Abstract
Structural features of neurons create challenges for effective production and distribution of essential metabolic energy. We investigated how metabolic energy is distributed between cellular compartments in photoreceptors. In avascular retinas, aerobic production of energy occurs only in mitochondria that are located centrally within the photoreceptor. Our findings indicate that metabolic energy flows from these central mitochondria as phosphocreatine toward the photoreceptor’s synaptic terminal in darkness. In light, it flows in the opposite direction as ATP toward the outer segment. Consistent with this model, inhibition of creatine kinase in avascular retinas blocks synaptic transmission without influencing outer segment activity. Our findings also reveal how vascularization of neuronal tissue can influence the strategies neurons use for energy management. In vascularized retinas, mitochondria in the synaptic terminals of photoreceptors make neurotransmission less dependent on creatine kinase. Thus, vasculature of the tissue and the intracellular distribution of mitochondria can play key roles in setting the strategy for energy distribution in neurons.
Keywords: energy metabolism, phototransduction
A significant energy distribution problem can arise from the relative locations of mitochondria, ion pumps, and synapses in neurons. In photoreceptors, ion pumps occupy the intervening space between the centrally located mitochondria and the synaptic terminal. Ion pumping in dark-adapted photoreceptors consumes ∼20× more energy than neurotransmission (1). Therefore, the pumps could intercept all the metabolic energy made by the mitochondria before it can reach the synaptic terminal. In the vascularized retinas of mice, rats, and humans (2–4) this problem is solved by the presence of additional mitochondria in the terminal. However, in the avascular retinas of zebrafish, salamanders, rabbits, and guinea pigs there are no mitochondria in the terminals (2, 4, 5), which creates a need to partition some of the energy made by the central mitochondria into a protected form that can bypass the ion pumps to support the essential energy demands of the synaptic terminal.
Energy consumption within retinal photoreceptors is compartmentalized and light-dependent. During illumination, phototransduction and light adaptation consume energy in the outer segment (OS). In darkness, energy is consumed by ion pumps in the inner segment and by glutamate release at the synaptic terminal (1). Energy demands and O2 consumption are far greater in darkness than in light (1, 6–8).
Metabolic energy is distributed in most cells as either ATP or phosphocreatine (PCr). There are 2 isoforms of creatine kinase (CK) in neurons, ubiquitous mitochondrial creatine kinase (uMtCK), and brain-type cytoplasmic creatine kinase (CK-B). uMtCK creates PCr from ATP at mitochondria (9), and CK-B can recreate ATP from PCr at sites of energy demand. In this way uMtCK and CK-B can collaborate to transfer metabolic energy between neuronal compartments (10, 11).
This paper describes how photoreceptor neurons solve the energy distribution problem. We found, unexpectedly, that CK-B in photoreceptors is sequestered in the synaptic terminal. We report evidence that metabolic energy flows as PCr to the terminal in darkness. In light, it flows as ATP to the OS.
Results
Distribution of Mitochondria in Photoreceptors Depends on Vascularization.
A comparison of synaptic terminals in some of the types of retinas we studied, mouse, salamander, and zebrafish, is shown in Fig. S1. Mitochondria are abundant in mouse photoreceptor terminals but absent from salamander and larval zebrafish terminals. This observation confirms previous findings that mitochondria infiltrate photoreceptor terminals in the vascularized retinas of mice and rats (2–4), but they are absent from the terminals in the avascular retinas of salamanders and zebrafish (2, 4, 5).
CK-B and uMtCK Are the only Sources of CK Activity in Mouse Retina.
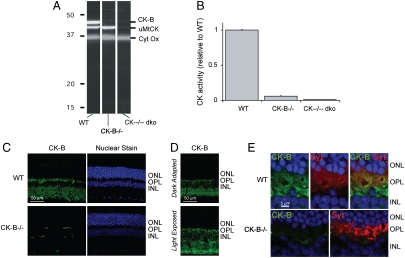
We used knockout mice to identify the enzymes that contribute CK activity to the retina. The immunoblot in Fig. 1A confirms that uMtCK and CK-B are present in WT mouse retinas (12, 13). The blot also shows that CK-B is absent in retinas from CK-B-/- mice (14) and that both isoforms are missing in retinas from CK-B-/-;uMtCK-/- (“CK--/-- dko”) mice (15). We found that normal WT mouse retina homogenates have 2.0 nmol/ min of CK activity per retina. Homogenates of CK-B-/- retinas have ∼5% and CK--/-- dko retinas have < 1% of normal activity (Fig. 1B).
Fig. 1.
Identification of the types of CK in mouse retina. (A) Immunoblot of retina homogenates from WT, CK-B-/-, and CK--/-- dko mice. The blots were probed with antibodies to CK-B and uMtCK. The pattern of labeling demonstrates the specificity of the antibodies. (B) CK activity in retinal homogenates from WT, CK-B-/-, and CK--/-- dko mice. The absence of CK activity in the dko retinas shows that no other active CK isoforms of CK are expressed at significant levels in retina. (C) CK-B antibody immunostaining (Top) in WT (Top) and in CK-B-/- knockout (Bottom). There is intense labeling is in the photoreceptor synapse layer. The signals in the CK-B-/- retina come from the secondary antibody reacting with an unknown antigen in the retinal capillaries. The panels on the right show nuclear staining to demonstrate that there is no retinal degeneration in CK-B-/- retinas. (D) Comparison of the distribution of CK-B in light- vs. dark-adapted mouse retinas. (E) Pre- and postsynaptic localization of CK-B in the photoreceptor terminal layer. Sections were double labeled with antibodies to CK-B (Green) and to a presynaptic marker, synaptotagmin (Red). The top three panels show the outer nuclear layer (ONL), outer plexiform layer (OPL), and inner nuclear layer (INL) of a WT mouse retina. The bottom two panels show labeling of a CK-B-/- retina.
CK-B is Confined in Photoreceptors to the Synaptic Terminal.
The upper left panel of Fig. 1C shows a section of a light-adapted mouse retina probed with an antibody to mammalian CK-B. Labeling in photoreceptors is strongest at the terminal and weakest in the OS. Immunoelectron microscopy provided additional evidence that CK-B is absent from the OS (Fig. S2E) (12). There is little difference in the distribution of CK-B in light vs. dark (Fig. 1D). Double labeling with an antibody to synaptotagmin, a presynaptic marker, showed that CK-B is present in both pre- and postsynaptic regions of the outer plexiform layer (Fig. 1E). Antibody specificity was confirmed by using CK-B-/- retinas (compare upper and lower panels of Fig. 1C).
To determine if there is any CK-B in other parts of the photoreceptor besides the terminal, images were intentionally overexposed. Under conditions where the CK-B signal in the terminals is well into saturation, some CK-B immunoreactivity could be detected throughout the photoreceptor (Fig. S2).
Analyses of serial retina sections confirmed this distribution in rat and salamander retinas. Flattened rat retinas were frozen, and 10-μm serial sections were cut and analyzed by immunoblotting (16) with antibodies that recognize specific isoforms of mammalian CK. The specificity of these antibodies is shown in Fig. 1A. Synaptotagmin (synaptic terminals), rhodopsin (OS), and uMtCK (mitochondria) served as landmarks. The results (Fig. S3A) confirm that CK-B is absent from OSs and enriched in the photoreceptor synaptic layer of this vascularized retina. The distribution of CK activity in rat serial sections (Fig. S3B) also is consistent with this localization. Antibodies to CK from avascular retinas were not available, so we instead measured CK activity. Flattened dark-adapted salamander retinas were frozen, and 5-μm serial sections were cut. Fig. S4D shows the distribution of CK activity in the salamander retina compared to the distributions of DNA (Fig. S4B), COX (Fig. S4C), and rhodopsin (Fig. S4E). A cross-section of a salamander retina is shown in Fig. S4A for orientation. CK activity in the salamander retina is lowest in the OS layer and highest in the ellipsoid and synaptic layers.
Model for Energy Distribution Within Photoreceptors.
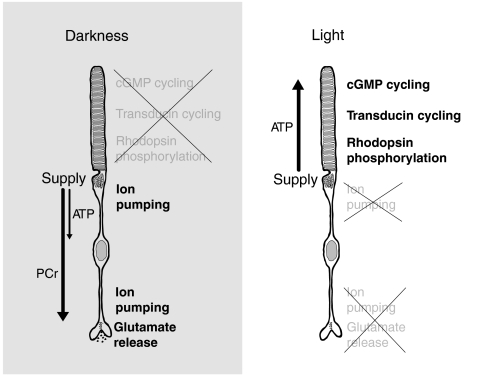
CK-B is abundant in the synaptic layer and absent from OSs. Therefore, the photoreceptor terminal, but not the OS, can convert PCr into ATP. This observation suggested a model for how energy is distributed in photoreceptors (Fig. 2). In light, energy flows from the cell body to the OS as ATP, but in darkness, it flows to the terminal as PCr. The model predicts that CK activity would not be required in light, whereas in darkness CK activity would be required for energy to flow to the synaptic terminal. Our model also predicts a more substantial need for CK activity in avascular retinas than in vascularized retinas, which have mitochondria within their synaptic terminals.
Fig. 2.
Model for energy distribution within photoreceptors. In darkness, metabolic energy is consumed primarily in the inner compartments of the photoreceptor. ATP produced by the central mitochondria is consumed by ion pumps before it can reach the synaptic terminal. The mitochondria also use uMtCK to make PCr, which is not a substrate for the pumps. The PCr bypasses the pumps and diffuses to the synaptic terminal where CK-B, which is localized specifically to the terminal, converts PCr back into ATP to support synaptic transmission. In light, energy consumption shifts to the OS where ATP meets the energy demands of phototransduction. The model shown is for avascular retinas. In vascularized retinas, there are additional mitochondria at the synaptic terminal that can produce ATP directly within the terminal.
1 Fluoro 2,4 Dinitrobenzene (FDNB) Is a Specific Inhibitor of CK.
We tested this model by using a cell-permeant inhibitor to alter CK activity. At low concentrations, FDNB specifically modifies a reactive cysteine on CK and inhibits the activity of CK (17, 18). Fig. S5A shows that 10 μM FDNB inhibits > 95% of CK activity in mouse retina homogenates. The same amount of FDNB does not affect cGMP-gated channel activity (Fig. S6 A and B), cGMP levels (Fig. S6C), high-energy nucleotide levels (Fig. S7 A–C), O2 consumption (Fig. S7D), or glucose uptake (Fig. S7E). These tests confirm that FDNB is a specific inhibitor of CK activity.
FDNB Does Not Affect Energy-Dependent Activities in the OS.
The model in Fig. 2 predicts that energy-dependent functions in the OS should be insensitive to FDNB. Processes in the OS that consume energy include phosphorylation of rhodopsin, reduction of all-trans retinal, and phototransduction. FDNB does not affect any of these OS activities (Figs. S5 B–D and S7F).
Effect of FDNB on the Dark Current.
If the model in Fig. 2 is correct, then energy-dependent activities in the terminal ought to be sensitive to FDNB. The dark current is a flow of ions into the OS, but it is sustained by ATP-dependent pumps in the inner segment and terminal (1, 19). The dark current is reduced ∼50% by 10 μM FDNB in both salamander (Fig. S5E) and mouse (Fig. S5F) rods. Partial inhibition of pump activity is consistent with the model in Fig. 2. Pumps in the cell body may use ATP directly, whereas those in the terminal may be more dependent on PCr.
Effects of FDNB on Synaptic Transmission.
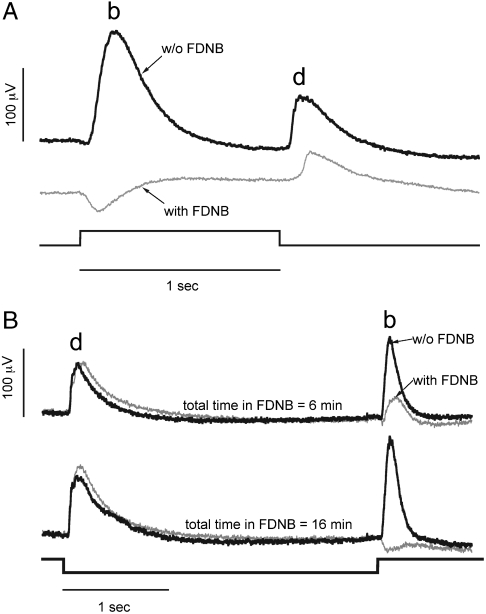
Our model predicts that synaptic transmission would be especially sensitive to inhibition of CK, particularly in avascular retinas. The synaptic terminal requires energy to refill and recycle vesicles, and it contains the highest concentration of CK-B in the cell. We tested whether FDNB influences components of the electroretinogram (ERG) that depend on synaptic transmission. Larval zebrafish eyes were used because their retinas are avascular and because they produce robust on and off ERG responses (20). The larval eye is permeable to small molecules such as FDNB (21). Fig. 3A (Black Trace) shows ERG responses of dark-adapted eyes from six day postfertilization (dpf) larvae exposed to a 1-sec step of bright light. When light suppresses glutamate release, the synapse is cleared by a glutamate transporter (22). Removal of glutamate depolarizes on bipolar cells (BPCs) and hyperpolarizes off BPCs, a response that contributes to the ERG signal known as the “b wave.” Returning the eye to darkness restores glutamate release, which hyperpolarizes on BPCs, depolarizes off BPCs, and contributes to the “d wave.” b and d waves represent combined BPC responses superimposed on field potentials from suppression and restoration of photoreceptor dark current (23). We used 2-amino-4-phosphonobutyrate and 6-cyano-7-nitroquinoxaline-2,3-dione to confirm that in the eyes of zebrafish larvae suppression of synaptic transmission leaves only the photoreceptor field potential contribution of the ERG (Fig. S8). We found that FDNB treatment in darkness similarly eliminates all of the components of both b and d waves associated with synaptic transmission (Fig. 3A, Grey Trace). Only the changes in field potential associated with the photoreceptor dark current remain. This result is consistent with FDNB choking off the flow of energy that supports glutamate release in darkness.
Fig. 3.
Darkness enhances the effect of FDNB on synaptic transmission. (A) ERG responses of eyes from zebrafish larvae to a step of intense white light. The eyes were treated either with control solution (Top Trace) or with the same solution including 20 μM FDNB (Bottom Trace). When the eyes are dark-adapted the on response, “b wave,” and the off response, “d wave,” are eliminated by FDNB. (B) Light adaptation reduces the effect of FDNB. Light-adapted zebrafish either were treated with control solution (Black) or with the same solution including 20 μM FDNB (Gray). Eyes were exposed to constant illumination during isolation. Then they were exposed to a pulse of darkness every 30 sec. Responses to the pulses of darkness at 6 and 16 min after the beginning of FDNB treatment are shown. Off responses are less sensitive to FDNB when the eyes are maintained under nearly continuous illumination.
Light reduces energy demand by suppressing the dark current and glutamate release. We hypothesized that the decreased energy demand during illumination would eliminate or reduce the need for CK activity. We tested this idea by analyzing effects of FDNB on light-adapted ERG responses. We dissected zebrafish larval eyes in bright light and continued to illuminate them during FDNB treatment. After 5 min of constant illumination, we recorded responses to 3-sec pulses of darkness once every 30 sec. The traces in Fig. 3B show that d waves (off responses) are unaffected by FDNB. Apparently, continuous illumination during FDNB treatment reduces the energy demand from ion pumping and slows vesicle recycling enough that a trickle of ATP is sufficient to fill vesicles. The onset of darkness releases the stored glutamate to generate the d wave. FDNB still eliminates the b wave because the energy demands of vesicle refilling in darkness cannot be supported even for 3 sec. These results confirm that FDNB affects the flow of energy to the synapse rather than the mechanism of neurotransmission.
Paired Cell Recordings.
Paired cell recordings provide a more precise way to analyze the relationship between CK activity and synaptic transmission. We used salamander retinas for these experiments because their unusually large neurons have robust electrical properties.
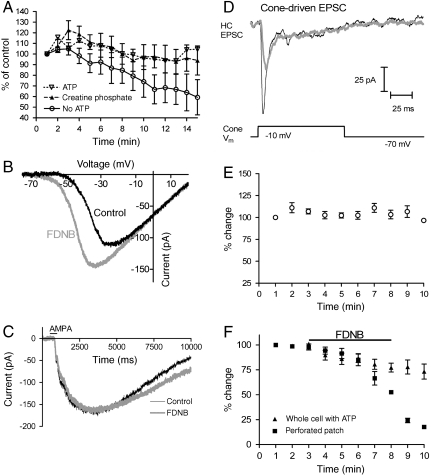
We first established that CK in salamander cones is functional. A whole cell patch was established on a horizontal cell (HC) to record excitatory postsynaptic currents (EPSCs) evoked by electrically depolarizing an upstream cone. A separate whole cell patch also was established on the cone to control its membrane potential. Depolarization of the cone from -70 to -10 mV evoked an EPSC in the HC. When the pipette on the photoreceptor contained no high-energy metabolite, EPSC responses waned (Fig. 4A). EPSC responses stayed constant for up to 20 min when the pipette contained 10 mM ATP. To determine if photoreceptors can use endogenous CK to convert PCr into ATP, we substituted PCr for ATP in the pipette. Patch pipettes with PCr (filled triangles) sustain EPSC responses longer than pipettes with no high-energy metabolites (open circles) (Fig. 4A). Importantly, the ability of PCr to sustain responses shows that CK is present and active in photoreceptors.
Fig. 4.
CK activity is essential for synaptic transmission by salamander cones. (A) Confirmation of CK activity in salamander cones. The amplitudes of EPSCs evoked in HCs by depolarizing voltage clamped cones (100-ms step from -70 to -10 mV) are shown. The patch pipette on the HC always contained 10 mM ATP. The patch pipette on the cone contained either ATP, PCr, or neither ATP nor PCr as indicated. Without ATP or PCr, EPSCs waned (n = 12). However, when either 10 mM ATP (n = 4) or 10 mM PCr (n = 9) were included, the responses were stable. The stability of the PCr response shows there is CK activity in cones that can convert PCr from the pipette into ATP to support sustained synaptic transmission. (B) FDNB causes the inward Ca2+ current in cones to activate at more negative potentials. The Ca2+ current was measured (n = 8) by using a ramp voltage protocol (0.5 mV/ms). The passive input resistance, measured between -70 and -80 mV, was subtracted post hoc. (C) FDNB does not affect glutamate receptor currents evoked in HCs by AMPA (100 μM) ejected by pressure from a puffer pipette (n = 4). ATP (10 mM) was included in the HC whole cell patch pipette. (D) The effect of 10 μM FDNB on EPSCs recorded from a HC in response to depolarization (100-ms step from -70 to -10 mV) of a simultaneously voltage-clamped cone in the salamander retina. Black trace: Control conditions; gray trace: after bath application of FDNB for 5 min. The whole cell patch pipette on the HC included 10 mM ATP. The cone was clamped with a perforated patch pipette to ensure reliance on endogenous nucleotides. Responses to depolarization were stable when the cone was voltage clamped by using a perforated patch (E). FDNB blocked these responses within minutes (filled squares in F) (n = 4). By contrast, when 10 mM ATP was introduced into the cone by using ruptured patch whole cell recordings, FDNB had little effect (filled triangles in F) (n = 6).
In the course of these experiments we identified an effect of FDNB that might not be related to its action on CK. FDNB shifts the voltage dependence of the cone Ca2+ current by about -10 mV (Fig. 4B). Although we did not identify the cause of this shift, we made sure it does not affect our analysis by using only voltage steps from -70 to -10 mV. These voltages activate Ca2+ currents similarly with and without FDNB (Fig. 4B).
HCs Reliably Report Glutamate Release from Photoreceptors.
Because we relied on HCs to report glutamate release, we made sure that FDNB does not affect the ability of HCs to respond to stimulation (24). Fig. 4C shows that 10 μM FDNB does not influence HC currents induced by AMPA. Thus, HCs are reliable reporters of glutamate release from photoreceptors. HC patch pipettes in all experiments included 10 mM ATP to bypass any need HCs may have for CK.
Sustained Glutamate Release from Photoreceptor Terminals Requires CK Activity.
Having established that CK is active in photoreceptors and that HCs reliably report glutamate release, we could test whether endogenous CK activity is required to sustain glutamate release. We used a nystatin-induced perforated patch electrode on the photoreceptor to control its membrane potential and to ensure that the photoreceptor relied on endogenous mechanisms for energy distribution. Depolarization of a cone from -70 to -10 mV induced glutamate release, which caused a transient inward synaptic current in the HC (Fig. 4D). In the absence of FDNB, the amplitude of these responses remained constant over 10 min (Fig. 4E). FDNB progressively attenuated the EPSCs in HCs (Filled Squares, Fig. 4F). Fig. S9 A–C shows the corresponding experiments for depolarization of rods. Our findings show that CK activity is needed to maintain a flow of metabolic energy sufficient to sustain repetitive glutamate release at the photoreceptor terminal.
We also used paired cell recording to confirm that there are no relevant off-target effects of FDNB. We included 10 mM ATP in the whole cell patch pipettes on both the cone and HC, which ensured that neither cell would depend on PCr for energy distribution. FDNB should be ineffective under these conditions if it has no relevant target other than CK. We used an ATP-filled pipette to depolarize the cone and recorded postsynaptic currents in the HC. With ATP in the pipette 10 μM FDNB had little effect (Filled Triangles, Fig. 4F), confirming the absence of off-target effects.
Role of Creatine Kinases in Vascularized Retinas.
Mitochondria migrate to the photoreceptor terminals in vascularized retinas where O2 is available from the inner retina (Fig. S1). These mitochondria could provide energy needed to support synaptic transmission and make photoreceptors in vascularized retinas less dependent on CK. We tested whether PCr and CK are as essential for synaptic transmission in vascularized mouse retinas as they are in the avascular zebrafish and salamander retinas.
We evaluated the role of CK in phototransduction and synaptic transmission in mouse retinas by comparing flash-induced ERG responses of WT and CK-B-/- mice. The a wave of the dark-adapted mouse ERG reports phototransduction in the OS, and the b wave reports synaptic transmission and bipolar cell responses. There is little difference between ERG responses of CK-B-/- (Fig. S9D) vs. WT mice. These findings are consistent with the synaptic mitochondria in these vascularized retinas providing sufficient energy to the terminal to support synaptic transmission under the conditions of these ERG analyses.
CK either could play no role at all in vascularized retinas or it could provide partial support for normal function of the synaptic terminal. If it plays a supporting role, then CK deficiency might cause retinas to compensate in some way for the loss of energy at the terminal. Previous analyses of other tissues showed that CK deficiency can evoke a compensation in metabolic capacity (14, 15, 25–30). We found evidence for such compensation in the retinas of CK-deficient mice (Fig. S9E). COX I levels are 48 ± 5% higher than normal in CK-B-/- retinas, and uMtCK is 38 ± 5% higher. COX I also is 24 ± 5% higher than normal in CK--/-- dko retinas. It is possible that less compensation is needed in dko retinas because they do not convert any ATP into PCr.
Discussion
Our findings support a model in which PCr is used to solve the fundamental energy distribution problem in photoreceptors. In darkness, PCr from the central mitochondria of the photoreceptor flows past the highly active ion pumps to the synaptic terminal where it is made back into ATP. In light, energy flows as ATP to the OS. We proposed this model on the basis of our observation of the distribution of CK in the retina. We then confirmed its physiological importance by using both biochemical and electrophysiological measurements.
Distribution of CK in the Retina.
A previous study detected CK-B in isolated bovine rod OS (13), but other studies of mouse (12) and chicken (31) retinas found CK-B only in photoreceptor inner segments and terminals. We used both immunological methods and biochemical activity measurements to confirm that CK is sequestered in the synaptic terminals of photoreceptors and absent from the OSs.
Energy Routing in Photoreceptors.
The polarized distribution of CK in photoreceptors suggests that PCr enhances energy flow only in one direction, toward the synaptic terminal. The effects of the CK inhibitor FDNB are consistent with this suggestion. FDNB does not influence the OS activities we measured. In contrast, it blocks neurotransmission from photoreceptors in zebrafish and salamander retinas. The photoreceptor terminals in these avascular retinas have no mitochondria, so they are especially dependent on PCr from mitochondria in the central domain of the photoreceptor.
Solutions to the Neuronal Energy Distribution Problem.
In darkness, ion pumps in a photoreceptor can consume as much as 108 ATP/ sec (1). These pumps reside in the plasma membrane between the central mitochondria and synaptic terminal (19). There they consume ATP ∼20 times faster than the terminal. If the rate of ATP production in the central photoreceptor is close to the rate at which the pumps consume it, then very little ATP can reach the terminal.
Our findings show that neurons solve this fundamental problem in distinct ways depending on morphology, environment, and availability of nutrients. Photoreceptors in avascular retinas divert a portion of mitochondrial ATP into a protected pathway. uMtCK makes PCr from ATP. The energy stored in PCr is protected because it cannot be used by the pumps. At the terminal CK-B converts the PCr back into ATP to support neurotransmission. Photoreceptors in vascular retinas solve the problem in a different way. Because O2 is available from inner retina capillaries, mitochondria migrate to the terminal for on-site production of ATP.
Energy Requirements for Synaptic Transmission.
Release of glutamate from photoreceptor terminals in darkness requires a continuous flow of energy to support recycling and refilling of vesicles. Each vesicle requires ∼10,000 ATP to reload with glutamate (32) and ∼500 vesicles fuse per second in darkness (33). These estimates suggest that the overall ATP consumption in the steady state could be as high as ∼5 × 106 ATP per second. Our direct measurements of CK activity in mouse retina homogenates and the distribution of CK activity show that there is enough CK even in a mouse photoreceptor terminal to make ∼108 ATP per second, more than enough to meet this demand.
Additional Perspectives.
Our findings provide a framework for understanding energy distribution in spatially polarized neurons. Additional studies will be needed to determine if regulation of either uMtCK or CK-B influences the efficiency and directionality of energy flow. Our study was extensive, but we focused it only on aerobic energy production. Further studies will be needed to address the equally important contributions of glycolysis to photoreceptor energy metabolism in light and darkness (34, 35).
PCr and ATP production could be regulated so it is possible that inappropriate regulation could be a fundamental cause of photoreceptor pathology. Creatine also can have neuroprotective activity (36). PCr disperses throughout the photoreceptor (37) even though it is used mainly at the synaptic terminal. The entire cytoplasm is available to store PCr as an energy buffer. In principle, the reservoir of creatine and PCr in the cytoplasm could help to reduce the shock and stress of frequent and rapid changes in the magnitude and location of energy demand in photoreceptors between darkness and light.
Experimental Procedures
Please refer to SI Text for detailed descriptions of the methods.
Serial Sectioning/Immunoblots.
Retina dissection and serial sectioning were as described (38).
Photoreceptor Responses and Dark Current Measurements.
Light-evoked currents from mouse and salamander rod photoreceptors were measured with suction electrodes from finely chopped pieces of retinal tissue as described (39).
Paired Whole Cell Recordings.
Paired pre- and postsynaptic recordings from rods or cones with horizontal or off bipolar cells were obtained by using retinal slices from the aquatic tiger salamander as described elsewhere (40).
Electroretinography.
Mouse ERGs were performed as described (41), and zebrafish ERGs were recorded from eyes isolated from six dpf zebrafish larvae as described by Wong et al. (21).
Supplementary Material
Acknowledgments.
We thank Martin Kushmerick for insightful and helpful discussions. We also thank Ed Parker for electron micrographs of mouse and zebrafish retinas. This study was supported by National Institutes of Health Grants R01 EY017863 and R01 EY06641 and the Core Grant for Vision Research P30EY1730.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1002471107/-/DCSupplemental.
References
- 1.Okawa H, Sampath AP, Laughlin SB, Fain GL. ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr Biol. 2008;18(24):1917–1921. doi: 10.1016/j.cub.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bentmann A, et al. Divergent distribution in vascular and avascular mammalian retinae links neuroglobin to cellular respiration. J Biol Chem. 2005;280(21):20660–20665. doi: 10.1074/jbc.M501338200. [DOI] [PubMed] [Google Scholar]
-
3.Johnson JE, Jr, et al. Spatiotemporal regulation of ATP and
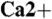 dynamics in vertebrate rod and cone ribbon synapses. Mol Vis. 2007;13:887–919. [PMC free article] [PubMed] [Google Scholar]
dynamics in vertebrate rod and cone ribbon synapses. Mol Vis. 2007;13:887–919. [PMC free article] [PubMed] [Google Scholar] - 4.Stone J, van Driel D, Valter K, Rees S, Provis J. The locations of mitochondria in mammalian photoreceptors: relation to retinal vasculature. Brain Res. 2008;1189:58–69. doi: 10.1016/j.brainres.2007.10.083. [DOI] [PubMed] [Google Scholar]
- 5.Townes-Anderson E, MacLeish PR, Raviola E. Rod cells dissociated from mature salamander retina: Ultrastructure and uptake of horseradish peroxidase. J Cell Biol. 1985;100(1):175–188. doi: 10.1083/jcb.100.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ames A, III, Li YY, Heher EC, Kimble CR. Energy metabolism of rabbit retina as related to function: High cost of Na+ transport. J Neurosci. 1992;12(3):840–853. doi: 10.1523/JNEUROSCI.12-03-00840.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: Fundamental and clinical aspects. Arch Ophthalmol. 2003;121(4):547–557. doi: 10.1001/archopht.121.4.547. [DOI] [PubMed] [Google Scholar]
- 8.Yu DY, Cringle SJ. Outer retinal anoxia during dark adaptation is not a general property of mammalian retinas. Comp Biochem Phys A. 2002;132(1):47–52. doi: 10.1016/s1095-6433(01)00528-1. [DOI] [PubMed] [Google Scholar]
- 9.Speer O, et al. Octameric mitochondrial creatine kinase induces and stabilizes contact sites between the inner and outer membrane. Biochem J. 2005;385(Pt 2):445–450. doi: 10.1042/BJ20040386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR. Functions and effects of creatine in the central nervous system. Brain Res Bull. 2008;76(4):329–343. doi: 10.1016/j.brainresbull.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 11.Meyer RA, Sweeney HL, Kushmerick MJ. A simple analysis of the “phosphocreatine shuttle”. Am J Physiol. 1984;246(5 Pt 1):C365–C377. doi: 10.1152/ajpcell.1984.246.5.C365. [DOI] [PubMed] [Google Scholar]
- 12.Sistermans EA, et al. Tissue- and cell-specific distribution of creatine kinase B: A new and highly specific monoclonal antibody for use in immunohistochemistry. Cell Tissue Res. 1995;280(2):435–446. doi: 10.1007/BF00307817. [DOI] [PubMed] [Google Scholar]
- 13.Hemmer W, Riesinger I, Wallimann T, Eppenberger HM, Quest AF. Brain-type creatine kinase in photoreceptor cell outer segments: Role of a phosphocreatine circuit in outer segment energy metabolism and phototransduction. J Cell Sci. 1993;106(Pt 2):671–683. doi: 10.1242/jcs.106.2.671. [DOI] [PubMed] [Google Scholar]
- 14.Jost CR, et al. Creatine kinase B-driven energy transfer in the brain is important for habituation and spatial learning behaviour, mossy fibre field size and determination of seizure susceptibility. Eur J Neurosci. 2002;15(10):1692–1706. doi: 10.1046/j.1460-9568.2002.02001.x. [DOI] [PubMed] [Google Scholar]
- 15.Streijger F, et al. Structural and behavioural consequences of double deficiency for creatine kinases BCK and UbCKmit. Behav Brain Res. 2005;157(2):219–234. doi: 10.1016/j.bbr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Sokolov M, et al. Massive light-driven translocation of transducin between the two major compartments of rod cells: A novel mechanism of light adaptation. Neuron. 2002;34(1):95–106. doi: 10.1016/s0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- 17.Buechter DD, Medzihradszky KF, Burlingame AL, Kenyon GL. The active site of creatine kinase. Affinity labeling of cysteine 282 with N-(2,3-epoxypropyl)-N-amidinoglycine. J Biol Chem. 1992;267(4):2173–2178. [PubMed] [Google Scholar]
- 18.Tombes RM, Shapiro BM. Metabolite channeling: A phosphorylcreatine shuttle to mediate high energy phosphate transport between sperm mitochondrion and tail. Cell. 1985;41(1):325–334. doi: 10.1016/0092-8674(85)90085-6. [DOI] [PubMed] [Google Scholar]
-
19.Molday LL, Wu WW, Molday RS. Retinoschisin (RS1), the protein encoded by the X-linked retinoschisis gene, is anchored to the surface of retinal photoreceptor and bipolar cells through its interactions with a
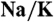 ATPase-SARM1 complex. J Biol Chem. 2007;282(45):32792–32801. doi: 10.1074/jbc.M706321200. [DOI] [PubMed] [Google Scholar]
ATPase-SARM1 complex. J Biol Chem. 2007;282(45):32792–32801. doi: 10.1074/jbc.M706321200. [DOI] [PubMed] [Google Scholar] - 20.Van Epps HA, Yim CM, Hurley JB, Brockerhoff SE. Investigations of photoreceptor synaptic transmission and light adaptation in the zebrafish visual mutant nrc. Invest Ophth Vis Sci. 2001;42(3):868–874. [PubMed] [Google Scholar]
- 21.Wong KY, Gray J, Hayward CJ, Adolph AR, Dowling JE. Glutamatergic mechanisms in the outer retina of larval zebrafish: Analysis of electroretinogram b- and d-waves using a novel preparation. Zebrafish. 2004;1(2):121–131. doi: 10.1089/zeb.2004.1.121. [DOI] [PubMed] [Google Scholar]
- 22.Hasegawa J, Obara T, Tanaka K, Tachibana M. High-density presynaptic transporters are required for glutamate removal from the first visual synapse. Neuron. 2006;50(1):63–74. doi: 10.1016/j.neuron.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Ueno S, et al. Contribution of retinal neurons to d-wave of primate photopic electroretinograms. Vision Res. 2006;46(5):658–664. doi: 10.1016/j.visres.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Yang JH, Maple B, Gao F, Maguire G, Wu SM. Postsynaptic responses of horizontal cells in the tiger salamander retina are mediated by AMPA-preferring receptors. Brain Res. 1998;797(1):125–134. doi: 10.1016/s0006-8993(98)00373-4. [DOI] [PubMed] [Google Scholar]
- 25.in 't Zandt HJ, et al. Cerebral creatine kinase deficiency influences metabolite levels and morphology in the mouse brain: A quantitative in vivo 1H and 31P magnetic resonance study. J Neurochem. 2004;90(6):1321–1330. doi: 10.1111/j.1471-4159.2004.02599.x. [DOI] [PubMed] [Google Scholar]
- 26.Kuiper JW, Oerlemans FT, Fransen JA, Wieringa B. Creatine kinase B deficient neurons exhibit an increased fraction of motile mitochondria. BMC Neurosci. 2008;9:73, 83. doi: 10.1186/1471-2202-9-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steeghs K, et al. Cytoarchitectural and metabolic adaptations in muscles with mitochondrial and cytosolic creatine kinase deficiencies. Mol Cell Biochem. 1998;184(1–2):183–194. [PubMed] [Google Scholar]
- 28.Steeghs K, Oerlemans F, Wieringa B. Mice deficient in ubiquitous mitochondrial creatine kinase are viable and fertile. Biochim Biophys Acta. 1995;1230(3):130–138. doi: 10.1016/0005-2728(95)00044-j. [DOI] [PubMed] [Google Scholar]
- 29.Streijger F, et al. Mice lacking the UbCKmit isoform of creatine kinase reveal slower spatial learning acquisition, diminished exploration and habituation, and reduced acoustic startle reflex responses. Mol Cell Biochem. 2004;256–257(1–2):305–318. doi: 10.1023/b:mcbi.0000009877.90129.e3. [DOI] [PubMed] [Google Scholar]
- 30.Veksler VI, et al. Muscle creatine kinase-deficient mice. II. Cardiac and skeletal muscles exhibit tissue-specific adaptation of the mitochondrial function. J Biol Chem. 1995;270(34):19921–19929. doi: 10.1074/jbc.270.34.19921. [DOI] [PubMed] [Google Scholar]
- 31.Wallimann T, Wegmann G, Moser H, Huber R, Eppenberger HM. High content of creatine kinase in chicken retina: Compartmentalized localization of creatine kinase isoenzymes in photoreceptor cells. Proc Natl Acad Sci USA. 1986;83(11):3816–3819. doi: 10.1073/pnas.83.11.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21(10):1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Jackman SL, et al. Role of the synaptic ribbon in transmitting the cone light response. Nat Neurosci. 2009;12(3):303–310. doi: 10.1038/nn.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winkler BS. Glycolytic and oxidative metabolism in relation to retinal function. J Gen Physiol. 1981;77(6):667–692. doi: 10.1085/jgp.77.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winkler BS, Starnes CA, Twardy BS, Brault D, Taylor RC. Nuclear magnetic resonance and biochemical measurements of glucose utilization in the cone-dominant ground squirrel retina. Invest Ophth Vis Sci. 2008;49(10):4613–4619. doi: 10.1167/iovs.08-2004. [DOI] [PubMed] [Google Scholar]
- 36.Chaturvedi RK, Beal MF. Mitochondrial approaches for neuroprotection. Ann NY Acad Sci. 2008;1147:395–412. doi: 10.1196/annals.1427.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Azeredo FA, Lust WD, Passonneau JV. Light-induced changes in energy metabolites, guanine nucleotides, and guanylate cyclase within frog retinal layers. J Biol Chem. 1981;256(6):2731–2735. [PubMed] [Google Scholar]
- 38.Song H, Sokolov M. Analysis of protein expression and compartmentalization in retinal neurons using serial tangential sectioning of the retina. J Proteome Res. 2009;8(1):346–351. doi: 10.1021/pr800631d. [DOI] [PubMed] [Google Scholar]
- 39.Sampath AP, et al. Recoverin improves rod-mediated vision by enhancing signal transmission in the mouse retina. Neuron. 2005;46(3):413–420. doi: 10.1016/j.neuron.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Cadetti L, Tranchina D, Thoreson WB. A comparison of release kinetics and glutamate receptor properties in shaping rod-cone differences in EPSC kinetics in the salamander retina. J Physiol. 2005;569(Pt 3):773–788. doi: 10.1113/jphysiol.2005.096545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seeliger MW, et al. New views on RPE65 deficiency: The rod system is the source of vision in a mouse model of Leber congenital amaurosis. Nat Genet. 2001;29(1):70–74. doi: 10.1038/ng712. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.