Abstract
Increasing the reward value of behavioral goals can facilitate cognitive processes required for goal achievement. This facilitation may be accomplished by the dynamic and flexible engagement of cognitive control mechanisms operating in distributed brain regions. It is still not clear, however, what are the characteristics of individuals, situations, and neural activation dynamics that optimize motivation-linked cognitive enhancement. Here we show that highly reward-sensitive individuals exhibited greater improvement of working memory performance in rewarding contexts, but exclusively on trials that were not rewarded. This effect was mediated by a shift in the temporal dynamics of activation within right lateral prefrontal cortex, from a transient to predominantly tonic mode, with an additional anticipatory transient boost. In contexts with intermittent rewards, a strategy of proactive cognitive control may enable globally optimal performance to facilitate reward attainment. Reward-sensitive individuals appear preferentially motivated to adopt this resource-demanding strategy, resulting in paradoxical benefits selectively for nonrewarded events.
Keywords: executive function, personality, working memory, dopamine, mixed blocked/event-related fMRI
In some task situations, successful behavioral performance leads to the potential for a highly rewarding outcome (e.g., gambling games, college entrance exams, sales contests) . When motivational salience is high, the increased value of the behavioral goal to be achieved needs to be translated into an optimal cognitive strategy (1–3). Previous experimental evidence suggests that such a translation does occur, because both cognitive performance and brain activity are enhanced in behavioral situations paired with motivational incentives (e.g., monetary rewards) (4–11). Importantly, these behavioral and neural enhancements have been found to be associated with the potential reward value available on specific trials. However, there is still very little knowledge regarding the specific behavioral situations, neural mechanisms, and individual trait factors that are critical for such enhancement effects.
We have postulated a theoretical framework, known as the Dual Mechanisms of Control (DMC; ref. 12), that distinguishes two cognitive control modes, proactive and reactive (Fig. S1A). The former is characterized by sustained active maintenance and/or anticipatory implementation of behavioral goals in the lateral prefrontal cortex (lPFC) (13, 14), whereas the latter is characterized by transient, bottom-up updating of goal-relevant information within a wider brain network (15). In previous work, we have demonstrated that the DMC model predicts age-related and incentive-dependent shifts in activation dynamics in the lPFC (16–18). However, a limitation of the prior work has been the lack of a conclusive demonstration that experimental and individual differences effects in cognitive control modes are both functionally mediated by a shift in the activation dynamics within lPFC. In the current study, we provide such a demonstration, focusing on the effects of motivational context and reward-related personality traits.
The DMC framework predicts that a proactive strategy of cognitive control implemented in lPFC will be most dominant in individuals and situations characterized by a reward-focused motivational orientation (12, 16). Moreover, a counterintuitive prediction of the framework is that adoption of a proactive control strategy, because it involves preparatory maintenance and updating of task goals, would globally facilitate performance in rewarding motivational contexts, and not just on the particular events that are directly linked to immediate reward.
We tested the predictions of the DMC framework by examining both experimentally manipulated and individual difference effects of reward expectancy on brain activation dynamics during a demanding cognitive task involving working memory. Working memory tasks are widely agreed to involve executive control processes that serve to flexibly update the short-term storage of task-relevant information in accordance with task goals, and drive top-down attentional mechanisms that use such maintained information as a basis for the selection of task-appropriate responses (12, 14, 19, 20). As such, the task allowed us to examine how such control processes might be modulated during reward-focused motivational contexts, as has been demonstrated in previous studies (7, 9, 11).
Human participants (n = 31) performed the working memory task during functional magnetic resonance imaging (fMRI) scanning, in both reward (R+) and nonreward (R-) contexts (Fig. 1A and Fig. S1B; Methods). In the R+ context, monetary bonuses were provided for fast and accurate performance, indicated by postresponse visual feedback. Further, within the R+ context, individual trials randomly varied in reward value (high, low, or none), and participants were informed of this value before each trial onset. We then identified brain regions that were sensitive to the reward manipulations, predicting that the R+ context would be associated with a shift toward proactive control, and marked by a particular neural signature of increased sustained, anticipatory maintenance. Then, we examined effects of individual differences in trait sensitivity to reward between participants (21, 22), using a composite index derived from standard personality assessments (23–25). We predicted that variability in such traits would additionally modulate brain activation dynamics and performance.
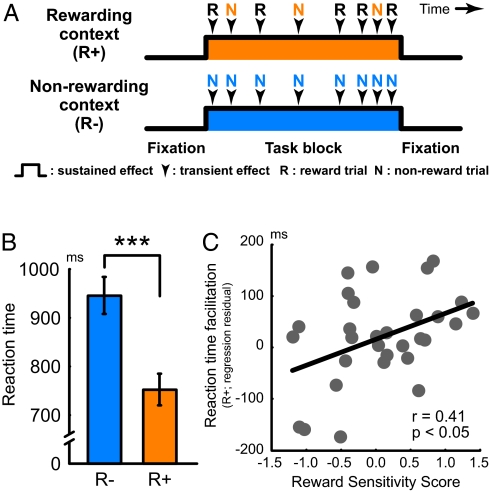
Fig. 1.
Experimental design, behavioral, and individual difference effects. (A) A mixed blocked/event-related fMRI design enabled dissociation of transient/sustained activity dynamics across motivational contexts. In the reward block (R+; orange), reward trials (R) and nonreward trials (N) were pseudorandomly intermixed, whereas only nonreward trials were presented in the nonreward block (R-; blue). For transient (trial-by-trial) effects, only nonreward trials were analyzed (orange “N” vs. blue “N”) to examine the impact of motivational context on otherwise identically matched trials. The sustained (block-wise) effect (orange rectangle vs. blue rectangle) isolated persistently increased activation during task blocks, independent of trial-related effects. (B) Reaction times were faster in the R+ context than the R- context. ***, P < 0.001. (C) The RT effect was plotted against a personality trait score for reward sensitivity (z score normalized composite index). The vertical axis indicates the RT facilitation in R+ context with the RTs in R- context partialled out, and the horizontal axis indicates the reward sensitivity score. The contextual RT facilitation of incentive was greater in individuals with higher reward sensitivity score.
Results
Behavioral Results.
Reaction times (RT) showed the expected trial-by-trial enhancement associated with increased reward values (F(1,30) = 24.4, P < 0.001; Fig. S2A; SI Methods and SI Results), suggesting that cognitive performance is modulated by potential rewards, and consistent with previous findings of motivational performance enhancement during working memory (9, 11). Moreover, as shown in Fig. 1B, there was an additional performance enhancement due to motivational context: RT on nonreward trials in the R+ context were >20% faster (≈200 msec) than those in the R- context [t(30) = 9.0, P < 0.001]. The magnitude of this contextual enhancement also showed a significant positive correlation with the trait reward-sensitivity of participants (ρ = 0.41, P < 0.05; Fig. 1C; SI Methods), whereas the trial-related effects of reward value within the R+ context did not (P > 0.23; Fig. S2 B and C; SI Results). These correlation results suggest that highly reward-sensitive individuals showed greater performance enhancement, but specifically due to the motivational context, rather than to the trials with the highest reward value. Accuracy was at ceiling, and so it was not affected by experimental manipulations or individual differences (SI Results).
Imaging Results.
Examination of brain activity dynamics provided more direct support for the DMC predictions. One of the central predictions is that a shift toward proactive control in the R+ context should be reflected as an increase in sustained activity, and a corresponding decrease in reactive-type transient activity, indicating the reduced need for control engagement during the probe and response period. We first identified brain regions exhibiting context-related changes in both transient and sustained activity, but importantly, without assumptions regarding the direction of effects (SI Methods). The event-related analysis focused only on nonreward trials because these were matched across both contexts. Across the whole brain, two regions in the right hemisphere were identified, lPFC [Brodmann area (BA) 46/9 (41, 21, 28)] and posterior parietal cortex [BA 40/7; (39, −51, 47)] (Fig. 2A and Table S1). Although a conjunction approach was used, each contrast was individually significant at P < 0.05, corrected for multiple comparisons in the whole brain (ref. 26; SI Methods and SI Results). Based on our a priori theoretical hypotheses, related to the DMC framework, of lPFC involvement in proactive and reactive control, our subsequent analyses focused on the lPFC region of interest (ROI). Nevertheless, supplemental exploratory analyses were also conducted on the posterior parietal region. None of the brain-behavior and personality effects described below were significant in the parietal ROI (SI Results).
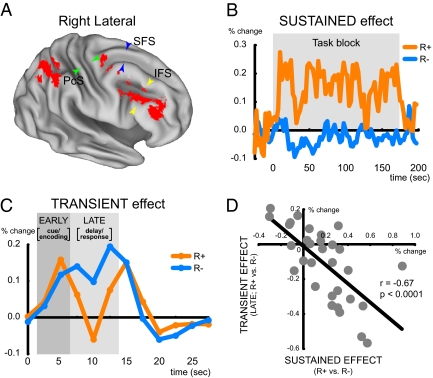
Fig. 2.
Localization and dynamics of motivational context effects on brain activity. (A) Brain regions showing shifts in activity dynamics between the R+ and R- context were colored in red, projected onto a 3D brain surface representation. The prefrontal and parietal activations each consisted of one large contiguous cluster representing each region, as listed in Table S1. Green, blue, and yellow arrowheads indicate precentral sulcus (PcS), superior frontal sulcus (SFS), and inferior sulcus (IFS), respectively. (B) Time course of sustained activity in the right lPFC region during the R+ and R- blocks. (C) Time course of transient activity in the right lPFC region during the R+ and R- trials. The early transient effect (EARLY) and late transient effect (LATE) were extracted from the time course (Fig. S3A). Note that sustained effects (B) were not present in these time courses. (D) Scatter plot of correlations for sustained and late-transient transient activity during the R+ blocks/trials. Each dot indicates one participant. The negative correlation indicates that higher sustained activity was associated with a reduced late-trial transient response.
As shown Fig. 2B, in the lPFC ROI, sustained activation was significantly increased in the R+ context (R+: t(30) = 4.0, P < 0.001; R+ vs. R-: t(30) = 4.2; P < 0.001; see also Fig. S3A and Table S1). Conversely, the event-related response revealed a transient decrease in activation on R+ relative to R- trials, primarily in the later period of the trial (Fig. 2C). To conduct more direct comparisons, the transient effect was quantified by separating trial-related activation into early and late periods (Fig. S2C; SI Methods). The early-late decomposition of the transient activity allowed us to examine within-trial distinctions between proactive and reactive strategies. More specifically, larger late-transient activity can be considered to reflect the recruitment of reactive control (i.e., more related to the period of probe processing and response selection), whereas the early-trial period may reflect anticipatory updating and maintenance of task-rule information based on integration with the current memory set. Importantly, these two transient components were statistically independent (SI Methods), and moreover, empirically double-dissociated in terms of reward context effects (F(1,30) = 14.4, P < 0.001; see also Fig. S3A), consistent with the idea that the two trial components reflect distinct components of task control. In the early-trial period, there was significant activation in both contexts (R+: t(30) = 3.0, P < 0.01; R-: t(30) = 5.0, P < 0.001), but no difference related to the reward effect [t(30) = 0.34, P = 0.71] (Fig. S3A), suggesting that both of the R+ and R- trials recruited anticipatory control processes during this period. In contrast, the context effect was significant in the late-trial period, with robust activity in the R- context (t(30) = 4.2, P < 0.001) with a significant decrease in the R+ context (R+ vs. R-: t(30) = −2.9, P < 0.01) (Fig. 2C and Fig. S3A). The double dissociation of brain activity dynamics (sustained and late-transient) and reward context (R+ and R-) in the lPFC was statistically reliable (F(1,30) = 13.1, P < 0.01; Fig. S3A).
Interestingly, the activation dynamics in this lPFC region was not affected by trial-by-trial fluctuations in reward value within the R+ context (P > 0.26; Fig. S3, SI Results), suggesting that the shift in activation dynamics was purely contextual in rewarding situations. Nevertheless, it is worth noting that such transient effects of trial reward value have been observed together with sustained effects (6), and indeed were observed in other brain regions in this dataset (27).
The observed patterns suggest a shift from a reactive-type transient activation mode in the R- context, to a more predominately tonic mode of activation in the R+ context, consistent with an increased reliance on proactive control as a result of high motivational salience. This dynamic shift was confirmed at a between-subjects level, in terms of a significant negative correlation between the transient and sustained effects (r = −0.67, P < 0.0001; Fig. 2D). Thus, the reduction in late-trial transient activation was greatest in participants that showed a stronger increase in sustained activation. It is important that these effects were not due to artifacts of statistical collinearity related to the estimation method (SI Methods).
Brain–Behavior Relationships.
The DMC framework suggests that proactive control facilitates optimal performance in demanding cognitive tasks. Consequently, there should be a relationship between the neural signature of proactive control and task performance. Indeed, within the lPFC ROI, there was a significant correlation between the context-dependent reduction in late-trial activity and enhancement in task performance (P < 0.05, corrected within the lPFC ROI; Fig. 3A). Importantly, the correlation involved the nonrewarded trials in R+ context, and thus refers exclusively to the contextual effect. Indeed, there were no correlations between transient activity in lPFC and trial-by-trial performance enhancements related to reward value (Fig. S4; SI Results). Moreover, the late-trial period reflects when responses were generated, which lends support to the plausibility of a causal relationship between activity and performance. Interestingly, the contextual performance enhancements were associated with a larger reduction—not increase—in late-trial activity, suggesting decreased cognitive demands in this period in participants exhibiting enhanced performance. However, the correlation is consistent with the DMC model, in suggesting that global optimization of performance in the rewarding context results from a shift away from a reactive-type transient pattern of activation (Fig. S1A; refs. 16–18).
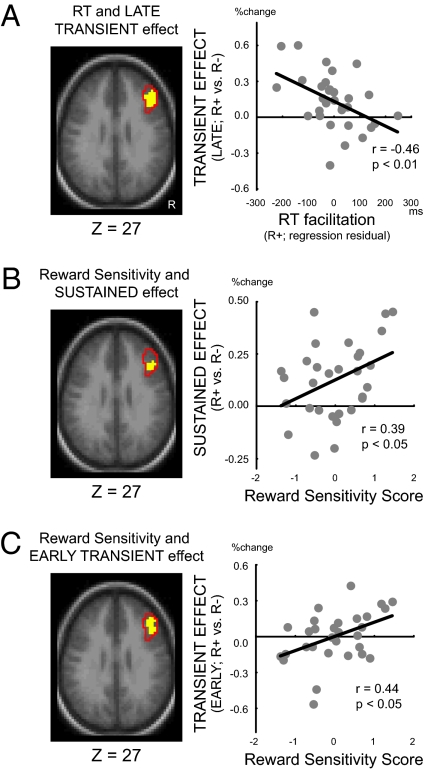
Fig. 3.
Statistical correlation maps and plots between behavioral measurements and brain activity components within the right lPFC ROI. Significant regions were colored in yellow on the transverse anatomical section at the labeled coordinate (P < 0.05 corrected for multiple comparison; Left). The area enclosed by red lines indicates the lPFC ROI identified from whole-brain analyses. The activity and the behavioral measurements of individual participants are plotted to demonstrate: (A) the significant negative correlation that was observed between RT contextual facilitation and late transient activity in R+ trial, indicating that faster RTs were associated with reduced activity; (B) individuals with higher reward sensitivity score exhibited greater sustained activity during R+ block; and (C) the higher-score individuals also exhibited greater transient activity during the early period of R+ trial.
Reward-Related Individual Differences.
We then tested whether such shifts in activation dynamics induced by reward context also covaried with individual differences in trait sensitivity to reward (SI Methods). Again, significant correlations were observed within the lPFC ROI. Specifically, reward-sensitivity was positively correlated with the degree of sustained activation increase in the R+ context (P < 0.05, corrected within the lPFC ROI; Fig. 3B). Additionally, although there were no contextual effects in early-trial activation at the group level, another positive between-subjects correlation was observed between reward-sensitivity and the context-related increase in early-trial activity (P < 0.05, corrected within the lPFC ROI; Fig. 3C). Thus, highly reward-sensitive individuals were the ones most likely to exhibit a pattern of lPFC dynamics typified by high levels of sustained activity plus an additional boost of transient activity in the early-trial period. The early-trial transient activation boost is also highly characteristic of increased goal-driven anticipatory updating and maintenance of task information in working memory (16–18).
Mediation Analysis.
Given that reward sensitivity was also positively correlated with improved performance in the R+ context, we aimed to provide more direct and comprehensive evidence that such performance enhancements were tied to the stronger shifts in lPFC activity dynamics observed in high reward-sensitive individuals. A path model approach was employed to test whether the behavioral relationship between reward sensitivity and performance was statistically mediated by the sustained and transient activation dynamics observed in the R+ context (28). As shown in Fig. 4A, a successful model was identified, in which reward sensitivity was positively associated with increased sustained and early-trial activity, which were, in turn, associated with reduced late-trial activity, that led to enhanced task performance (χ2 = 3.7, P = 0.16, AGFI = 0.67). When this indirect pathway of activity components was included, the direct pathway from reward sensitivity to performance dropped to a nonsignificant level, indicating statistical mediation (indirect effect: 0.15, P < 0.05). To ensure that these results were not biased by the use of individual correlation tests to define the region of potential mediation, the successful model was again tested by defining the mediation region purely based on the group-wise sustained and transient activation contrasts, without any reference to the individual correlation tests. Importantly, the mediation effect was again confirmed (Fig. S5A; SI Results).
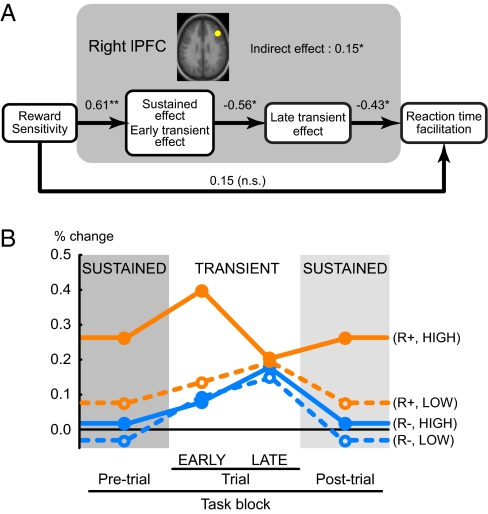
Fig. 4.
LPFC activity dynamics mediates the relationship between individual differences and behavioral performance. (A) The path diagram illustrates the direct and indirect relations among the brain activity, reward sensitivity score, and task performance. The indirect effect was mediated by lPFC brain activity dynamics, comprised of the state/early-transient component and the late-transient component. The beta values indicated beside the arrows were simultaneously estimated in a multivariate regression. **, P < 0.01; *, P < 0.05. (B) For each task period (pretrial, and early/late-task periods), activation magnitudes were separately calculated for 10 highest (HIGH) and 10 lowest (LOW) individuals, in terms of reward sensitivity score. The vertical axis indicates the activity level relative to fixation block (i.e., transient and sustained activations are cumulative). In the R- context the two groups are similar (blue solid and dashed lines). However, in the R+ context, the HIGH group dynamically modulates cognitive control processes in the right lPFC (orange solid lines). The enhancement of sustained and early-transient activity results in the decrease in late-transient activity. On the other hand, the LOW group exhibits a qualitatively different profile in activity dynamics, with very little effect of the R+ context (orange dashed lines).
Alternative models were also tested but were not found to be successful, indicating that the indirect path effects were highly specific (Figs. S5B and S6; SI Results). In particular, we found that the mediation effect was only significant when each of the early, late, and sustained components were included in the model in their temporally correct sequence. This finding increases confidence that the mediation pattern was due to the full shift in activation dynamics rather than a simple change in activation magnitude.
The statistical mediation model makes more concrete the idea that individual differences in reward sensitivity may reflect qualitatively different cognitive control strategies used for task performance. Fig. 4B shows the magnitudes of sustained, early- and late-trial transient components for the 10 highest and 10 lowest individuals defined in terms of reward-sensitivity score (Fig. 4B). High reward-sensitive individuals are characterized by greater sustained activity in the R+ context, supplemented by an additional transient activation boost in the early-trial period, which reduces the late-trial activation. In contrast, for low reward-sensitive individuals the smaller R+ increase in tonic and early-trial activation resulted in the continued need for a strong boost in activity during the late-trial period.
Discussion
This study identified critical factors that contribute to the behavioral performance enhancement observed in demanding cognitive situations involving reward expectation. First, the motivational context appears to exert a significant influence over behavior and brain activity, because these were modulated even on nonrewarded trials performed within a rewarding motivational context. Second, this contextual effect was accomplished by a shift in the temporal dynamics of brain activity, and not just by a simple change in activation magnitude. Finally, the personality trait of reward sensitivity specifically modulated the contextual effect, with greater performance enhancement and modulation of activity dynamics occurring in highly reward-sensitive individuals.
The pattern of activation dynamics observed in high and low reward-sensitive individuals illustrates well the distinction between proactive and reactive cognitive control strategies. Specifically, working memory tasks such as this one can be performed reasonably successfully with a reactive strategy that involves just-in-time transient reaccessing of task rules and configurations for encoding, maintenance, and response selection in a stimulus-triggered manner, following the presentation of memory set and probe items (12). In contrast, an optimal proactive control strategy would involve sustained maintenance of task set and anticipated rule use across trials (i.e., even during intertrial intervals) to facilitate the transient encoding and translation of memory set information into a prospective expectancy regarding the upcoming probe (i.e., prepare a target response if the probe is one of the expected memory set items) before its onset. Our results are consistent with general findings regarding right fronto-parietal cortex in preparatory attention (15) and our prior empirical work (16–18) in demonstrating that the distinction between proactive and reactive control modes occurs in terms of activation dynamic shifts.
The reactive control mode may often times be sufficient for producing high accuracy rates. However, to maximize reward attainment, reactive control is not an optimal cognitive strategy, at least for conditions in which it is critical that responses are not only accurate but also fast (12). The specific task paradigm used in this study may be ideal for the detection of such individual difference effects precisely because such a high degree of performance was possible even with a reactive control strategy. It might be interesting, however, to contrast the pattern observed here with reward-context effects and individual differences present in paradigms that make stronger control demands (8, 10). One possible prediction is that, although group-average effects of reward context might be larger, individual difference effects might be attenuated because, in paradigms with very high control demands, it may be necessary to shift to proactive control to obtain successful performance, whereas in the current paradigm it may have been a more optional strategy.
The lateral prefrontal cortex has consistently been implicated during performance of working memory tasks, exhibiting persistent neuronal activity during delay periods (14, 19, 20). However, the increased lPFC activity observed here was present not only during the early-trial period in which memory set items were encoded and maintained, but also extended to intertrial intervals that did not involve retention of memory set items. It is thus unlikely that this lPFC region is primarily involved in the short-term storage of memory set items, but instead is more related to maintenance of goal-related information needed for successful performance, such as task sets or rules (29). Indeed, recent empirical work has suggested that lateral PFC is preferentially involved in active maintenance of goal-related information (30–35). Interestingly, PFC neurons also code information related to the reward context (3, 7, 36). Thus, a primary implication of our findings is that the right lPFC represents goal-relevant information (e.g., task rules; ref. 29) that incorporates the potential reward value of task goal achievement, according to the current motivational context (3, 20).
Previous studies have explored neural mechanisms that underlie reward expectancy effects across a range of cognitive domains (4–11). These studies have consistently demonstrated that increasing trial reward value is associated with increasing transient activity on such trials. A recent study further suggests that effective connectivity between lateral and medial PFC changes as a function of motivational factors (8). Importantly, in that study, the shift in lateral-medial connectivity was related to block-related rather than trial-related changes in motivational value, consistent with our findings. Another study found both transient and sustained modulations in lPFC regions in accordance with reward values (that were modulated and reinforced in a blockwise, rather than trial-specific manner) as well as to personality traits of reward drive (6). One possibility of these modulations is that the lPFC integrates task information with accumulated reward value in both a transient and sustained fashion, when this information must be internally maintained and updated both across motivational contexts and trials. Like the current findings, these results point to the importance of contextual and sustained, as well as trial-specific and transient effects of motivation.
An important question raised by our findings is why individual differences in reward-related personality traits, which have been shown to modulate activity in brain reward circuitry (37), can also be specifically linked to contextual changes in cognitive control. One possibility is that such traits reflect the persistency of reward-triggered behaviors regulated by the mesocortical dopamine (DA) system (21, 38). Indeed, the midbrain DA system plays a central role in the processing of reward and motivational information through both phasic and tonic signaling (39, 40). Moreover, genetic variation in the DA system is associated with stable individual differences in both affective dimensions of personality (41) and reward-related cognitive processes (42). The DA projection to lateral PFC, known to modulate working memory functions (43), enables proactive control by regulating phasic and tonic DA signals (44). Thus, high reward-sensitive individuals might possess a DA system that provides the optimal tonic-phasic balance required for proactive control (21, 45, 46). However, this interpretation does not rule out a possibility that the contextual effect, especially the increased sustained activity in the lPFC, is maintained by each individual instance of reward delivery.
In summary, the findings presented here highlight key dimensions relevant to investigations of the neural bases of personality, motivation, and cognitive control. We have suggested that reward-sensitivity, an affective component of personality (21, 41, 47, 48), points to a particular endophenotype in which lPFC cognitive control mechanisms are selectively modulated in rewarding situations. Another dimension is the finding that it is the temporal dynamics of brain activity that mediates cognitive control and personality effects, and not just the simple magnitude of brain activity. A last critical dimension is that motivational variables can enable reprioritization of behavioral goals in cognitive situations (1–3, 20). Thus, utilization of motivational manipulations may be a powerful means of isolating, dissociating, and characterizing the various components of cognitive control.
Methods
Task and Procedure.
Participants perfromed a Sternberg-type working memory task (ref. 47; Fig. S1). Incentives were indicated via a reward cue presented at the beginning of each trial, indicating the amount of potential monetary reward for a correct response faster than a pre-specified threshold. There were three different possible reward cues indicating a 75-cent (high), 25-cent (low), or no potential reward. A 5-word memory set was then presented on the screen, followed by a delay period that served as a retention interval. After the delay, a probe word was presented, and participants had to decide whether the probe word was included in the memory set. Post-response visual feedback indicated if the response was rewarded.
A mixed blocked and event-related fMRI design was used (Fig. 1A; ref. 48). Two types of task blocks were administered, the rewarding block (R+) and nonrewarding block (R-). The R+ block consisted of both rewarding and nonrewarding trials, whereas the R- block consisted of only nonreward trials. The critical components were the blocked (sustained) effect between the R+ and R- blocks and the trial-by-trial (transient) effect between the nonreward trials in the two blocks. The transient effect was examined based on contrasting nonreward trials across the two block types. Before the start of each functional run, participants were instructed regarding the block (R+ vs. R-), and further in the R+ block, about the value and variety of rewards available (high, low, none) to minimize the degree of reward prediction error experienced when encountering different trial reward values across contexts.
Data Analysis.
A general-linear model approach was used to estimate signal magnitudes for both transient and sustained activity. The sustained and transient effects are simultaneously but independently coded within the same GLM (48). For the sustained effect, R+ and R- blocks were coded by a box-car function using an assumption of a fixed-shape response. For the event-related effects, R+ and R- trials were coded by a series of delta-function regressors. Because the transient regressors are sparsely distributed within a task block, any negative correlation between transient and sustained activity (Fig. 2D) should not be attributable to the collinearity.
The event-related and sustained estimates for the imaging data were then submitted to a group analysis by using a voxel-wise random-effects model. Whole brain exploratory analysis was first performed to identify brain regions that revealed a shift in brain activity dynamics between R+ and R- block/trials in terms of both of the sustained and transient effects, P < 0.05 corrected for multiple comparisons across the whole brain. Brain regions were reported significant only if the conjunction null hypothesis was rejected (26).
ROI analyses were then performed to examine profiles of the activity dynamics. Because each trial consisted of multiple events, two activity components of interests (early, late; Fig. 2C) were extracted from the time course of the transient effect (see ref. 49 for similar approach). The early-trial component likely includes activity that is primarily related to the presentation of reward cue and encoding of the memory set, whereas the late-trial component primarily includes maintenance of the word set and the response to the probe, but likely not any reward feedback effects.
Brain–Behavior and Individual Difference Analyses.
Brain–behavior relationships were examined by exploring individual differences in personality traits, behavioral performance, and brain activity within the lPFC ROI identified in the whole brain exploratory analysis above. Voxel-wise correlation coefficients were computed between the behavioral or personality measurements and activity components (sustained, early-transient, and late-transient). Significant correlations were reported above the threshold of P < 0.05, corrected for multiple comparisons within the ROI.
Mediation Analyses.
To test whether the brain activity components can reliably account for the covariation of the two behavioral measurements [reward sensitivity and reaction time (RT)], a mediation analysis was performed (28). The independent (predictor) and dependent (predicted) variables were the reward sensitivity score and the RT enhancement in the R+N trial, respectively. The present model constitutes a single mediator model, with the mediator consisting of two activity components in series (Fig. 4A). Specifically, one component consists of the sustained activity and early transient activity, and the other component consists of late transient activity, constituting a step-wise mediator, based on the temporal order of the task. All of the regression coefficients in the model were estimated simultaneously in a multivariable regression. The appropriate model was further tested by using a different ROI definition to test the robustness of the mediation effect. In a separate control analysis, to examine the specificity of this model, several possible alternative models were tested.
See also SI Methods for full descriptions.
Supplementary Material
Acknowledgments
Funding was provided by National Institutes of Health Grant R01 MH66078 (to T.S.B.) and a Research Fellowship from the Uehara Memorial Foundation (K.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1002007107/-/DCSupplemental.
References
- 1.Niv Y, Joel D, Dayan P. A normative perspective on motivation. Trends Cogn Sci. 2006;10:375–381. doi: 10.1016/j.tics.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Pessoa L. How do emotion and motivation direct executive control? Trends Cogn Sci. 2009;13:160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watanabe M, Sakagami M. Integration of cognitive and motivational context information in the primate prefrontal cortex. Cereb Cortex. 2007;17:101–109. doi: 10.1093/cercor/bhm067. [DOI] [PubMed] [Google Scholar]
- 4.Adcock RA, Thangravel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JDE. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 5.Delgado MR, Gillis MM, Phelps EA. Regulating the expectation of reward via cognitive strategies. Nat Neurosci. 2008;11:880–881. doi: 10.1038/nn.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engelmann JB, Damaraju E, Padmala S, Pessoa L. Combined effect of attention and motivation on visual task performance: Transient and sustained motivational effects. Front Hum Neurosci. 2009;3:4. doi: 10.3389/neuro.09.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ichihara-Takeda S, Funahashi S. Activity of primate orbitofrontal and dorsolateral prefrontal neurons: Effect of reward schedule on task-related activity. J Cogn Neurosci. 2008;20:563–579. doi: 10.1162/jocn.2008.20047. [DOI] [PubMed] [Google Scholar]
- 8.Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci. 2009;12:939–945. doi: 10.1038/nn.2321. [DOI] [PubMed] [Google Scholar]
- 9.Krawczyk DC, Gazzaley A, D’Esposito M. Reward modulation of prefrontal and visual association cortex during an incentive working memory task. Brain Res. 2007;1141:168–177. doi: 10.1016/j.brainres.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto K, Suzuki W, Tanaka K. Neuronal correlates of goal-based motor selection in the prefrontal cortex. Science. 2003;301:229–232. doi: 10.1126/science.1084204. [DOI] [PubMed] [Google Scholar]
- 11.Taylor SF, et al. A functional neuroimaging study of motivation and executive function. Neuroimage. 2004;21:1045–1054. doi: 10.1016/j.neuroimage.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 12.Braver TS, Gray JR, Burgess GC. In: Variation in Working Memory. Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. New York: Oxford Univ Press; 2007. pp. 76–106. [Google Scholar]
- 13.Duncan J, Emslie H, Williams P, Johnson R, Freer C. Intelligence and the frontal lobe: The organization of goal-directed behavior. Cognit Psychol. 1996;30:257–303. doi: 10.1006/cogp.1996.0008. [DOI] [PubMed] [Google Scholar]
- 14.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;21:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 15.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 16.Locke HS, Braver TS. Motivational influences on cognitive control: Behavior, brain activation, and individual differences. Cogn Affect Behav Neurosci. 2008;8:99–112. doi: 10.3758/cabn.8.1.99. [DOI] [PubMed] [Google Scholar]
- 17.Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proc Natl Acad Sci USA. 2009;106:7351–7356. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jimura K, Braver TS. Age-related shifts in brain activity dynamics during task switching. Cereb Cortex. doi: 10.1093/cercor/bhp206. in press 10.1093/cercor/bhp206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- 20.Courtney SM. Attention and cognitive control as emergent properties of information representation in working memory. Cogn Affect Behav Neurosci. 2004;4:501–516. doi: 10.3758/cabn.4.4.501. [DOI] [PubMed] [Google Scholar]
- 21.Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of motivation, and extraversion. Behav Brain Sci. 1999;22:491–569. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- 22.Zelenski JM, Larsen RJ. Susceptibility to affect: A comparison of three personality taxonomies. J Pers. 1999;67:761–791. doi: 10.1111/1467-6494.00072. [DOI] [PubMed] [Google Scholar]
- 23.Ball SA, Zuckerman M. Sensation seeking, Eysenck’s personality dimensions and reinforcement sensitivity in concept formation. Pers Individ Dif. 1990;11:343–353. [Google Scholar]
- 24.Carver CS, White T. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. J Pers Soc Psychol. 1994;67:319–333. [Google Scholar]
- 25.Higgins ET, et al. Achievement orientations from subjective histories of success: Promotion pride versus prevention pride. Eur J Soc Psychol. 2001;31:3–23. [Google Scholar]
- 26.Nichols T, Brett M, Anderson J, Wager T, Poline J-B. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Beck SM, Locke HS, Savine AC, Jimura K, Braver TS. Primary and secondary rewards differentially modulate neural activity dynamics during working memory. PLoS One. 2010;5:e9251. doi: 10.1371/journal.pone.0009251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakai K. Task set and prefrontal cortex. Annu Rev Neurosci. 2009;31:219–245. doi: 10.1146/annurev.neuro.31.060407.125642. [DOI] [PubMed] [Google Scholar]
- 30.Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- 31.Bunge SA, Kahn I, Wallis JD, Miller EK, Wagner AD. Neural circuits subserving the retrieval and maintenance of abstract rules. J Neurophysiol. 2003;90:3419–3428. doi: 10.1152/jn.00910.2002. [DOI] [PubMed] [Google Scholar]
- 32.Dosenbach NUF, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montojo CA, Courtney SM. Differential neural activation for updating rule versus stimulus information in working memory. Neuron. 2008;59:173–182. doi: 10.1016/j.neuron.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rushworth MFS, et al. Attentional selection and action selection in the ventral and orbital prefrontal cortex. J Neurosci. 2005;25:11628–11636. doi: 10.1523/JNEUROSCI.2765-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakai K, Rowe JB, Passingham RE. Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nat Neurosci. 2002;5:479–484. doi: 10.1038/nn846. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe M, Hikosaka K, Sakagami M, Shirakawa S. Coding and monitoring of motivational context in the primate prefrontal cortex. J Neurosci. 2002;22:2391–2400. doi: 10.1523/JNEUROSCI.22-06-02391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hahn T, et al. Neural response to reward anticipation is modulated by Gray’s impulsivity. Neuroimage. 2009;46:1148–1153. doi: 10.1016/j.neuroimage.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 38.Shultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- 39.Niv Y. Cost, benefit, tonic, phasic: What do response rates tell us about dopamine and motivation? Ann N Y Acad Sci. 2007;1104:357–376. doi: 10.1196/annals.1390.018. [DOI] [PubMed] [Google Scholar]
- 40.Robbins TW. Chemical neuromodulation of frontal-executive functions in humans and other animals. Exp Brain Res. 2000;133:130–138. doi: 10.1007/s002210000407. [DOI] [PubMed] [Google Scholar]
- 41.Benjamin J, et al. Population and familial association between the D4 dopamine receptor gene and measures of Novelty Seeking. Nat Genet. 1996;12:81–84. doi: 10.1038/ng0196-81. [DOI] [PubMed] [Google Scholar]
- 42.Klein TA, et al. Genetically determined differences in learning from errors. Science. 2007;318:1642–1645. doi: 10.1126/science.1145044. [DOI] [PubMed] [Google Scholar]
- 43.Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: Involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- 44.Cohen JD, Braver TS, Brown JW. Computational perspectives on dopamine function in prefrontal cortex. Curr Opin Neurobiol. 2002;12:223–229. doi: 10.1016/s0959-4388(02)00314-8. [DOI] [PubMed] [Google Scholar]
- 45.Cloninger CR, Svrakic DM, Przybeck TRA. Psychobiological model of temperament and character. Arch Gen Psychiatry. 1993;50:975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- 46.Gray JA. In: Explorations in Temperament. Strelau J, Angleitner A, editors. New York: Plenum; 1991. pp. 105–128. [Google Scholar]
- 47.Sternberg S. High-speed scanning in human memory. Science. 1996;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- 48.Visscher KM, et al. Mixed blocked/event-related designs separate transient and sustained activity in fMRI. Neuroimage. 2003;19:1694–1708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- 49.Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.