24 species for the ability to synthesize lipids de novo and collected additional data from the literature. We found the majority of adult parasitoid species to be incapable of synthesizing lipids, and phylogenetic analyses showed that the evolution of lack of lipogenesis is concurrent with that of parasitism in insects. Exploitive host manipulation, in which the host is forced to synthesize lipids to the benefit of the parasitoid, presumably facilitates loss of lipogenesis through environmental compensation. Lipogenesis re-evolved in a small number of parasitoid species, particularly host generalists. The wide range of host species in which generalists are able to develop may impede effective host manipulation and could have resulted in regaining of lipogenic ability in generalist parasitoids. As trait loss through environmental compensation is unnoticed at the phenotypic level, it may be more common than currently anticipated, especially in species involved in intricate symbiotic relationships with other species.
Keywords: coevolution, environmental compensation, fat reserves, host manipulation, parasitoids
Evolutionary changes are frequently associated with the acquisition of novel traits, but loss of traits can also curb the course of evolution (1, 2). Trait loss can be the result of negative selection for a specific phenotype, for example to reduce costs associated with the trait (3). Alternatively, a trait may become selectively neutral due to ecological or evolutionary shifts which render the phenotype associated with the trait redundant. Well-known examples are the loss of eyes in cave-dwelling organisms (4) and the loss of wing function in birds and insects (5). In these examples, respectively, vision and ability for flight have become redundant, resulting in mutation accumulation and loss of function.
The evolutionary loss of a trait, however, is not inevitably accompanied by a loss of the phenotype associated with that trait. If the loss of function is compensated by environmental or biotic factors, the phenotype will be maintained. For instance, several plant species have lost the ability for photosynthesis (6), although the photosynthetic requirements are met through exploitation of other plants. Similarly, despite the loss of vitamin C production in humans, vitamin C is still an essential vitamin for human health but it is provided through dietary intake (7, 8). Environmental compensation can therefore release traits from selection because the phenotype is not affected by loss of such traits. Moreover, if a trait is energetically costly, environmental compensation can even result in selection against expression of this trait, because reduced expression will save energetic expenditure.
Environmental compensation of trait loss is frequently observed in species that are involved in symbiotic, coevolutionary relationships, such as occurs in instances of mutualism or parasitism. The supply of essential resources by a symbiotic partner makes the production of such resources superfluous in the receiving organism, and renders the genes involved prone to mutation accumulation. As a consequence, coevolution may lead to a loss of genes that is unnoticed at the phenotypic level. To illustrate this point, many obligate endosymbionts such as Buchnera and Baumannia have undergone massive genome reduction, although which genes are lost depends on their specific host (9, 10). Other relevant examples include the parasitic fungi, which have lost several genes involved in metabolism (11). Trait loss or a reduction in trait functioning is only expected to evolve in long-term, stable coevolutionary physiological relationships. However, the relative frequent occurrence of such tight interspecific interactions implies that environmental compensation for trait loss may be much more prevalent than currently appreciated (12–14).
Here we unravel a case of parallel evolution of compensated trait loss in insect parasitoids. The exceptional lifestyle of parasitoids provides unique opportunities to study environmental compensation for trait loss (15). Parasitoids develop in or on arthropod hosts during the larval stage; the larvae are therefore completely dependent on their host for nutrient acquisition (16). Various ways have evolved in which parasitoid larvae can manipulate their host's physiology to increase nutrient availability, including host exploitation for lipids (17, 18). For example, parasitism by the hymenopteran Euplectrus separatae results in a release of fat particles from the host's fat body and an increase in hemolymph free fatty acids of the host (19). Direct uptake of lipids from the host tissue is highly advantageous for parasitoid larvae because they can avoid substantial metabolic costs that are associated with lipogenesis (20). In addition, it has been shown that some parasitoid species are unable to accumulate lipids as adults due to the absence of de novo lipid synthesis (21, 22), even under conditions that would induce enhanced lipogenesis in other animals (23).
We propose that the loss of lipogenesis is an evolutionary consequence of the parasitoid lifestyle, because parasitism facilitates redundancy of traits that are involved in lipid production. So far, lack of adult lipid accumulation has been found in two parasitic dipterans and nine closely related parasitic hymenopterans (22). To demonstrate phylogenetic congruence between loss of lipogenesis and a parasitoid lifestyle, data are needed from multiple independent phylogenetic groups. We used a two-pronged approach to obtain these data. First, we performed an exhaustive survey of the literature and acquired data on lipogenic ability of 70 species. Second, we tested an additional 24 species for their lipogenic ability by conducting feeding experiments and physiological measurements. The final dataset included almost 30 parasitoid species from three different orders, which enabled us to answer the question of whether the evolution of parasitism results in loss of lipogenic ability in insects. Furthermore, we tested for correlated evolution between lipogenic ability and key parasitoid traits associated with the parasitoid lifestyle.
Results
Feeding Experiments and Lipogenic Ability.
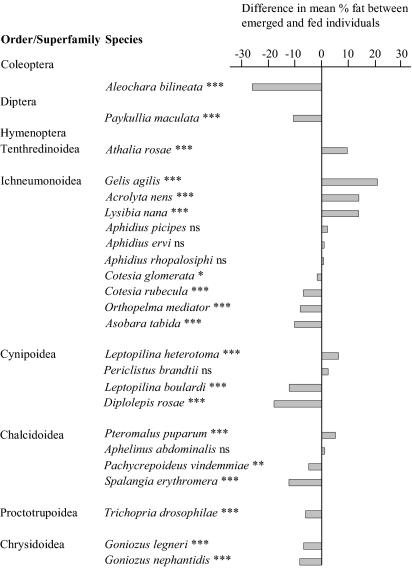
The lipogenic ability of species was determined by comparing the lipid levels of individuals at emergence and after several days of feeding. Of the 24 species tested, 18 were demonstrated to lack lipid accumulation in a situation of excess food. The staphylinid beetle Aleochara bilineata (Coleoptera) and the rhinophorid fly Paykullia maculata (Diptera) showed a significant decrease in lipid levels between newly emerged individuals and fully fed individuals, which is in-dicative of lack of lipogenesis (Fig. 1 and Table S1). Furthermore, nearly all hymenopteran parasitoids tested lacked lipid accumulation. Individuals in the feeding treatments either had decreased lipid levels or failed to increase their lipid levels, despite the fact that they had access to a surplus of carbohydrates. Only five parasitoid species proved an exception to this pattern: Gelis agilis, Lysibia nana, Acrolyta nens, Pteromalus puparum, and Leptopilina heterotoma were shown to significantly increase their lipid levels during their lifetimes when fed. The only nonparasitoid species in our feeding experiment was the symphytan Athalia rosae, belonging to the most basal lineage within Hymenoptera. This species significantly increased its lipid levels when fed (Fig. 1 and Table S1).
Fig. 1.
Difference in mean lipid content (%) between emerged and fed individuals as measured in the feeding experiments. Asterisks indicate significant differences between emerged and fed individuals: *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant. Sample sizes for each treatment and species are listed in Table S1.
Evolution of Lack of Lipid Accumulation in Relation to Parasitism.
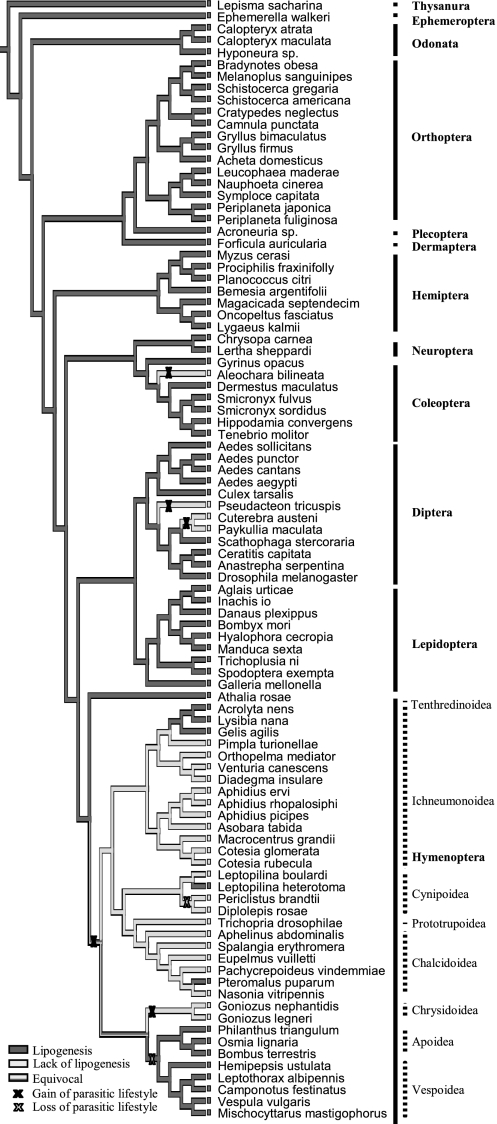
Our literature survey and feeding experiments resulted in data on the lipogenic ability of 94 species. Twenty-six species exhibited a lack of lipid accumulation, including 1 coleopteran parasitoid, 3 dipteran parasitoids, and 20 hymenopteran parasitoids. All other, nonparasitoid hymenopteran species showed an increase in lipid levels when fed, with the exception of the gall wasp Diplolepis rosae and its inquiline Periclistus brandtii (Fig. 1 and Table S1). Phylogenetic analysis of lipogenic ability is complicated by uncertainties in the hymenopteran phylogeny (24, 25). We therefore consider three possible consensus trees, but the conclusions of our analyses are qualitatively the same for all trees. The parasitoid lifestyle can be inferred to have evolved independently four times and lost twice when lack of lipogenesis is considered the ancestral state at the root of the tree (for the consensus trees in which Ichneumonoidea and Aculeata, as well as Proctotrupomorpha and Aculeata, are considered sister groups). The evolution of lack of lipid accumulation is concurrent with that of parasitism at least one time in Coleoptera, two times in Diptera, and one time in Hymenoptera, demonstrating that lack of lipid accumulation has evolved four times independently in parasitoid lineages throughout the insects. Lack of lipogenesis is significantly more likely to originate on parasitic than on nonparasitic branches in the phylogeny (concentrated changes test, P < 0.001). One of the consensus trees, in which Ichneumonoidea and Proctotrupomorpha are considered sister groups, shows the ancestral state at the root of the hymenopteran tree to be equivocal. The ancestral state could be either nonparasitic or parasitic and lipogenic ability could be absent or present (Fig. 2). We compared all possible combinations of character evolution and found lack of lipogenesis to have evolved concurrently with parasitism three, four, or five times. Each comparison showed concurrent evolution between lack of lipogenesis and parasitism to be significant (concentrated changes test, P < 0.05 for each comparison using Benjamini and Hochberg's (26) false discovery rate as a correction for multiple testing).
Fig. 2.
Phylogeny based on morphological and molecular data, showing inferred gains ( ) and losses (
) and losses ( ) in parasitic lifestyle. Character tracing for lipogenic ability is shown, in which dark gray branches refer to an ability to accumulate lipids, whereas light gray branches refer to a lack of lipid accumulation in adults. In this phylogeny, Ichneumonoidea and Proctotrupomorpha are considered sister groups.
) in parasitic lifestyle. Character tracing for lipogenic ability is shown, in which dark gray branches refer to an ability to accumulate lipids, whereas light gray branches refer to a lack of lipid accumulation in adults. In this phylogeny, Ichneumonoidea and Proctotrupomorpha are considered sister groups.
Evolution of Lipogenesis in Relation to Parasitoid Traits.
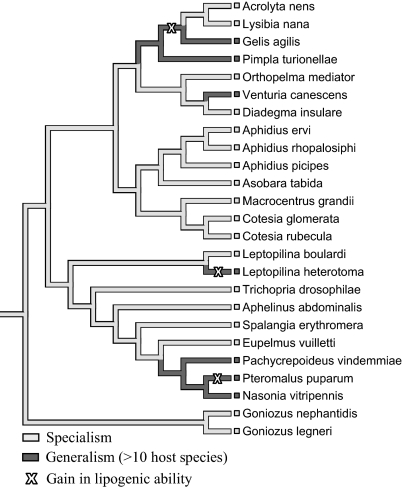
Not all parasitoid species showed a lack of lipogenic ability. Within the parasitic Hymenoptera, five parasitoid species were found to be capable of lipogenesis (Fig. 1 and Table S1), showing an exception to the general pattern. Phylogenetic reconstruction of lipogenic ability in insects suggested the ability for lipogenesis in these five species to be a secondarily derived character rather than an ancestral trait, suggesting they have regained lipogenic ability (Fig. 2). To identify possible selection pressures that may have caused a reversal to lipogenic ability, we analyzed the conditional probability of reversed lipogenesis for several parasitoid traits. For each of the consensus trees, as shown in Table 1, no significant relationship was found with developmental characters (mode of parasitism, host stage attacked, host developmental arrest, and facultative hyperparasitism), nor with adult diet (host-feeding behavior) when lack of lipogenesis is the ancestral state at the root of the tree. Reversals from lack of lipogenesis to lipogenesis are not significantly associated with these parasitoid traits. When Ichneumonoidea and Proctotrupomorpha are considered sister groups, lipogenesis could have been the ancestral state at the root of the tree. In that case, similar results are obtained, except for pupal host stage attacked and facultative hyperparasitism, which are significantly correlated with the ability for lipogenesis (Table 1). In contrast, reversals to lipogenesis were concentrated to lineages with a generalist host species range, that is, which attacked 10 or more host species (Fig. 3) when either lack of lipogenesis or lipogenesis are considered the ancestral state. Parasitoids attacking many different host species seem to be capable of lipogenesis, as opposed to more specialist parasitoids, attacking relatively few hosts that lack lipogenesis. In addition to defining generalists by the number of host species they parasitize, generalists can also be defined by the number of host families they attack (i.e., 5 or more host families). We found a similar correlation between species adopting a wide host family range and lipogenesis (Table 1) when lipogenesis is considered ancestral. However, when lack of lipogenesis is considered the ancestral state, reversals to lipogenesis are not significantly concentrated to hymenopteran parasitoid lineages adopting a wide host family range.
Table 1.
Results of concentrated changes tests, to test for correlated evolution between parasitoid traits and lipogenic ability in hymenopteran parasitoids in one of the consensus trees
| Ichneumonoidea and Proctotrupomorpha as sister groups |
|||||
| Lack of lipogenesis ancestral |
Lipogenesis ancestral |
||||
| Traits | Gains* | P value | Gains* | Losses* | P value |
| Developmental characters | |||||
| Ectoparasitic | 2 | 0.295 | 2 | 1 | 0.138 |
| Pupal host stage attacked | 2 | 0.207 | 2 | 0 | 0.027† |
| Idiobiont | 2 | 0.404 | 2 | 2 (or 1) | 0.270 (0.175) |
| Hyper and facultative hyper | 2 | 0.097 | 2 | 0 | 0.015† |
| Host range | |||||
| Generalism (>5 host families) | 2 | 0.068 | 2 | 0 | 0.012† |
| Generalism (>10 species) | 3 | 0.005† | 3 | 0 | <0.001† |
| Adult feeding | |||||
| Non-host-feeding | 2 | 0.537 | 2 | 0 | 0.066 |
| Host-feeding | 2 | 0.366 | 2 | 2 | 0.412 |
Results for the other two consensus trees are in agreement with the results obtained if lack of lipogenesis is ancestral.
*Number of gains and losses occurring on branches with aforementioned trait state.
†Significant differences after correction for multiple testing using Benjamini and Hochberg's false discovery rate.
Fig. 3.
Phylogeny showing inferred character states for host range (specialist/generalist) for hymenopteran parasitoids, in which gains ( ) in lipogenic ability are shown. In this phylogeny, Ichneumonoidea and Proctotrupomorpha are regarded as sister groups.
) in lipogenic ability are shown. In this phylogeny, Ichneumonoidea and Proctotrupomorpha are regarded as sister groups.
Discussion
Our phylogenetic study has yielded two key results. The first result is the evidence of a concurrent loss of lipogenesis with the evolution of parasitism in insects. Lack of lipogenesis has evolved repeatedly and independently in four insect lineages with a parasitic lifestyle. The notion that lack of lipid accumulation is restricted to parasitic species is substantiated by the presence of full lipogenic abilities in Symphyta, a sister group of Hymenoptera, and nonparasitic aculeates. Parallel evolution of compensated trait loss in insect parasitoids is possibly produced by the tight interspecific interactions between host and parasitoid and, to hypothesize, by the physiological manipulation that allows loss of lipogenic function without burdening the energetic state of the parasitoid larvae or emerging adults. The second key result reveals that lipogenic ability can re-evolve in species adopting large host ranges, that is, host species generalists. Insufficient host specialization prevents the fine-tuned physiological matching that is needed for host manipulation and optimal exploitation of the host, thus requiring the need to synthesize lipids.
Lack of Lipogenic Ability and Adult Performance.
As a preface to discussing the adaptive evolution of lack of lipogenesis, we start by considering alternative explanations for the lack of lipid accumulation in well-fed parasitoids. Lipid synthesis is generally regarded as an involuntary dose-dependent physiological process when there is a surplus of carbohydrates available in the diet (20). Nevertheless, the net increase in lipid reserves hinges on the rate at which lipids are metabolized. If lipids are broken down at an equal or higher rate than they are produced, then the effect on lipid accumulation would be similar to the pattern observed in our feeding experiments. Even though we deem such a scenario unlikely due to the low energetic efficiency this would entail, we cannot exclude this possibility because we did not measure the rate of lipogenesis and lipid breakdown directly. Our case is corroborated, however, by a radiotracer study in the parasitoid Eupelmus vuilletti which showed that ingestion of radioactively labeled glucose was not incorporated into lipid reserves, excluding the possibility of de novo lipogenesis (21). Additional studies on other parasitoid species that lack adult lipid accumulation are needed to verify the generality of this finding.
Another assumption that requires consideration is the ability of species to ingest carbohydrate-rich food sources. Evolutionary reduction of the mouthparts or digestive organs limits nutrient uptake and hence restrains lipid accumulation. The species used in the feeding experiments have been shown to use the source of carbohydrates presented (SI Text), with the exception of the gall wasp Diplolepis rosae (27) and the parasitic fly Cuterebra austeni (28), which possess fully developed mouthparts but have not been shown to ingest food. From these findings, we conclude that the lack of lipid accumulation can clearly occur with full feeding abilities.
Lack of lipogenic ability in adults may appear to be highly disadvantageous, hence challenging the assumption of selective neutrality that is required for the evolution of trait loss. However, the necessity of acquiring additional lipid reserves as adults can be evaded if sufficient resources are carried over from the larval stage to sustain lipid use during adult life (29). From this perspective, additional lipid synthesis as an adult is even unwanted because the production of lipids from carbohydrates is less efficient than the direct metabolism of carbohydrates, due to energetic conversion costs. Indeed, for some species that lack lipogenesis, capital lipid reserves can mount to more than 30%. Parasitoids can also economize on lipid use if acute energy requirements can be met by regular ingestion of sugars through feeding on nectar, honeydew, or carbohydrate-rich oviposition substrates (30). For example, in the parasitoid Venturia canescens, nectar feeding occurs frequently in the field, postponing the moment of lipid depletion despite relatively low capital lipid levels (31).
Adult females also face a high demand for lipids from egg production (32); hence we expected a lack of lipogenesis to favor pro-ovigenic reproductive strategies, in which a large complement of eggs is already mature at emergence (16, 33). Alternatively, host feeding may have evolved to provide an income resource of lipids for egg maturation (34), as it is known that host hemolymph contains nontrivial, albeit low, amounts of lipids (35). However, our results showed that neither of these reproductive traits was significantly associated with lipogenic ability. It has been suggested that most parasitoids are time-limited, that is, energy resources are depleted before all eggs have been deposited (36, 37). Presumably, such allocation strategies helped parasitoids to overcome the constraints of lacking lipogenic ability as adults.
Larval Exploitation and Host Range.
Adaptation by parasitoids to their host has resulted in highly specific mechanisms to increase lipid levels and optimize host exploitation (19, 38, 39). In agreement with the specialized nature of host–parasitoid interactions, our phylogenetic analysis indicates that breadth of host range is a key trait in the evolution of lipogenic abilities. Lack of lipogenesis is predominantly found in specialist parasitoids, whereas parasitoids adopting large host ranges have re-evolved lipogenic ability. Larval lipogenesis may no longer be redundant in these generalist species because the physiological manipulation of different host species is expected to be problematic, leading to poor host exploitation and lower capital lipid reserves in adults.
Nonetheless, some generalist parasitoids were found incapable of synthesizing lipids as adults, such as Nasonia vitripennis and Pachycrepoideus vindemmiae. In N. vitripennis, it has been shown that although this species can develop on over 60 different host species, it has a preference for sarcophagous flies (40). From this perspective, it is a specialist because the injection of venom during oviposition can only increase lipid levels in Sarcophaga species (38). A certain level of specialism, in which parasitoids have adapted to specific hosts to manipulate their physiology, is thus required to obtain sufficient resources during development.
It is this unique ability to manipulate their hosts that distinguishes parasitoids from predacious species, and confines the evolution of lack of lipogenesis to parasitoids. Predators can maximize energetic gain by increasing the number of prey or selecting more nutritious prey, but they are unable to attain higher resource availability once a prey has been captured. A special case of host manipulation and redundancy of lipogenesis may be found in gall wasps. Gall wasps have evolved phytophagy secondarily within Hymenoptera (41, 42). Similar to parasitoid–host interactions, they are able to manipulate their host plant's physiology to increase lipid levels in cells surrounding the developing larva (43). Indeed, these species also have intimate physiological relationships with their host plants, and these intricate coevolutionary relationships have thus resulted in loss of lipid accumulation as adults.
Toward Further Understanding of the Evolutionary Loss of Traits.
The evolutionary loss of lipogenesis in parasitic insects is one of few examples of repeated trait loss through environmental compensation (6–8). This study demonstrates that compensated trait loss of physiological traits can be masked by parasitoid–host interactions. We found that species with superficially similar developmental strategies can vary considerably in physiological dependence on their host. Closer examination of other stable coevolutionary relationships can be expected to yield more such examples.
Parallel evolution of traits might be the result of quite different underlying genetic mechanisms. For example, similar coat patterns and pigmentation in mice species have been shown to have evolved through different genetic mechanisms (44). Similar to the evolution of novel traits, parallel trait loss can also be expected to occur through various genetic mechanisms. Further genome analysis is needed to assess whether specific genes have been preferred targets of mutation accumulation. A prime candidate to explain lack of lipogenesis would be fatty acid synthase (FAS), which has a central role in the production of lipids. FAS has previously been documented to be absent in the genome of a parasitic fungus that is dependent on external lipids for growth (11). Recent completion of the genome of the parasitoid N. vitripennis (45) has revealed a homolog to the FAS gene of Drosophila, although its functionality remains to be tested.
A fundamental question regarding the evolutionary loss of traits is whether physiological abilities that have been lost can be regained. Indeed, a striking finding is that lipogenesis has re-evolved three times within Hymenoptera, a fact which challenges several widespread views regarding trait loss. Trait loss is usually thought to involve one-way directional evolution, in which a lost trait does not re-evolve (46). However, exceptions to this general rule have been found (47, 48), and lipogenic ability may be one of them. We propose that after the loss of lipogenic ability much of the lipogenic pathway may remain intact, perhaps due to pleiotropic effects on other physiological pathways. Conversion of carbohydrates to triglycerides employs key enzymes that are functional in other physiological processes, such as pyruvate metabolism, the citrate cycle, and biosynthesis of secondary metabolites (49). The lipogenesis pathway may be protected from severe degradation due to shared components with essential metabolic pathways, hence enabling a fully functional lipid synthesis to re-evolve. It is essential to identify the underlying genetic and physiological mechanisms involved to understand the evolutionary dynamics of the loss of this trait.
Materials and Methods
Insects.
All insects were obtained from existing laboratory cultures or field collections, either as larvae and pupae or inside their host. The following species were obtained from existing cultures: Cotesia glomerata, P. puparum, A. nens, L. nana, and G. agilis (NIOO-KNAW, The Netherlands), Cotesia rubecula (University of Wageningen, The Netherlands), Leptopilina boulardi (University of Leiden, The Netherlands), Aphidius ervi and Aphelinus abdominalis (Koppert BV, The Netherlands), A. rosae (University of Bielefeld, Germany), P. vindemmiae (University of Lyon 1, France), A. bilineata, Aphidius rhopalosiphi, Aphidius picipes, Leptopilina heterotoma, Asobara tabida, Spalangia erythromera, and Trichopria drosophilae (University of Rennes 1, France), and Goniozus nephantidis and Goniozus legneri (University of Nottingham, United Kingdom). Galls containing D. rosae, Orthopelma mediator, and P. brandtii were collected at several sites near Wassenaar (The Netherlands) in September and October 2007. Porcellio scaber, hosts of P. maculata, were collected at several sites in the provinces of North and South Holland (The Netherlands) during January, February, and March 2008. All insects were kept at 20 °C, relative humidity (RH) 75%, and L:D 12:12, except A. ervi and P. puparum, which were kept at RH 50%, and C. glomerata, G. agilis, L. nana, A. nens, L. boulardi, P. vindemmiae, and C. rubecula, which were kept at 23 °C, RH 75%, and L:D 12:12. Galls containing D. rosae, O. mediator, and P. brandtii were kept subsequently at 20 °C, 10 °C, 5 °C, 10 °C, and 20° C until emergence to mimic temperature conditions in their natural environment. Vials and galls were inspected daily for newly emerged females.
Feeding Experiments.
To determine lipogenic ability, newly emerged females were randomly assigned to two treatments: emergence, in which females were frozen directly after emergence, or fed, in which individuals were allowed to feed on honey ad libitum for approximately half their average lifespan. During the experiments only adult females were used and were provided with water on cotton wool in addition to honey. An exception is A. bilineata, for which sex could not be determined and both sexes were used in experiments. A. bilineata was, furthermore, allowed to feed on Drosophila food medium, as this species is predacious rather than nectivorous. Medium consisted of 20 g agar, 50 g saccharose, 35 g yeast, 9 g kalmus (10 parts acidum tartaricum, 4 parts ammonium sulfate, 3 parts potassium phosphate, and 1 part magnesium sulfate), and 10 mL nipagin (100 g 4-methyl hydroxyl benzoate per L of ethanol) per L of water. At the end of the experiment, females were frozen at −20 °C until further processing.
Fat content was determined based on the method by David et al. (50). Whole insects were freeze-dried for 2 days, after which dry weight was determined. Individuals were subsequently placed in a glass tube containing 4 mL of ether. After 24 h, the ether was removed and insects were washed with fresh ether. Insects were freeze-dried for 2 days after ether extraction and dry weight was determined again. For A. bilineata, P. maculata, D. rosae, O. mediator, P. brandtii, and A. rosae, freeze-drying was extended to 4 days and the quantity of ether was 8 mL because of their increased cuticle toughness and larger size. Fat content was calculated by subtracting dry weight after ether extraction from dry weight before ether extraction, and then converted to the percentage of lipids to correct for differences in body size among individuals.
Reconstruction of Phylogenetic Relationships.
Relationships between insect orders within the Hemimetabola are based on morphological data according to Kristensen (51), and the phylogeny of the endopterygotes was completed with molecular data on relationships between Holometabola, as reported in Castro and Dowton (52). New insights suggest an alternative placement of Hymenoptera within the Holometabola, but vary considerably between studies (53–55). The phylogeny used in this study has been constructed with regard to the more conventional placement of Hymenoptera as a sister group of Mecopterida (Diptera + Lepidoptera). However, alternative placement of Hymenoptera does not affect our findings. Inference of phylogenetic relationships at the superfamily, family, and genus level has been made using available morphological and molecular data (SI Text). The enormous diversity of hymenopteran parasitoids poses difficulties in determining phylogenetic relationships, mainly at the superfamily level. Consensus between phylogenies based on morphology and molecular data has not yet been reached satisfyingly, and relationships among hymenopteran superfamilies are thus regarded as polytomous in this study. Within Hymenoptera, data on lipogenic ability have been obtained for parasitoids of the superfamilies Proctotrupoidea, Cynipoidea and Chalcidoidea (Proctotrupomorpha), and Ichneumonoidea and Chrysidoidea (Aculeata) (22, 56, 57). As the analysis does not allow polytomies, three alternative consensus trees were used for phylogenetic testing within Hymenoptera. The three trees assumed Ichneumonoidea and Aculeata, Proctotrupomorpha and Aculeata, or Ichneumonoidea and Proctotrupomorpha to be sister groups, respectively.
Statistical Analysis.
Scatterplots and regression lines of dry weight before ether extraction compared with dry weight after ether extraction were used to find potential outliers in the data. Outliers were only removed if they deviated from a 99% confidence interval of the linear regression line. Normality of the data was determined by looking at the error structure of the residuals of the data. Nonnormal data were transformed to normality using a log or square-root transformation. Treatments showing normally distributed data were compared using independent sample t test if variances were equal and Welch's t test if variances were unequal. If data were not normally distributed, the nonparametric Kruskal–Wallis test was used. Statistical ana-lyses were done using R project Version 2.9 (The R Foundation).
Phylogenetic tests were performed with each of the three consensus trees using all 94 insect species as a target clade. Lipogenic ability and parasitic lifestyle were scored as presence/absence characters. To test for correlations between parasitic lifestyle (independent character) and lipogenic ability (dependent character), ancestral states were reconstructed using parsimony for the three consensus trees. Concentrated changes tests (58) using minstate and maxstate simulations with a sample size of 10,000 were carried out using MacClade Version 4.08 (59).
Correlated evolution between parasitoid traits (independent characters) and lipogenic ability (dependent character) was tested using a phylogeny consisting only of parasitic Hymenoptera. Data on the presence or absence of parasitoid traits were obtained from the literature and each character was scored binomially, that is, ectoparasitoid or endoparasitoid (0/1), idiobiont or koinobiont (0/1), other host stage attacked or larval host stage attacked (0/1), pupal host stage attacked or other host stage attacked (0/1), other host stage attacked or adult host stage attacked (0/1), solitary or gregarious (0/1), primary or hyper and facultative hyperparasitoid (0/1), non-host-feeding or host-feeding (0/1), pro-ovigenic or synovigenic (0/1), and specialist or generalist (0/1). As clear definitions of generalism and specialism are lacking for hymenopteran parasitoids, we used two criteria based on Stireman (60): The first criterion assumes generalists to attack 5 or more host families and specialists 4 or fewer host families. The second criterion assumes generalists to attack 10 or more host species and specialists 9 or fewer host species. When reconstructing generalism and specialism, the ancestral state of the clade comprising A. nens, L. nana, G. agilis, and P. turionellae was equivocal. This has been resolved to generalism being the ancestral state of this clade, as the majority of species within the subfamily Cryptinae are known to be generalists (61, 62). Ancestral states were reconstructed using parsimony and analyses were carried out using concentrated changes tests with exact count. For several trait states the sample sizes were low. A power analysis showed that even if maximal concurrence between trait state and lipogenic ability was assumed, a lack of significance was found; hence, for these traits, the power of the analyses was too low and they were excluded from the analyses. These trait states included endoparasitism, koinobiosis, larval host stage attacked, solitary, primary, synovigenic, and specialism. Concentrated changes tests were not performed if fewer than two gains occurred on branches of the trait state in question, which was the case for adult host stage attacked and gregarious and pro-ovigenic trait states.
Supplementary Material
Acknowledgments
We thank Caroline Müller, Anne-Marie Cortesero, Ian Hardy, Femmie Kraaijeveld, Roland Allemand, and Hans Smid for providing us with insects. Eric Kok, Mirte Fritz, and Louis Boumans we would like to thank for their efforts in obtaining exceptionally large amounts of rose galls and woodlice necessary to provide sufficient material for testing. We are grateful to Donald Quicke and Mark Dowton for their information on hymenopteran phylogenetic relationships, Dan Hahn for his help in finding laboratory cultures of parasitoids, and two anonymous referees for their comments on the earlier draft of the manuscript. B.V. was funded by Netherlands Organisation for Scientific Research (NWO) ALW Grant 816.01.013.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1001744107/-/DCSupplemental.
References
- 1.Fong DW, Kane TC, Culver DC. Vestigialization and loss of nonfunctional characters. Annu Rev Ecol Syst. 1995;26:249–268. [Google Scholar]
- 2.Porter ML, Crandall KA. Lost along the way: The significance of evolution in reverse. Trends Ecol Evol. 2003;18:541–547. [Google Scholar]
- 3.Lahti DC, et al. Relaxed selection in the wild. Trends Ecol Evol. 2009;24:487–496. doi: 10.1016/j.tree.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Poulson TL, White WB. Cave environment. Science. 1969;165:971–981. doi: 10.1126/science.165.3897.971. [DOI] [PubMed] [Google Scholar]
- 5.Roff DA. The evolution of flightlessness: Is history important? Evol Ecol. 1994;8:639–657. [Google Scholar]
- 6.Krause K. From chloroplasts to “cryptic” plastids: Evolution of plastid genomes in parasitic plants. Curr Genet. 2008;54:111–121. doi: 10.1007/s00294-008-0208-8. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee IB. Evolution and biosynthesis of ascorbic acid. Science. 1973;182:1271–1272. doi: 10.1126/science.182.4118.1271. [DOI] [PubMed] [Google Scholar]
- 8.Ohta Y, Nishikimi M. Random nucleotide substitutions in primate nonfunctional gene for L-gulono-γ-lactone oxidase, the missing enzyme in L-ascorbic acid biosynthesis. Biochim Biophys Acta. 1999;1472:408–411. doi: 10.1016/s0304-4165(99)00123-3. [DOI] [PubMed] [Google Scholar]
- 9.Dale C, Moran NA. Molecular interactions between bacterial symbionts and their hosts. Cell. 2006;126:453–465. doi: 10.1016/j.cell.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Wu D, et al. Metabolic complementarity and genomics of the dual bacterial symbiosis of sharpshooters. PLoS Biol. 2006;4:1079–1092. doi: 10.1371/journal.pbio.0040188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J, et al. Dandruff-associated Malassezia genomes reveal convergent and divergent virulence traits shared with plant and human fungal pathogens. Proc Natl Acad Sci USA. 2007;104:18730–18735. doi: 10.1073/pnas.0706756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cairney JWG. Evolution of mycorrhiza systems. Naturwissenschaften. 2000;87:467–475. doi: 10.1007/s001140050762. [DOI] [PubMed] [Google Scholar]
- 13.Houck MA, OConnor BM. Ecological and evolutionary significance of phoresy in the Astigmata. Annu Rev Entomol. 1991;36:611–636. [Google Scholar]
- 14.Stewart FJ, Cavanaugh CM. Symbiosis of thioautotrophic bacteria with Riftia pachyptila. Prog Mol Subcell Biol. 2006;41:197–225. doi: 10.1007/3-540-28221-1_10. [DOI] [PubMed] [Google Scholar]
- 15.Godfray HCJ. Parasitoids: Behavioural and Evolutionary Ecology. Princeton, NJ: Princeton Univ Press; 1994. [Google Scholar]
- 16.Jervis MA, Ellers J, Harvey JA. Resource acquisition, allocation, and utilization in parasitoid reproductive strategies. Annu Rev Entomol. 2008;53:361–385. doi: 10.1146/annurev.ento.53.103106.093433. [DOI] [PubMed] [Google Scholar]
- 17.Rivers DB, Denlinger DL. Redirection of metabolism in the flesh fly, Sarcophaga bullata, following envenomation by the ectoparasitoid Nasonia vitripennis and correlation of metabolic effects with the diapause status of the host. J Insect Physiol. 1994;40:207–215. [Google Scholar]
- 18.Vinson SB, Iwantsch GF. Host regulation by insect parasitoids. Q Rev Biol. 1980;55:143–165. [Google Scholar]
- 19.Nakamatsu Y, Tanaka T. Venom of Euplectrus separatae causes hyperlipidemia by lysis of host fat body cells. J Insect Physiol. 2004;50:267–275. doi: 10.1016/j.jinsphys.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Garrett R, Grisham C. Biochemistry. Orlando, FL: Saunders; 1999. [Google Scholar]
- 21.Giron D, Casas J. Lipogenesis in an adult parasitic wasp. J Insect Physiol. 2003;49:141–147. doi: 10.1016/s0022-1910(02)00258-5. [DOI] [PubMed] [Google Scholar]
- 22.Visser B, Ellers J. Lack of lipogenesis in parasitoids: A review of physiological mechanisms and evolutionary implications. J Insect Physiol. 2008;54:1315–1322. doi: 10.1016/j.jinsphys.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Zinke I, Schutz CS, Katzenberger JD, Bauer M, Pankratz MJ. Nutrient control of gene expression in Drosophila: Microarray analysis of starvation and sugar-dependent response. EMBO J. 2002;21:6162–6173. doi: 10.1093/emboj/cdf600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dowton M, Austin AD. Simultaneous analysis of 16S, 28S, COI and morphology in the Hymenoptera: Apocrita-evolutionary transitions among parasitic wasps. Biol J Linn Soc Lond. 2001;74:87–111. [Google Scholar]
- 25.Sharkey MJ. Phylogeny and classification of Hymenoptera. In: Zhang ZQ, Shear WA, editors. Linnaeus Tercentenary: Progress in Invertebrate Taxonomy. Vol. 1668. Magnolia Press, Auckland: Zootaxa; 2007. pp. 521–548. [Google Scholar]
- 26.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 27.Randolph S. The Natural History of the Rose Bedeguar Gall and Its Insect Community. Suffolk, UK: The British Plant Gall Society; 2005. [Google Scholar]
- 28.Kemp DJ, Alcock J. Lifetime resource utilization, flight physiology, and the evolution of contest competition in territorial insects. Am Nat. 2003;162:290–301. doi: 10.1086/376890. [DOI] [PubMed] [Google Scholar]
- 29.Colasurdo N, Gelinas Y, Despland E. Larval nutrition affects life history traits in a capital breeding moth. J Exp Biol. 2009;212:1794–1800. doi: 10.1242/jeb.027417. [DOI] [PubMed] [Google Scholar]
- 30.Eijs IEM, Ellers J, Van Duinen GJ. Feeding strategies in drosophilid parasitoids: The impact of natural food resources on energy reserves in females. Ecol Entomol. 1998;23:133–138. [Google Scholar]
- 31.Casas J, et al. Energy dynamics in a parasitoid foraging in the wild. J Anim Ecol. 2003;72:691–697. doi: 10.1046/j.1365-2656.2003.00740.x. [DOI] [PubMed] [Google Scholar]
- 32.Ellers J, van Alphen JJM. Life history evolution in Asobara tabida: Plasticity in allocation of fat reserves to survival and reproduction. J Evol Biol. 1997;10:771–785. [Google Scholar]
- 33.Jervis MA, Ferns PN. The timing of egg maturation in insects: Ovigeny index and initial egg load as measures of fitness and of resource allocation. Oikos. 2004;107:449–460. [Google Scholar]
- 34.Giron D, Pincebourde S, Casas J. Lifetime gains of host-feeding in a synovigenic parasitic wasp. Physiol Entomol. 2004;29:436–442. [Google Scholar]
- 35.Beenakkers AMT, Vanderhorst DJ, Vanmarrewijk WJA. Insect lipids and lipo-proteins, and their role in physiological processes. Prog Lipid Res. 1985;24:19–67. doi: 10.1016/0163-7827(85)90007-4. [DOI] [PubMed] [Google Scholar]
- 36.Ellers J, Sevenster JG, Driessen G. Egg load evolution in parasitoids. Am Nat. 2000;156:650–665. doi: 10.1086/316990. [DOI] [PubMed] [Google Scholar]
- 37.Rosenheim JA. An evolutionary argument for egg limitation. Evolution. 1996;50:2089–2094. doi: 10.1111/j.1558-5646.1996.tb03595.x. [DOI] [PubMed] [Google Scholar]
- 38.Rivers DB, Denlinger DL. Venom-induced alterations in fly lipid-metabolism and its impact on larval development of the ectoparasitoid Nasonia vitripennis (Walker) (Hymenoptera, Pteromalidae) J Invertebr Pathol. 1995;66:104–110. [Google Scholar]
- 39.Suzuki M, Tanaka T. Development of Meteorus pulchricornis and regulation of its noctuid host, Pseudaletia separata. J Insect Physiol. 2007;53:1072–1078. doi: 10.1016/j.jinsphys.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Desjardins CA, Perfectti F, Bartos JD, Enders LS, Werren JH. The genetic basis of interspecies host preference differences in the model parasitoid Nasonia. Heredity. 2010;104:270–277. doi: 10.1038/hdy.2009.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grimaldi D, Engel MS. Evolution of Insects. New York: Cambridge Univ Press; 2005. [Google Scholar]
- 42.Quicke DJL. Parasitic Wasps. London: Chapman & Hall; 1997. [Google Scholar]
- 43.Harper LJ, Schonrogge K, Lim KY, Francis P, Lichtenstein CP. Cynipid galls: Insect-induced modifications of plant development create novel plant organs. Plant Cell Environ. 2004;27:327–335. [Google Scholar]
- 44.Steiner CC, Rompler H, Boettger LM, Schoneberg T, Hoekstra HE. The genetic basis of phenotypic convergence in beach mice: Similar pigment patterns but different genes. Mol Biol Evol. 2009;26:35–45. doi: 10.1093/molbev/msn218. [DOI] [PubMed] [Google Scholar]
- 45.Werren JH, et al. Nasonia Genome Working Group Functional and evolutionary insights from the genomes of three parasitoid Nasonia species. Science. 2010;327:343–348. doi: 10.1126/science.1178028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bull JJ, Charnov EL. On irreversible evolution. Evolution. 1985;39:1149–1155. doi: 10.1111/j.1558-5646.1985.tb00455.x. [DOI] [PubMed] [Google Scholar]
- 47.Cruickshank RH, Paterson AM. The great escape: Do parasites break Dollo's law? Trends Parasitol. 2006;22:509–515. doi: 10.1016/j.pt.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 48.Bely AE, Sikes JM. Latent regeneration abilities persist following recent evolutionary loss in asexual annelids. Proc Natl Acad Sci USA. 2009;107:1464–1469. doi: 10.1073/pnas.0907931107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.David J, Cohet Y, Gouillet P. Physiology of starvation and use of reserves in Drosophila melanogaster adults. (Translated from French.) Arch Zool Exp Gen. 1975;116:579–590. [Google Scholar]
- 51.Kristensen NP. Phylogeny of endopterygote insects, the most successful lineage of living organisms. Eur J Entomol. 1999;96:237–253. [Google Scholar]
- 52.Castro LR, Dowton M. The position of the Hymenoptera within the Holometabola as inferred from the mitochondrial genome of Perga condei (Hymenoptera: Symphyta: Pergidae) Mol Phylogenet Evol. 2005;34:469–479. doi: 10.1016/j.ympev.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Savard J, et al. Phylogenomic analysis reveals bees and wasps (Hymenoptera) at the base of the radiation of Holometabolous insects. Genome Res. 2006;16:1334–1338. doi: 10.1101/gr.5204306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Timmermans MJTN, Roelofs D, Marien J, van Straalen NM. Revealing pancrusta-cean relationships: Phylogenetic analysis of ribosomal protein genes places Collembola (springtails) in a monophyletic Hexapoda and reinforces the discrepancy between mitochondrial and nuclear DNA markers. BMC Evol Biol. 2008;8:83. doi: 10.1186/1471-2148-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wiegmann BM, et al. Single-copy nuclear genes resolve the phylogeny of the holometabolous insects. BMC Biol. 2009;7:34. doi: 10.1186/1741-7007-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buckner JS, Hagen MM. Triacylglycerol and phospholipid fatty acids of the silverleaf whitefly: Composition and biosynthesis. Arch Insect Biochem Physiol. 2003;53:66–79. doi: 10.1002/arch.10086. [DOI] [PubMed] [Google Scholar]
- 57.Cripps C, Blomquist GJ, Derenobales M. De novo biosynthesis of linoleic acid in insects. Biochim Biophys Acta. 1986;876:572–580. [Google Scholar]
- 58.Maddison WP. A method for testing the correlated evolution of two binary characters: Are gains or losses concentrated on certain branches of a phylogenetic tree? Evolution. 1990;44:539–557. doi: 10.1111/j.1558-5646.1990.tb05937.x. [DOI] [PubMed] [Google Scholar]
- 59.Maddison WP, Maddison DR. MacClade 4: Analysis of Phylogeny and Character Evolution. Sunderland, MA: Sinauer Associates; 2003. Version 4.08. [Google Scholar]
- 60.Stireman JO. The evolution of generalization? Parasitoid flies and the perils of inferring host range evolution from phylogenies. J Evol Biol. 2005;18:325–336. doi: 10.1111/j.1420-9101.2004.00850.x. [DOI] [PubMed] [Google Scholar]
- 61.Shwarz M, Shaw MR. Western palearctic Cryptinae (Hymenoptera: Ichneumonidae) in the national museums of Scotland, with nomenclatural changes, taxonomic notes, rearing records and special reference to the British check list. Part 3. Tribe Phygadeuontini, subtribes Chiroticina, Acrolytina, Hemitelina and Gelina (excluding Gelis), with descriptions of new species. Entomol Gaz. 2000;51:147–186. [Google Scholar]
- 62.Laurenne N, Karatolos N, Quicke DLJ. Hammering homoplasy: Multiple gains and losses of vibrational sounding in cryptine wasps (Insecta: Hymenoptera: Ichneumonidae) Biol J Linn Soc Lond. 2009;96:82–102. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.