Abstract
We report that adrenocorticotropic hormone (ACTH) protects against osteonecrosis of the femoral head induced by depot methylprednisolone acetate (depomedrol). This therapeutic response likely arises from enhanced osteoblastic support and the stimulation of VEGF by ACTH; the latter is largely responsible for maintaining the fine vascular network that surrounds highly remodeling bone. We suggest examining the efficacy of ACTH in preventing human osteonecrosis, a devastating complication of glucocorticoid therapy.
Keywords: osteoporosis, osteoclast, osteoblast
The use of glucocorticoids for medical conditions as diverse as asthma, ulcerative colitis, kidney diseases, and rheumatologic disorders causes not only a variety of metabolic and medical complications, including diabetes and osteoporosis, but also a painful debilitating condition, osteonecrosis, usually affecting the femoral head (1). Osteonecrosis almost invariably requires surgical debridement of dead bone and contributes to approximately 10% of the more than 500,000 hip replacements annually in the United States (2). In addition, 30–50% patients on long-term glucocorticoids sustain a hip fracture with a 2- to 2.5-fold increased risk (3).
Osteocyte apoptosis is thought to be the key determinant of glucocorticoid-induced cortical bone loss (4). Reduced osteoblast function manifesting in attenuated bone formation has also been documented in trabecular bone in rodents and humans (5). In contrast to glucocorticoid-induced osteoporosis, the pathogenesis of glucocorticoid-induced osteonecrosis is unclear (6). It resembles the osteonecrosis caused by traumatic damage to the artery that supplies the femoral head, hence the name, avascular necrosis (3), but the necrosis actually begins as regional trabecular death (6), likely from osteoblast and osteocyte apoptosis. However, there is strong evidence for an ischemic component. For example, studies using a rat model of Legg-Calve-Perthe's disease suggest that the intracortical blockade of lateral epiphyseal arteries that supply approximately 80% of the femoral head (7) can, in part, be attributed to their anatomical predisposition. It is nonetheless unclear whether ischemia is the initiating event or is secondary to local cellular or vascular bed damage (8).
It is further surprising that osteonecrosis is not a cardinal feature of adrenocorticotropic hormone (ACTH)-producing adenomas (9), where glucocorticoid excess is profound. A question therefore arises—does ACTH protect against glucocorticoid-induced osteonecrosis? Indeed, one of our groups has documented functional ACTH receptors (MC2Rs) on osteoblasts; their activation enhances cell proliferation (10). These data are consistent with the presence of receptors for other anterior pituitary hormones, FSH and TSH, on bone cells, as well as with the description of another pituitary-bone axis, in which these hormones bypass traditional endocrine targets to regulate bone mass directly (11–13).
We were thus prompted to investigate whether glucocorticoid-induced osteonecrosis could, in fact, be prevented by exogenous ACTH and, if so, whether any such protection might arise from an effect of ACTH on osteoblast function and/or survival. Mechanistically, as glucocorticoid-induced osteonecrosis appears to have an ischemic component (14), we surmised that ACTH might also enhance vascularity, for example, by increasing VEGF production. If our assumptions were correct, they would not only extend our description of a pituitary-bone axis (13), but might also relate a suppressed ACTH to the pathophysiology of the osteonecrosis that has hitherto been attributed solely to glucocorticoid excess.
Results
To examine the effect of ACTH in preventing glucocorticoid-induced osteonecrosis, we used a rabbit model that we have previously described and validated (6). Namely, the treatment of rabbits with depot methylprednisolone acetate (depomedrol) at 10 mg/kg/day for 28 days produced reproducible and consistent damage to the femoral head without, at this relatively short time, femoral head collapse or secondary changes. We treated female rabbits averaging 4.48 kg with depomedrol alone or depomedrol plus ACTH (1–24) (cosyntropin), 0.2 μg/kg/day as an s.c. injection. This ACTH dose yields a roughly normal ACTH serum level for about 2.5–3 h although producing only a spike in endogenous cortisol (15). Rabbits were killed at day 24 to assure that no animal had weight loss exceeding 20% (average weight, 3.95 kg). The weight loss, 12 ± 5% of body mass, was similar in all animals. At necropsy, animals treated with ACTH had normally appearing adrenal glands, whereas the depomedrol-only group, as expected, had atrophied glands that were difficult to locate within in the retroperitoneal fat.
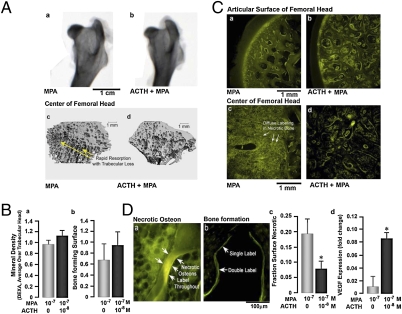
Examination of the femoral heads revealed no gross differences, except for a somewhat greater “moth eaten” appearance in methylprednisolone acetate-treated rabbits compared with those treated with methylprednisolone acetate plus ACTH (Fig. 1A). This difference correlated with increased consolidation of regions with trabecular loss obvious on μCT in the methylprednisolone acetate plus ACTH animals, although focal trabecular damage occurred in all cases (Fig. 1). Quantitative DXA and tetracycline labeling, respectively, showed that overall femoral head density and subarticular bone forming surfaces were approximately 10% and 30% greater, on average, in the methylprednisolone acetate plus ACTH group compared with the methylprednisolone acetate-only rabbits (Fig. 1B), although the differences did not reach statistical significance.
Fig. 1.
ACTH protects the rabbit femoral head from glucocorticoid-induced focal osteonecrosis. Rabbits (approximately 4.5 kg) were treated with 10 mg/kg depot methylprednisolone acetate (MPA), and half of the animals also received 0.2 μg/kg ACTH (1–24) (cosyntropin) daily. (A) Dual energy x-ray absorptiometry (DXA) scans of femoral heads after 24 days of MPA with or without ACTH (a and b) showed regions of nonuniform density that were slightly more apparent in MPA-only group. Microcomputed tomography (c and d) revealed focal damaged trabeculae, indicating rapid resorption in the MPA-treated group (arrow). (B) Femoral head bone mineral density measurements showed only a minor overall difference; however, there was a 30% increase in the subarticular bone-forming surfaces on tetracycline labeling in the MPA plus ACTH group (n = 4, mean ± SD, not significant). (C) Although tetracycline labels bone forming surfaces (strong linear labeling), it is taken up passively by necrotic bone as shown (6). The region from the articular cartilage, 2- to 3-mm deep, showed only surface tetracycline labeling and was protected from necrosis (a and b). In contrast, in the centers of the femoral heads, all animals had focal necrotic bone with diffuse tetracycline labeling of osteons (c and d), extending in many cases entirely through trabeculae. In the group without ACTH (c), however, there were prominent consolidated areas of necrosis, in which marrow adipocytes were also necrotic, causing lipolysis that also bound calcium and, thus, labeled with tetracycline. The results were in keeping with the discrete lesions noted on μCT in the MPA-only group (Ac). With ACTH, areas of necrosis were smaller and generally not consolidated with less prominent labeling of necrotic adipocytes. (D) As noted, tetracycline labels bone formation at the mineralizing surface, but necrotic bone loses its isolation from extracellular fluid, causing diffuse labeling of the necrotic bone (a). There was a highly significant approximately 50% reduction in the necrotic surface area in femora from rabbits treated with MPA plus ACTH compared with those given MPA alone (c); these data demonstrate a clear protection of the focal osteonecrosis by ACTH. Statistics: Student's t test; *P < 0.05; n = 4; mean ± SD). Effect of ACTH on VEGF mRNA expression measured by quantitative PCR in MPA-treated rabbits (d). Statistics: Student's t test; *P < 0.05; in triplicate; mean ± SD).
We have previously demonstrated deep tetracycline adsorption in dead osteons arising from glucocorticoid exposure (6): this unique lamellar pattern arises from increased bone matrix permeability and hydroxyapatite exposure. Relatively high doses of tetracycline (10 mg/kg) were therefore used, as in previous studies (6), to label necrotic bone. Fig. 1C a and b show similar amounts of surface labeling immediately adjacent to the articular cartilage of the femoral head, with the two groups having the same appearance. In the central femoral head, however, there was a profound increase in diffuse tetracycline uptake into necrotic bone in the methylprednisolone acetate-only group. The methylprednisolone acetate plus ACTH group, in contrast, showed evidence of appositional labeling (cf. Fig. 1 C c vs. d and D a vs. b). Thus, morphometry of necrotic trabeculae displayed an approximately 50% reduction in necrotic surface in the methylprednisolone acetate plus ACTH group compared with the methylprednisolone acetate-only group (Fig. 1Dc). The striking differences correlated with lesser lipolysis and diffuse labeling in marrow fat—often termed “marrow edema”—which was most notable in Fig. 1Cc. Furthermore, quantitative PCR revealed a significant increase in VEGF mRNA expression in bone marrow of rabbits treated with methylprednisolone acetate plus ACTH compared with those receiving methylprednisolone acetate alone (Fig. 1Dd).
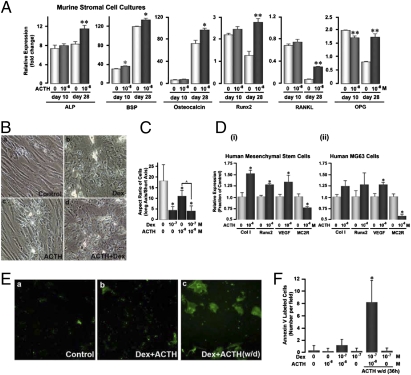
The results suggest that ACTH supports osteoblast activity, perhaps through VEGF expression, ultimately maintaining the viability of multicellular osteons that comprise the bone matrix. To investigate this hypothesis, we studied the effect of ACTH on murine bone marrow stromal cells and human mesenchymal stem cells. After removal of hematopoietic stem cells from murine whole bone marrow, the adherent stromal cells were cultured with glycerol-2-phosphate and ascorbate for up to 28 days. At approximately 10 days, the maturing cells form colonies positive for alkaline phosphatase, which is followed at approximately 21 days by the formation of mineralizing colonies. We thus quantified changes in key osteoblast maturation marker genes by quantitative PCR (Fig. 2A). ACTH exposure stimulated the expression of the alkaline phosphatase, bone sialoprotein, osteocalcin, and Runx2 genes, as well as those of the osteoclast differentiation cytokine RANKL and its soluble decoy receptor, osteoprotegerin (OPG). Importantly, all of these differences were seen only after 28 days of culture, not after 10 days (Fig. 2A), suggesting that the effects of ACTH on osteoblast differentiation, at least in the mouse, were exerted during the relatively late, rather than early, stages of differentiation. Not surprisingly, the results also demonstrate that the effect of ACTH is exerted through the up-regulation of Runx2 expression. Effects on RANK-L and OPG are similarly not surprising, as both cytokines are known to be comodulated by a variety of stimuli, including those downstream of G protein coupled receptors (16).
Fig. 2.
ACTH promotes osteoblast differentiation and protects against glucocorticoid-induced apoptosis. (A) ACTH (10−8 M) increases the expression of genes associated with osteoblast maturation and activity, including alkaline phosphatase (ALP), bone sialoprotein (BSP), osteocalcin, the key transcription factor for osteoblast differentiation Runx2, as well as the osteoclastogenic cytokine RANK-L and its decoy receptor, osteoprotegerin (OPG), after 28 but not 10 days of culture in medium containing 100 μM ascorbate and 10 mM glycerol phosphate (“differentiation medium”). Statistics: Student's t test, *P < 0.05, **P < 0.01; mean ± SEM, in triplicate. (B) Human mesenchymal stem cells in differentiation medium for 28 days showed typical morphological changes with ACTH and dexamethasone (Dex), namely cuboidal cell transformation and rapid mineralization (17) (fields: 440 μm2), measured as the cell's aspect ratio (long/short axis). Statistics: Student's t test, mean ± SD, n = 10, *P < 0.05, ^P = 0.012 (C). (D) Quantitative PCR on similar 28-day cultures showed effects on osteoblast maturation genes consistent with those in murine bone stromal cells (A), notably increases in type 1 COLLAGEN (Col1), RUNX2, and VEGF mRNA. However, the expression of the ACTH receptor MC2R was attenuated (i). The overall response of the transformed osteoblast-like cell line MG63 to ACTH was qualitatively similar to that of nontransformed cells (ii), although the response magnitudes were smaller and, in some cases, did not reach significance (as shown). MG63 cells display features of early osteoblastic maturation, but typically do not mineralize. Statistics for C and D: Student's t test, *P < 0.05; mean ± SD. (E and F) Human mesenchymal stem cell cultures in differentiation medium were treated with ACTH (10−8 M) and/or dexamethasone (Dex, 10−7 M) (as shown) for 28 days, and apoptosis was measured by labeling with fluorescent annexin V. Only small numbers of apoptotic cells were seen per field (field: 220 μm2). However, when ACTH was withdrawn (w/d) for 36 h, the numbers of annexin V-positive cells increased dramatically, suggesting that ACTH protects against glucocorticoid-induced apoptosis.
Parallel studies with human mesenchymal cell cultures incubated for 28 days with glycerol-2-phosphate and ascorbate showed evidence of nodule formation. Under these culture conditions, dexamethasone is known to stimulate osteoblast mineralization (17), which we quantified as reduced aspect ratio (long axis/short axis); the same occurred with ACTH and dexamethasone plus ACTH (Figs. 2 B and C). Quantitative PCR showed results in keeping with those obtained with murine cells, namely, ACTH increased expression of COLLAGEN type 1, RUNX2, and VEGF, while reducing expression of the ACTH receptor, MC2R (Fig. 2Di). Adding to our confidence was the observation that the transformed human osteoblast-like cell line MG63 displayed largely similar effects as primary nontransformed cells (Fig. 2Dii) but with a smaller response magnitude, consistent with its early maturation phenotype.
In addition to an effect on osteoblast maturation, we surmised that ACTH might support osteoblast survival, particularly considering that the fundamental known mechanism for the initiation of osteonecrosis is focal osteoblast/osteocyte apoptosis (6). Human mesenchymal cells were thus incubated with either dexamethasone or ACTH or a combination of the two and, in extended experiments, ACTH withdrawn from the ACTH-only or ACTH plus dexamethasone cultures followed by annexin V labeling. Whereas dexamethasone with or without ACTH was without major effects on cell viability, the withdrawal of ACTH from dexamethasone plus ACTH cultures, leaving cells in the presence of dexamethasone alone for 36 h, dramatically increased annexin V labeling (Figs. 2 E and F). This showed that ACTH inhibited dexamethasone-induced apoptosis in differentiating mesenchymal cell cultures, although it should be noted that cells within these cultures are at different stages of differentiation.
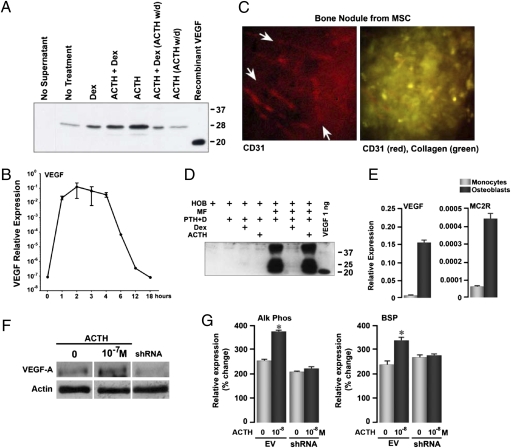
Because a key function of ACTH in the adrenal is the induction of VEGF synthesis, which we replicated in human mesenchymal cell cultures (Fig. 2D) and ACTH-treated rabbits (Fig. 1Dd), and as VEGF is central to bone development and homeostasis (18, 19), we examined the VEGF response to ACTH in considerably greater depth. A low level of VEGF was detected in 28-day osteoblast culture supernatants on immune precipitation, with a slight increase with dexamethasone and a more substantial increase with ACTH (Fig. 3A). The latter response was abrogated selectively upon the withdrawal of ACTH for the final 36 h; importantly, this coincided with enhanced apoptosis shown in Fig. 2E. The major ACTH fragment noted is a well-recognized form (20), as are 37- and approximately 18-kDa (final cleaved) fragments.
Fig. 3.
ACTH stimulates VEGF production from osteoblasts. (A) Immune precipitation of VEGF from nontransformed human osteoblast culture supernatants, showing that VEGF production is responsive to ACTH (10−8 M) and, to a lesser extent, to dexamethasone (Dex, 10−7 M). Media were changed for the last 36 h in the 4-week culture, during which time ACTH was withdrawn (w/d) and VEGF production receded to unstimulated levels. The major ACTH fragment is a well-recognized 28-kDa fragment (20); recombinant VEGF is shown as the approximately 18-kDa fragment. (B) Mineralizing human osteoblasts were rendered quiescent overnight in serum-free medium and treated for the times indicated (hours) with 10−8 M ACTH. VEGF expression, measured by quantitative PCR, was induced strongly within 1 h, but dropped after 3–4 h. The level of expression was similar to that seen in long-term ACTH-stimulated cultures (see E), although the rapid induction by ACTH of VEGF is seen more clearly in quiescent cells. Basal VEGF production (as in A) may thus be stimulated by serum cytokines or by low levels of ACTH in serum used in osteoblast cultures. (C) Mineralizing nodules formed in human mesenchymal stem cell (MSC) cultures incubated in media permitting osteoblast differentiation for 28 days also display CD31-expressing endothelial cells in capillary patterns similar to the rete of capillaries surrounding bone trabeculae in vivo. Red channel: CD31 label (arrows point to capillaries); overlay of red with the strong collagen signal (green) of the nodule (representative images; fields: 220 μm2). (D) Western blots showing very high VEGF expression in mixed human monocyte (MF)/osteoblast (HOB) cultures for 10 days treated with parathyroid hormone (PTH) and 1.25-(OH)2 vitamin D (PTH+D), dexamethasone (10−7 M, Dex) and/or ACTH (10−8 M). This VEGF production is abrogated by Dex. The importance of this finding is that VEGF production due to monocyte-osteoblast interaction in bone is profoundly down-regulated by glucocorticoids, heightening the potential sensitivity of VEGF production to reduced circulating ACTH. (E) Human monocytes in culture bear low levels of the ACTH receptor MC2R and produce small amounts of VEGF, confirming that the major production is from MC2R-bearing osteoblastic cells. (F) Western blot showing the effect of ACTH (10−7 M) on VEGF-A production in MC3T3.E1 osteoblastic cells and its knock down by an shRNA. (G) Quantitative PCR showing a lack of effect of ACTH (10−8 M) on alkaline phosphatase (Alk Phos) or bone sialoprotein (BSP) expression in VEGF-A shRNA-treated cells compared with empty vector-treated cells (EV). Statistics: Student's t test, mean ± SEM, triplicate, *P < 0.01.
That unstimulated osteoblasts produced measurable VEGF and that the culture medium contained 10% serum, which would include ACTH and other growth factors, led us to isolate the ACTH effect more definitively. Mineralizing human osteoblasts were cultured overnight in serum-free medium and then exposed to a saturating ACTH concentration (10−7 M), with VEGF mRNA measured over time by quantitative PCR. Under these conditions, VEGF mRNA was essentially absent in untreated cultures but was strongly up-regulated by ACTH within 1 h and began declining after 4 h, despite the presence of ACTH (Fig. 3B).
Because mesenchymal stem cells are multipotent and osteoblasts produce VEGF, we queried whether the bone nodules formed in vitro, as in Fig. 2B, may induce endothelial cell differentiation. CD31-expressing precursor cells were indeed found in capillary-like patterns within collagen-positive nodules (Fig. 3C). This appearance is reminiscent of the fine capillary network surrounding bone trabeculae in vivo (21). For this reason, the apoptotic cells noted in cultures with ACTH withdrawn (Fig. 2Ec) could at least in part represent VEGF-dependent cells, such as CD31 cells.
In addition, because there are several precedents for ACTH production by monocytes or macrophages (19), we questioned whether osteoblasts might also produce VEGF when stimulated by other cells, specifically the abundant monocytes in close proximity. Indeed, large amounts of VEGF protein were detected by Western immunoblotting of supernatant from 10-day, mixed monocytes/osteoblast cocultures (Fig. 3D). This monocyte-dependent VEGF stimulation in cocultures is distinct from the basal VEGF production by human mesenchymal cells noted in immunoprecipitation experiments, which increased slightly with dexamethasone (Fig. 3A). Although we currently remain uncertain whether the monocyte-stimulated VEGF increase is ACTH-dependent, it is worth reemphasizing that it was abrogated by dexamethasone (Fig. 3D, lane 5). This constitutes direct evidence for the inhibitory effect of a widely used glucocorticoid—dexamethasone—on endogenous VEGF production in a human bone cell population, wherein osteoblasts and monocytes lie in close proximity.
To substantiate further that the VEGF was likely released from osteoblasts, rather than from monocytes in the cocultures, we measured VEGF levels in both cell types. Monocytes expressed VEGF only at approximately 5% of that expressed by osteoblasts (Fig. 3E), indicating that monocytic VEGF was an unlikely contributor to the massive VEGF response noted in Fig. 3D. It was also unlikely that the VEGF response to ACTH (Fig. 3A) was mediated through the monocyte MC2R, as the monocyte MC2R level was only approximately 15% that of the osteoblast (Fig. 3E). Taken together, therefore, the results suggest that ACTH stimulates the osteoblastic production of VEGF, and that dexamethasone profoundly inhibits the monocyte-induced VEGF production, probably as one of several mechanisms that initiate osteonecrosis.
To explore whether ACTH-induced osteoblast differentiation was VEGF-mediated, at least in part, we knocked down VEGF production in MC3T3.E1 osteoblastic cells using VEGF-A shRNA to a point where cell proliferation was attenuated (Fig. 3F and Fig. S1). Although ACTH stimulated VEGF production in empty vector-treated cells (Fig. 3F) and enhanced the expression of the osteoblast differentiation markers alkaline phosphatase and bone sialoprotein, shRNA-treated cells failed to respond to ACTH (Fig. 3G). This experiment provided direct in vitro evidence that a substantial component of ACTH action on osteoblast differentiation was in fact dependent upon the production of VEGF.
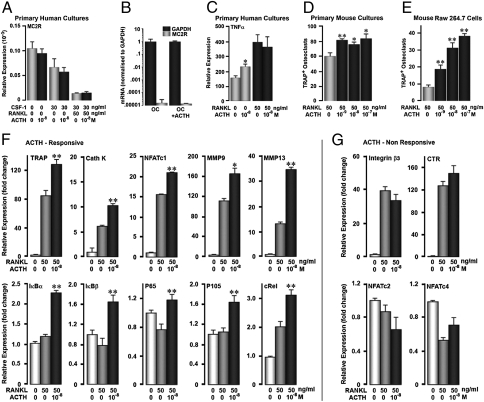
Because monocytes express the MC2R, albeit at significantly lower levels than in osteoblasts (Fig. 3E), we studied the effect of ACTH on the differentiation of osteoclast precursors. We therefore first measured MC2R expression in affinity-purified CD14 human monocytes. Fig. 4A shows that compared with untreated cells or those treated with M-CSF alone, cells induced to mature with RANK-L plus M-CSF displayed significantly lower MC2R expression. The declining MC2R expression as a function of differentiation suggests that any effects of ACTH on osteoclastogenesis must be exerted early, rather than later during maturation. This was further confirmed by the almost complete absence of MC2R mRNA in mature human osteoclasts, the minimal expression of which, we found, was also nonresponsive to ACTH (Fig. 4B). In fact, MC2R amplified only after 30 cycles; we believe that these trivial amounts probably reflect MC2R in contaminating cells. We have also done six gene screens for mature osteoclasts for MC2R. All were negative, with p values from 0.3 to 0.9 (not shown). The lack of receptors for ACTH on mature osteoclasts makes an effect on bone resorption highly unlikely.
Fig. 4.
Modest effects of ACTH on osteoclast precursors. (A) Human CD14 osteoclast precursors express significant amounts of mRNA for the ACTH receptor MC2R, although much less than that expressed on human osteoblasts (see Fig. 3E). However, during RANKL-induced differentiation, osteoclasts lose MC2R and would thus be expected to respond poorly to ACTH. (B) Very low levels of MC2R expression measured by quantitative PCR (qPCR) in mature human osteoclasts, with or without ACTH (10−8 M). (C) Human CD14 cells show a modest increase in TNFα mRNA with ACTH, suggesting a small effect on osteoclastogenesis. (D) In keeping with the response of human cells, a 5-day treatment of murine bone marrow cells with ACTH (concentrations as shown) caused a modest but clear increase in numbers of tartrate-resistant acid phosphatase- (TRAP-) positive osteoclasts, but without a clear concentration-dependence. (E) In contrast, RAW264.7 cells displayed a clear concentration-dependent increase in osteoclast formation. This transformed cell line retains large numbers of division-competent uncommitted precursor cells, even in the presence of RANKL. (F) qPCR for early osteoclast differentiation genes in response to RANK-L or RANK-L plus ACTH, namely tartrate-resistant acid phosphatase (TRAP), cathepsin K (Cath K), NFATc1, metalloproteinase (MMP) 9 and 13, and the inhibitors (IκBα and IκBβ) and subunits (p65, p 105, and c-Rel) of NF-κB. (G) qPCR for late differentiation markers, such as integrin β3 and the calcitonin receptor (CTR), and control proteins, such as NFATc2 and NFATc4. Statistics: Student's t test, *P < 0.05, **P < 0.01, qPCR in triplicate; TRAP+ osteoclasts (n = 8 wells per treatment).
To establish a function of ACTH earlier, as opposed to later during differentiation, we measured TNFα expression in untreated CD14 cells versus those treated with RANK-L for 12 days. Whereas a modest but reproducible elevation in TNFα expression was noted in untreated cells, cells treated with RANK-L failed to respond to ACTH (Fig. 4C). To confirm further an effect of ACTH on early osteoclast differentiation, we used two complementary murine models: (i) RANK-L- and M-CSF-treated primary bone marrow cultures, in which a large proportion of monocytic precursors are undergoing differentiation, and (ii) rapidly-proliferating RAW264.7 monocyte precursors, a small proportion of which undergo differentiation upon RANK-L exposure. Expectedly, only a modest osteoclastogenic effect was noted in RANK-L-treated, relatively mature, murine bone marrow cultures that failed to display a concentration-dependence to ACTH (Fig. 4D). In contrast, clear dose-dependency of the ACTH effect was noted with RAW264.7 cells (Fig. 4E). Finally, the expression of osteoclast differentiation genes was measured by quantitative PCR. The expression of early-onset genes, namely TRAP, cathepsin K, metalloproteinase-9 and -13, NFATc1, and the NF-κB subunits and inhibitors, was increased in response to ACTH (cf. RANK-L alone, Fig. 4F). However, the relatively late-onset genes, including the calcitonin receptor and integrin β3, failed to rise with ACTH as did the nonresponsive NFAT isoforms c2 and c4 (Fig. 4G).
Discussion
We show that the experimental osteonecrosis induced by methylprednisolone acetate in normal rabbits is reduced dramatically with ACTH (cosyntropin) injection. No treatment for this debilitating condition, except for surgical debridement, is currently available (3). We also show that ACTH stimulates VEGF production in vivo and supports the maturation and survival of osteoblasts in vitro by inducing VEGF secretion by these cells through the ACTH receptor MC2R. These results are significant in that they not only expand the pituitary-bone axis to the pathogenesis of glucocorticoid-induced osteonecrosis, but also create a rationale for the extended use of cosyntropin in humans.
Our discovery that ACTH targets bone is consistent earlier studies on the skeletal actions of anterior pituitary hormones, namely TSH and FSH, which bypass traditional endocrine targets to affect bone mass directly with remarkable sensitivity (11–13). We have suggested that low TSH and high FSH contribute to the thyrotoxic and postmenopausal bone loss that has traditionally been attributed solely to reciprocal changes in master hormones (13, 16). Our recent findings that the posterior pituitary hormone oxytocin also targets the skeleton directly (23) and contributes to maternal hyperresorption during pregnancy (24) further testify to the direct regulation of the skeleton by pituitary hormones.
In this study, however, although warranted from its potent effect on the osteoblast, we have not purposefully investigated whether ACTH normally regulates bone mass. Clinical evidence shows, however, that patients with adrenal Cushing's syndrome, where ACTH levels are suppressed, experience greater bone loss than those with pituitary Cushing's with high serum ACTH levels (25). Likewise, patients with familial glucocorticoid deficiency with elevated ACTH levels, due to hypothalamic feedback, have a higher bone mass than age-matched controls (26). Taken together, the findings are consistent with at least a small anabolic effect of ACTH, which seemingly counteracts the bone loss due to cortisol.
Notwithstanding, the absence of mouse genetic data, the results show clearly that ACTH can be used as a drug to prevent osteonecrosis, a dreaded complication of chronic glucocorticoid therapy (3). The femoral head is a site of high bone turnover, with formation and resorption occurring continuously over a large fraction of the total surface area. This surface area is also embedded with an extensive capillary network (21); this means that the maintenance and regeneration of capillaries requires support by molecules such as VEGF. We show that ACTH stimulates VEGF production in methylprednisolone acetate-treated rabbits; this constitutes direct evidence for the effect of ACTH on VEGF expression in vivo. We also demonstrate that glucocorticoids eliminate VEGF production in osteoblast-monocyte cocultures; that ACTH stimulates the production of VEGF by the osteoblast and that the effect of ACTH is VEGF-dependent. Methylprednisolone acetate will inevitably inhibit VEGF synthesis and subject osteoblasts and the adjacent vascular network to severe damage, resulting in necrotic debris, degenerating capillaries and extravasated blood cells (Fig. S2). We term this microvascular necrosis—to distinguish it from the avascular necrosis that occurs with the traumatic avulsion of the artery supplying the femoral head. With that said, a number of other mechanisms for osteonecrosis have been proposed, which include lipid and clotting defects (27, 28), suppressed Wnt-β-catenin signaling, and PPARγ abnormalities (29, 30).
However, the observation that low ACTH is associated with microvascular osteonecrosis and that ACTH administration reduces such necrosis, even despite methylprednisolone acetate, provides a firm rationale not only for a de facto cause-effect relationship but also for the use of ACTH in humans to decrease the risk of osteonecrosis. Indeed, the synthetic, less antigenic fragment of ACTH, cosyntropin is approved for use in humans for the diagnosis of adrenocortical insufficiency (31). Once used as an anti-inflammatory agent, and for nonendocrine glucocorticoid-responsive disorders, the medical use of repository ACTH is now restricted to the treatment of infantile myoclonic seizures (32). It is not unreasonable, therefore, to envisage the future clinical use for cosyntropin to prevent human osteonecrosis. That said, the clinical utility of these observations remain to be proven, and more importantly, the findings presented require independent verification in other laboratories.
Materials and Methods
Skeletal Phenotyping.
We used a rabbit model that we have previously described and validated (6). We treated female rabbits averaging 4.48 kg with depot methylprednisolone acetate (Depomedrol, MPA) alone or MPA plus ACTH (1–24) (cosyntropin), 0.2 μg/kg, daily, as an s.c. injection. They were also administered 10 mg/kg tetracycline 5 days apart before sacrifice. The femoral heads were subject to dual energy absorptiometry (DXA) by a GE-Lunar Piximus, and to microcomputed tomography by SCANCO Viva40. Necrotic surfaces were quantitated by computer-assisted morphometry, as described previously (6).
Cell Culture Experiments.
Bone marrow-derived stromal cells were cultured up to 28 days in differentiation medium consisting of α-MEM and 100 μM ascorbate and 10 mM glycerol-2-phosphate, as described (12). Human mesenchymal cell cultures or transformed MG63 cells were similarly incubated for 28 days, as described (12). Immunoprecipitation and Western immunoblotting for VEGF expression was carried out on culture supernatants as previously described (12). Annexin V staining on osteoblasts was carried out as described by Abe et al. (11). For the osteoclast formation experiments, bone marrow hematopoetic stem cells were Ficoll-purified and incubated with RANK-L (50 ng/mL) and M-CSF (30 ng/mL) for 5 days, after which TRAP+ osteoclast formation was assessed using a kit (Sigma) (12). Quantitative PCR was performed with appropriate human and murine primers, as before (12). The mouse-derived osteoblastic cell line MC3T3-E1 was grown in α-MEM medium supplemented with 10% FCS at 37 °C, 5% CO2. VEGF was knocked down using shRNA lentivirus particles (Santa Cruz Biotechnology) targeting mouse VEGF-A, per manufacturers protocol. Five days after lentiviral transduction, stable clones expressing the shRNA were selected by Puromycin resistance. VEGF knockdown was confirmed by Western blot.
Supplementary Material
Acknowledgments
This research was supported by grants to M.Z., L.S., C.I., L.J.R., and H.C.B. from the National Institutes of Health. M.Z. in particular acknowledges National Institutes of Health Grants AG23176, DK70526, and DK80459. J.I. is grateful to the American Federation for Aging Research for generous support.
Footnotes
Conflict of interest statement: M.Z. is a named inventor of a pending patent application related to osteoclastic bone resorption filed by Mount Sinai School of Medicine. In the event the pending or issued patent is licensed, he would be entitled to a share of any proceeds Mount Sinai School of Medicine receives from the licensee.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0912176107/-/DCSupplemental.
References
- 1.Gebhard KL, Maibach HI. Relationship between systemic corticosteroids and osteonecrosis. Am J Clin Dermatol. 2001;2:377–388. doi: 10.2165/00128071-200102060-00004. [DOI] [PubMed] [Google Scholar]
- 2.Mankin HJ. Nontraumatic necrosis of bone (osteonecrosis) N Engl J Med. 1992;326:1473–1479. doi: 10.1056/NEJM199205283262206. [DOI] [PubMed] [Google Scholar]
- 3.Assouline-Dayan Y, et al. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum. 2002;32:94–124. [PubMed] [Google Scholar]
- 4.Silverman SL, Lane NE. Glucocorticoid-induced osteoporosis. Curr Osteoporos Rep. 2009;7:23–26. doi: 10.1007/s11914-009-0005-4. [DOI] [PubMed] [Google Scholar]
- 5.Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: Pathophysiology and therapy. Osteoporos Int. 2007;18:1319–1328. doi: 10.1007/s00198-007-0394-0. [DOI] [PubMed] [Google Scholar]
- 6.Eberhardt AW, Yeager-Jones A, Blair HC. Regional trabecular bone matrix degeneration and osteocyte death in femora of glucocorticoid- treated rabbits. Endocrinology. 2001;142:1333–1340. doi: 10.1210/endo.142.3.8048. [DOI] [PubMed] [Google Scholar]
- 7.Kikkawa M, Imai S, Hukuda S. Altered postnatal expression of insulin-like growth factor-I (IGF-I) and type X collagen preceding the Perthes’ disease-like lesion of a rat model. J Bone Miner Res. 2000;15:111–119. doi: 10.1359/jbmr.2000.15.1.111. [DOI] [PubMed] [Google Scholar]
- 8.Kerachian MA, Séguin C, Harvey EJ. Glucocorticoids in osteonecrosis of the femoral head: A new understanding of the mechanisms of action. J Steroid Biochem Mol Biol. 2009;114:121–128. doi: 10.1016/j.jsbmb.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko JY, Chen SH, Chen CE, Chen SH, Eng HL. Femoral head preservation in non-united femoral neck fracture and head osteonecrosis in Cushing's disease. J Formos Med Assoc. 2004;103:234–238. [PubMed] [Google Scholar]
- 10.Zhong Q, et al. Multiple melanocortin receptors are expressed in bone cells. Bone. 2005;36:820–831. doi: 10.1016/j.bone.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Abe E, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115:151–162. doi: 10.1016/s0092-8674(03)00771-2. [DOI] [PubMed] [Google Scholar]
- 12.Sun L, et al. FSH directly regulates bone mass. Cell. 2006;125:247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 13.Iqbal J, et al. Skeletal morphofunctional considerations and the pituitary-thyroid axis. Front Biosci. 2009;1:92–107. doi: 10.2741/S9. [DOI] [PubMed] [Google Scholar]
- 14.Boss JH, Misselevich I. Osteonecrosis of the femoral head of laboratory animals: The lessons learned from a comparative study of osteonecrosis in man and experimental animals. Vet Pathol. 2003;40:345–354. doi: 10.1354/vp.40-4-345. [DOI] [PubMed] [Google Scholar]
- 15.Martin LG, Behrend EN, Mealey KL, Carpenter DM, Hickey KC. Effect of low doses of cosyntropin on serum cortisol concentrations in clinically normal dogs. Am J Vet Res. 2007;68:555–560. doi: 10.2460/ajvr.68.5.555. [DOI] [PubMed] [Google Scholar]
- 16.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 17.Chang PL, et al. Comparison of fetal and adult marrow stromal cells in osteogenesis with and without glucocorticoids. Connect Tissue Res. 2006;47:67–76. doi: 10.1080/03008200600584074. [DOI] [PubMed] [Google Scholar]
- 18.Jacobsen KA, et al. Bone formation during distraction osteogenesis is dependent on both VEGFR1 and VEGFR2 signaling. J Bone Miner Res. 2008;23:596–609. doi: 10.1359/JBMR.080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schipani E, Maes C, Carmeliet G, Semenza GL. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J Bone Miner Res. 2009;24:1347–1353. doi: 10.1359/jbmr.090602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joukov V, et al. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 1997;16:3898–3911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blair HC, Zaidi M, Huang CL, Sun L. The developmental basis of skeletal cell differentiation and the molecular basis of major skeletal defects. Biol Rev Camb Philos Soc. 2008;83:401–415. doi: 10.1111/j.1469-185X.2008.00048.x. [DOI] [PubMed] [Google Scholar]
- 22.Lyons PD, Blalock JE. The kinetics of ACTH expression in rat leukocyte subpopulations. J Neuroimmunol. 1995;63:103–112. doi: 10.1016/0165-5728(95)00133-6. [DOI] [PubMed] [Google Scholar]
- 23.Tamma R, et al. Oxytocin is an anabolic bone hormone. Proc Natl Acad Sci USA. 2009;106:7149–7154. doi: 10.1073/pnas.0901890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, et al. Oxytocin deficiency impairs maternal skeletal remodeling. Biochem Biophys Res Commun. 2009;388:161–166. doi: 10.1016/j.bbrc.2009.07.148. [DOI] [PubMed] [Google Scholar]
- 25.Minetto M, et al. Bone loss is more severe in primary adrenal than in pituitary-dependent Cushing's syndrome. Osteoporos Int. 2004;15:855–861. doi: 10.1007/s00198-004-1616-3. [DOI] [PubMed] [Google Scholar]
- 26.Elias LL, et al. Tall stature in familial glucocorticoid deficiency. Clin Endocrinol (Oxf) 2000;53:423–430. doi: 10.1046/j.1365-2265.2000.01122.x. [DOI] [PubMed] [Google Scholar]
- 27.Ajmal M, Matas AJ, Kuskowski M, Cheng EY. Does statin usage reduce the risk of corticosteroid-related osteonecrosis in renal transplant population? Orthop Clin North Am. 2009;40:235–239. doi: 10.1016/j.ocl.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glueck CJ, Freiberg RA, Wang P. Heritable thrombophilia-hypofibrinolysis and osteonecrosis of the femoral head. Clin Orthop Relat Res. 2008;466:1034–1040. doi: 10.1007/s11999-008-0148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeh CH, Chang JK, Wang YH, Ho ML, Wang GJ. Ethanol may suppress Wnt/beta-catenin signaling on human bone marrow stroma cells: A preliminary study. Clin Orthop Relat Res. 2008;466:1047–1053. doi: 10.1007/s11999-008-0171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim TH, et al. Peroxisome proliferator-activated receptor-gamma gene polymorphisms are not associated with osteonecrosis of the femoral head in the Korean population. Mol Cell. 2007;24:388–393. [PubMed] [Google Scholar]
- 31.Pura M, Kreze A, Kentos P, Vanuga P. The low dose (1 μg) Cosyntropin Test (LDT) for primary adrenocortical insufficiency: Defining the normal cortisol response and report on first patients with Addison's disease confirmed with LDT. Exp Clin Endocirnol Diabetes. 2010;118:151–157. doi: 10.1055/s-0029-1202275. [DOI] [PubMed] [Google Scholar]
- 32.Wheless JW, Clarke DF, Arzimanoglou A, Carpenter D. Treatment of pediatric epilepsy: European expert opinion, 2007. Epileptic Disord. 2007;9:353–412. doi: 10.1684/epd.2007.0144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.