Abstract
Afferent neural transmission of temperature sensation from skin thermoreceptors to the central thermoregulatory system is important for the defense of body temperature against environmental thermal challenges. Here, we report a thermosensory pathway that triggers physiological heat-defense responses to elevated environmental temperature. Using in vivo electrophysiological and anatomical approaches in the rat, we found that neurons in the dorsal part of the lateral parabrachial nucleus (LPBd) glutamatergically transmit cutaneous warm signals from spinal somatosensory neurons directly to the thermoregulatory command center, the preoptic area (POA). Intriguingly, these LPBd neurons are located adjacent to another group of neurons that mediate cutaneous cool signaling to the POA. Functional experiments revealed that this LPBd–POA warm sensory pathway is required to elicit autonomic heat-defense responses, such as cutaneous vasodilation, to skin-warming challenges. These findings provide a fundamental framework for understanding the neural circuitry maintaining thermal homeostasis, which is critical to survive severe environmental temperatures.
Keywords: autonomic nervous system, feedforward, somatosensory, sympathetic, thermoregulation
Active thermoregulation inherent in homeothermic animals, including humans, that defends their body temperatures in warm (i.e., above thermoneutral) environments is important to preserve molecular and cellular functions critical for life, because disrupted cellular function arising from protein denaturation can begin at body core and brain temperatures only a few degrees Celsius above normal. The unexpected death tolls during recent heat-waves, in combination with the threat that such environmental challenges will increase as a consequence of global warming (1), indicate both the significance of heat defense for the maintenance of the homeostatic physiological functions necessary for survival and the urgent need for an increased understanding of the CNS mechanisms controlling physiological heat-defense responses, such as increased heat dissipation from the body surface and reduced heat production (thermogenesis) inside the body.
In the central mechanism for body temperature regulation, the preoptic area (POA) plays pivotal roles by receiving and integrating temperature information from the skin and from other thermoreceptive sites in the body and then by sending command signals to peripheral thermoregulatory effectors (2–6). The feedforward signaling of environmental temperature from the skin to the POA is important to evoke rapid physiological thermoregulatory responses that defend body temperature against changes in environmental temperature before they impact internal thermal homeostasis (3, 5–7). We recently identified a cool sensory pathway that mediates feedforward signaling for evoking thermoregulatory responses to environmental cooling challenges (8). The signals of cutaneous cool sensation that are transmitted from somatosensory neurons in the spinal dorsal horn activate neurons in the external lateral part of the parabrachial nucleus (LPBel), which then provide glutamatergic excitation to a midline portion of the POA that is centered within the median preoptic nucleus (MnPO) (8). Importantly, autonomous thermoregulation does not require thermosensory signaling in the spinothalamocortical pathway (8), which mediates the perception and discrimination of skin temperature changes (9).
In contrast, although primary (peripheral) and secondary (spinal) somatosensory neurons that transmit cutaneous innocuous warm sensation have been described (10, 11), the contribution of warm sensory signaling to heat defense remains unclear. The notion that heat-defense responses to elevated environmental temperature are elicited by increased warm sensory input from the skin to the POA is supported by the demonstration that autonomic heat-defense responses can be evoked by increases in skin temperature that do not affect hypothalamic temperature (12). However, the possibility remains that such heat-defense responses are a consequence of the waning cutaneous cool sensory signaling through the LPBel–POA pathway that normally suppresses heat-defense responses.
In the present study, we sought to elucidate the fundamental mechanism for feedforward heat-defense responses by identifying neuronal populations that provide cutaneous warm signals directly to the POA using in vivo electrophysiological and anatomical approaches. The functional role of these candidate neuronal populations in autonomic heat-defensive responses to skin warming then was investigated as the basis for cutaneous vasodilation that increases heat dissipation from the skin.
Results
Neurons Providing Warm Sensory Signals to the POA.
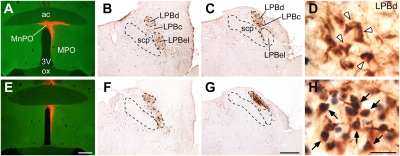
Candidate populations of neurons providing cutaneous warm signal inputs directly to the rat POA were identified as those both retrogradely labeled with cholera toxin b-subunit (CTb) injected into a POA subregion (MnPO and periventricular POA; Fig. 1 and Figs. S1 and S2) and expressing Fos protein, a marker of activated neurons (13), following exposure of the animals to a warm environment. CTb-labeled, POA-projecting neurons in the lateral parabrachial nucleus (LPB) formed two prominent clusters in the dorsal part of the LPB (LPBd) and in the LPBel, and the LPBd cluster was located slightly caudal to the LPBel cluster (Fig. 1 and Fig. S2). Exposure of the animals to a warm (36 °C) environment for 4 h induced remarkable Fos expression in a dense neuronal group of the CTb-positive cluster in the LPBd but not in the LPBel cluster (Fig. 1H and Figs. S1F and S2 D and F). In contrast, following cold exposure (4 °C), Fos expression was detected in the CTb-positive cluster in the LPBel as well as in some spread neurons in the central part of the LPB (LPBc) but was limited to very few neurons in the LPBd (Figs. S1 D and G and S2 D and G) (8). Following exposure to the control temperature (24 °C), only a few double-labeled neurons were seen in either the LPBd or the LPBel (Fig. 1D and Figs. S1E and S2 D and E). No brain regions other than the LPBd contained a substantial number of CTb-labeled neurons that also expressed Fos after warm exposure.
Fig. 1.
POA-projecting LPBd neurons are activated in a warm environment. Fos expression in LPB neurons that were retrogradely labeled with CTb injected into the MnPO (red, A and E) was examined in rats exposed to control (24 °C) (A–D) or warm (36 °C) (E–H) temperature. Many neurons in the LPBd of the warm-exposed animal exhibited both CTb (brown) and Fos (blue-black) immunoreactivities (arrows, H), whereas most CTb-labeled neurons in the LPBd of the control-exposed animal were negative for Fos immunoreactivity (arrowheads, D). [Scale bars, 0.5 mm (A–C and E–G); 30 μm (D and H).] 3V, third ventricle; ac, anterior commissure; MPO, medial preoptic area; ox, optic chiasm; scp, superior cerebellar peduncle. Some images from the animal exposed to 24 °C were adapted from our previous study (8).
Because LPB neurons project broadly to the whole POA (14–16), we further examined the extent to which warming-activated LPB neurons project to POA subregions lateral to the MnPO. Although many retrogradely labeled cells were found in the LPB following CTb injections in the medial or lateral POA, the percentages of Fos-positive cells in the CTb-labeled populations in the LPBd after warm exposure (7–18%) were markedly lower than when CTb injections were centered in the MnPO and periventricular POA (42–54%) (Fig. S3). These anatomical results indicate that neurons in the LPBd are the sole source of skin warming-activated inputs to the POA and that this LPB subregion provides warm sensory inputs more densely to the median subregion of the POA than to the medial and lateral POA subregions.
Unit Recording of LPBd Neurons Projecting to the POA.
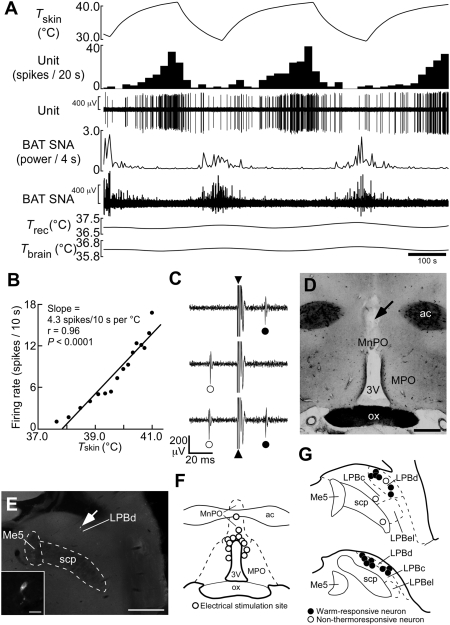
Using in vivo unit recording techniques, we determined the responses of single POA-projecting LPB neurons to warm sensory input from the skin (Fig. 2). The collision test was used to determine whether the recorded LPB neurons were antidromically activated by electrical stimulation in the MnPO (Fig. 2 C, D, and F). Of 17 antidromically identified LPB neurons projecting to the POA, 14 neurons increased their firing rates in response to trunk skin warming (resting, 5.5 ± 2.4 spikes/10 s; peak value during warming, 39.9 ± 9.5 spikes/10 s; P < 0.005, two-tailed paired t test; Fig. 2A and Fig. S4C; warm-responsive neuron), and their juxtacellularly labeled profiles were all localized in the LPBd (Fig. 2 E and G). The portion of the skin temperature ramp during which LPBd unit activity increased (i.e., the dynamic phase; SI Materials and Methods) was characterized by an onset temperature (mean: 34.7 ± 0.4 °C, n = 14) at which the unit firing rate began to increase and an offset temperature (38.0 ± 0.4 °C) above which the firing rate no longer increased but often maintained a plateau level of elevated activity (Fig. S4C) until recooling commenced. The skin warming-evoked increases in LPBd neuronal discharge were reversed rapidly following the onset of skin recooling (Fig. 2A and Fig. S4C). Sympathetic nerve activity (SNA) controlling brown adipose tissue (BAT) thermogenesis showed a discharge pattern that was complementary to the changes in LPBd neuronal firing rate (Figs. 2A and Fig. S4C). The average antidromic latency and estimated conduction velocity of these warm-responsive neurons were 19.4 ± 1.7 ms and 0.54 ± 0.06 m/s, respectively, values that were not significantly different from those of cool-responsive, POA-projecting neurons recorded in the LPBel (20.9 ± 0.9 ms and 0.45 ± 0.02 m/s, n = 11, respectively; P > 0.05, two-tailed unpaired t test) (8). The remaining three antidromically activated LPB neurons were not exclusively localized within the LPBd (Fig. 2G) and did not show thermoresponsive changes in their firing rate (prewarming, 34.5 ± 2.9 spikes/10 s; highest value during warming, 34.7 ± 3.6 spikes/10 s; P > 0.05; two-tailed paired t test; non-thermoresponsive neuron).
Fig. 2.
Skin warming-evoked response of single LPB neurons antidromically activated from the POA. (A) In vivo extracellular unit recording of the action potentials of a warm-responsive LPBd neuron (Unit) and changes in BAT SNA, rectal temperature (Trec), and brain temperature (Tbrain) in response to changes in trunk skin temperature (Tskin). Note that Trec and Tbrain do not exhibit any phasic changes during skin warming and recooling. (B) Linear regression analysis indicates a significant (Pearson's correlation test) positive correlation between skin temperature and the firing rate of the warm-responsive LPBd neuron shown in A during the middle skin-warming episode. (C) Collision test. Single-pulse stimulation in the POA (triangle) evoked a constant-onset latency (23 ms) response in this neuron (filled circle, top trace). POA stimulation at 22 ms after a spontaneous action potential (open circle) failed to evoke a response of this neuron (middle trace). POA stimulation at 24 ms after a spontaneous action potential evoked a constant-onset latency response of this neuron (bottom trace). All traces are superpositions of three stimulation trials. (D) Representative site, identified by a small scar (arrow), of electrical stimulation for collision tests. (E) Juxtacellular labeling allows visualization of a recorded LPBd neuron (arrow). (Inset) A magnified picture of this neuron. [Scale bars, 0.5 mm (D and E); 30 μm (Inset in E).] (F) Sites of electrical stimulation in the POA. (G) Locations of LPB neurons juxtacellularly labeled after unit recording. Me5, mesencephalic trigeminal nucleus.
Linear regression analysis revealed a significant, positive correlation of the firing rate of each warm-responsive LPBd neuron with skin temperature during the dynamic phase of the response to skin warming (Fig. 2B and Fig. S4 A and B). The average responsiveness (slope) of the relationship between the discharge frequency of warm-responsive neurons and skin temperature was 11.7 ± 2.9 spikes/10 s per °C. In contrast, none of the non-thermoresponsive LPBd neurons showed a significant correlation between firing rate and skin temperature.
Because the LPB also may mediate pain-related signals (17), we tested the responsiveness of the LPB units to noxious tail pinch. No response to noxious tail pinch was observed in 10 of 12 warm-responsive LPB neurons and in one of three non-thermoresponsive neurons, although a rapid pressor response was observed in all trials (Fig. S4D). The four neurons that responded to tail pinch showed a high-frequency burst discharge in response to pinching (Fig. S4E). These results indicate that neurons distributed in the LPBd are activated by warm signals from the skin and transmit these thermal signals directly to the POA but that the vast majority of these neurons do not mediate noxious mechanical signals.
Critical Role of the LPBd–POA Pathway in Heat Defense.
We tested in vivo the hypothesis that the LPBd neurons activated by warm signaling from the skin mediate physiological heat-defensive responses. When the body core (rectal) temperature of animals under quiescent conditions was set at 37–38 °C, sural sympathetic cutaneous vasoconstrictor (CVC) nerves regulating blood flow in the hind paw exhibited spontaneous activity synchronized with the respiratory cycle, and tail skin temperature, an indicator of tail skin blood flow, was just above 30 °C (Fig. S5 A and B). Warming the trunk skin for 200–300 s consistently evoked cutaneous vasodilation indicated by elimination of the spontaneous sural CVC activity and by a marked increase in tail skin temperature to 35–36 °C, a range near the core body temperature (Fig. S5A). That the spontaneous sural CVC activity arose entirely from sympathetic postganglionic fibers was confirmed by elimination of the discharge with hexamethonium, a ganglionic blocker (Fig. S5C). The properties of the sural CVC sympathetic nerves were consistent with previous characterizations of sympathetic CVC nerves (18, 19).
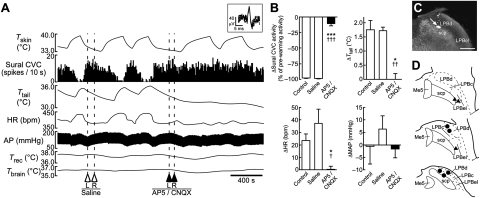
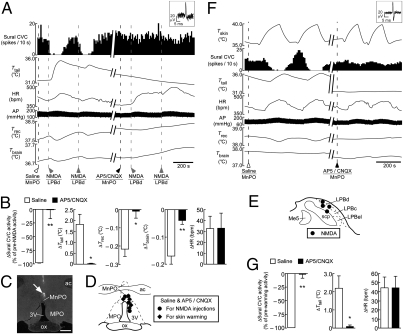
The skin warming-evoked inhibition of spontaneous sural CVC activity and the subsequent increase in tail skin temperature were completely abolished following bilateral nanoinjections of a mixture of the ionotropic glutamate receptor antagonists, DL-2-amino-5-phosphonopentanoic acid (AP5) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), into the LPBd (Fig. 3). Skin warming also increased heart rate (HR) but did not consistently change mean arterial pressure, and the skin warming-evoked increase in HR was eliminated following bilateral nanoinjections of AP5/CNQX into the LPBd (Fig. 3 A and B). The inhibition of sural CVC activity, the increase in tail skin temperature, and the tachycardia evoked by skin warming were not altered by either saline nanoinjections into the same LPBd sites or by bilateral nanoinjections of AP5/CNQX into the LPBel (Fig. 3). The skin warming-induced changes in these variables following AP5/CNQX nanoinjections into the LPBd and into the LPBel were significantly different (Table S1). Thus, glutamatergic activation of neurons in the LPBd, but not in the LPBel, is essential for physiological heat-defense responses to elevated environmental temperature.
Fig. 3.
Blockade of glutamatergic neurotransmission in the LPBd eliminates skin warming-induced heat-defense responses. (A) Trunk skin warming (Tskin)-evoked changes in sural CVC activity, tail skin temperature (Ttail), HR, arterial pressure (AP), Trec, and Tbrain before and after bilateral nanoinjections of saline or AP5/CNQX into the LPBd (dashed lines). Inset shows unitary action potentials recorded from the sural CVC nerve (superposition of five traces). (B) Average changes (n = 5) in sural CVC activity, Ttail, HR, and mean arterial pressure (MAP) in response to skin-warming episodes before injections (Control) or after saline or AP5/CNQX nanoinjections into the LPBd (injection sites shown by circles in D). *, P < 0.05; **, P < 0.01; ***, P < 0.001, compared with the control value; †, P < 0.05; ††, P < 0.01; †††, P < 0.001, compared with the saline value (two-tailed paired t test). (C) An injection site in the LPBd identified with a cluster of fluorescent beads (arrow). (Scale bar, 0.5 mm.) (D) Sites of injections in the LPB. Saline and AP5/CNQX were injected at the same sites. The right side of the symmetric bilateral injections is shown. AP5/CNQX injections into the LPBel (triangles) were significantly less effective on heat-defense responses to skin warming than those into the LPBd (circles) (Table S1).
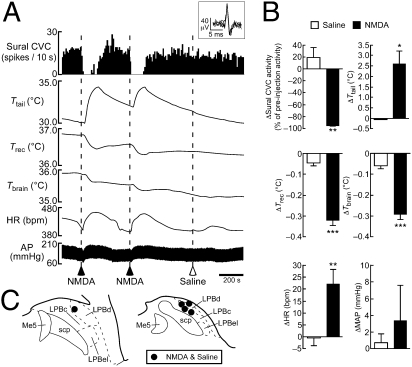
Further supporting this concept, stimulation of LPBd neurons with a local, unilateral nanoinjection of NMDA, but not saline, consistently inhibited spontaneous sural CVC activity and increased HR (Fig. 4). Each NMDA-elicited inhibition of sural CVC activity was associated with a marked increase in tail skin temperature and rapid drops in rectal and brain temperatures (Fig. 4), mimicking the physiological heat loss responses to skin warming. However, the effects of NMDA and saline nanoinjections into the LPBd on mean arterial pressure were not significantly different (Fig. 4B).
Fig. 4.
Stimulation of LPBd neurons evokes physiological responses that mimic heat-defense responses to skin warming. (A) Changes in sural CVC activity, Ttail, Trec, Tbrain, HR, and AP following unilateral nanoinjections of saline or NMDA into the LPBd (dashed lines). Inset shows unitary action potentials recorded from the sural CVC nerve (superposition of five traces). (B) Average changes (n = 5) in sural CVC activity, Ttail, Trec, Tbrain, HR, and MAP following saline or NMDA nanoinjection into the LPBd. *, P < 0.05; **, P < 0.01; ***, P < 0.001, two-tailed paired t test. (C) Injection sites. NMDA and saline were injected at the same sites.
In addition to eliciting heat-defense responses, activation of LPBd neurons also counteracted cooling-evoked thermogenic, metabolic, and cardiac responses. Cooling the trunk skin evoked increases in thermogenic (BAT SNA and BAT temperature), metabolic (expired CO2), and cardiac (HR) parameters (20), and unilateral nanoinjection of NMDA into the LPBd immediately reversed these skin cooling-evoked responses (Fig. S6). Following a similar nanoinjection with saline into the LPBd, these parameters continued to rise as long as the skin was cooled (Fig. S6B).
Because the results from our Fos and unit recording experiments revealed a direct input of cutaneous warm signals from LPBd neurons to the MnPO, we tested the effect of blockade of glutamatergic neurotransmission in the MnPO on the heat-defense responses evoked either by stimulation of LPBd neurons with NMDA or by skin warming. Following a saline nanoinjection into the MnPO, unilateral nanoinjections of NMDA into the LPBd consistently evoked heat defense-mimicking responses as observed in Fig. 4 (Fig. 5 A–E), and skin-warming episodes consistently evoked heat-defensive cutaneous vasodilation and tachycardia (Fig. 5 D, F, and G). Nanoinjection of AP5/CNQX into the MnPO prevented the effects of NMDA nanoinjection at the same LPBd site and of skin warming on spontaneous sural CVC activity and on tail skin, rectal, and brain temperatures (Fig. 5). Interestingly, even after AP5/CNQX nanoinjection into the MnPO, NMDA nanoinjections into the LPBd or skin warming still increased HR to levels comparable to those after saline nanoinjection into the MnPO (Fig. 5).
Fig. 5.
Cutaneous vasodilation evoked by stimulation of LPBd neurons or by skin warming is dependent on glutamatergic neurotransmission in the MnPO. (A) Changes in sural CVC activity, Ttail, HR, AP, Trec, and Tbrain that are evoked by unilateral NMDA nanoinjection into the LPBd (gray dashed lines) following nanoinjection of saline or AP5/CNQX into the MnPO (black dashed lines). Inset shows unitary action potentials recorded from the sural CVC nerve (superposition of five traces). (B) Average changes (n = 4) in sural CVC activity, Ttail, Trec, Tbrain, and HR that are evoked by NMDA nanoinjection into the LPBd following saline or AP5/CNQX nanoinjection into the MnPO (injection sites shown in D and E). *, P < 0.05; **, P < 0.01, two-tailed paired t test. (C) An injection site in the MnPO identified with a cluster of fluorescent beads (arrow). (Scale bar, 0.5 mm.) (D) Sites of saline and AP5/CNQX injections to examine their effects on responses evoked by NMDA injection into the LPBd (circles; see A and B) or those evoked by skin warming (diamonds; see F and G). Saline and AP5/CNQX were injected at the same sites. (E) Sites of NMDA injections. (F) Skin warming (Tskin)-evoked changes in sural CVC activity, Ttail, HR, AP, Trec, and Tbrain following saline or AP5/CNQX nanoinjection into the MnPO (dashed lines). Inset shows unitary action potentials recorded from the sural CVC nerve (superposition of five traces). (G) Average changes (n = 4) in sural CVC activity, Ttail, and HR that are evoked by skin warming following saline or AP5/CNQX nanoinjection into the MnPO (injection sites shown in D). *, P < 0.05; **, P < 0.01, two-tailed paired t test.
Discussion
Preventing the body core temperature from rising into a hyperthermic range in warm, supra-thermoneutral environments is one of the most important homeostatic functions in homeothermic animals. The present study explicates the central mechanism through which cutaneous thermal afferents elicit heat-defensive, autonomic effector functions in response to elevated environmental temperature to prevent increases in body core temperature. The present and previous findings are summarized in a schematic model (Fig. S7) of the neural pathways that drive thermoregulatory responses to environmental thermal challenges.
Our functional neuroanatomical experiments revealed that many POA-projecting neurons in the LPBd are activated (express Fos protein) in response to warm exposure. It seems unlikely that this Fos expression was induced by any secondary effects during warm exposure, such as dehydration, because the animals were allowed to access water freely, and, more importantly, the present in vivo unit recording identified POA-projecting neurons in the LPBd that are activated immediately in response to trunk skin warming. These results indicate that the LPBd contains neurons that transmit cutaneous warm signals directly to the POA. The idea that this activation of warm-responsive LPBd neurons is caused by a glutamatergic input from second-order somatosensory neurons in the spinal dorsal horn is supported by previous anatomical evidence: (i) There are numerous projections from the dorsal horn to the LPB (21), some of which terminate on POA-projecting LPB neurons (8); and (ii) a substantial population of dorsal horn neurons provide their axon collaterals both to the LPB and thalamus (22), and their terminals in the thalamus contain glutamate (23).
Somatosensory thalamocortical neurons in lamina I of the spinal dorsal horn, whose collaterals probably innervate the LPB (22), have been categorized into three classes: nociceptive-specific cells responding to noxious mechanical and heat stimuli; polymodal nociceptive cells responding to noxious mechanical, heat, and cold stimuli; and thermoreceptive-specific cells responding linearly to graded, innocuous warming or cooling stimuli and not activated further in the noxious temperature range (11, 24). Our in vivo unit recording study identified neurons in the LPBd that project directly to the POA and whose increase in discharge rate during innocuous skin warming, often reaching a plateau firing rate, paralleled that observed in thermoreceptive-specific warm-responsive lamina I neurons (11). The lack of responsiveness to noxious mechanical stimulation of the rat tail in most of the warm-responsive LPBd neurons recorded in our study also suggests that these neurons are activated primarily by thermoreceptive-specific lamina I neurons transmitting innocuous warm sensation.
In the present in vivo physiological study that examined the functional involvement of LPBd neurons in thermal homeostasis, blockade of glutamatergic inputs to the LPBd eliminated the heat-defense responses evoked by trunk skin warming: cutaneous vasodilation in the rat paws and tail. Furthermore, stimulation of LPBd neurons with the glutamatergic agonist, NMDA, evoked a remarkable cutaneous vasodilation that mimicked heat-defense responses evoked by skin warming. Together with the anatomical knowledge of the spinal–LPB glutamatergic projection, these results support the contention that LPBd neurons activated by cutaneous warm sensory, glutamatergic input from the dorsal horn mediate the feedforward thermal afferent signaling (Fig. S7) that is required to elicit the rapid homeostatic responses necessary to counter the challenges to core body temperature from environmental heat.
Intriguingly, a different LPB site centered at the LPBel contains a distinct neuronal population mediating cutaneous innocuous cool signaling to the POA that is required for eliciting cold-defense responses to lowered environmental temperature (8). In the present study, we compared the distributions of these two LPB neuronal populations that mediate cutaneous warm and cool signals to the POA and found that they are adjacent but are clearly segregated. This observation is consistent with the existence of peripheral and spinal mechanisms, including thermosensors, for the separate afferent transmissions of innocuous warm and cool sensations (11, 24–26) and indicates that the cutaneous warm and cool sensory signals from the spinal dorsal horn, both of which are required for driving feedforward thermoregulatory responses, are transmitted separately to the POA by LPBd and LPBel neurons, respectively (Fig. S7).
As evidenced by the present Fos study, warm-responsive LPBd neurons project to the midline portion of the POA, which is centered at the MnPO, rather than to the medial or lateral POA. Additionally, blockade of glutamatergic transmission in the MnPO eliminated cutaneous vasodilation evoked by LPBd neuronal stimulation or by skin warming. These results are consistent with an activation of MnPO neurons by glutamatergic inputs from the LPBd as a necessary step to elicit heat-defense responses to environmental warming. The MnPO also receives glutamatergic cutaneous cool inputs from LPBel neurons, and activation of MnPO neurons also is required for eliciting cold-defense responses to skin cooling (8, 27). These findings support a model (Fig. S7) in which cutaneous warm and cool signals from the LPBd and the LPBel, respectively, activate distinct populations of neurons in the MnPO.
In the central control of autonomic thermoregulatory effectors, such as BAT and CVCs, descending GABAergic projection neurons in the POA are thought to provide a tonic inhibition to their sympathoexcitatory efferent mechanisms when cold-defense responses are not needed (4, 6). Nanoinjection mapping in POA subregions with inhibitory agents to evoke cold-defense responses has suggested that such inhibitory, descending projection neurons are distributed mostly in the medial POA (28, 29), although some of these neurons controlling CVCs also might be distributed in the MnPO (29). Our previous results support a local POA mechanism for eliciting cold-defense responses in which cutaneous cool signaling from the LPBel activates GABAergic interneurons in the MnPO, which then inhibit the tonic activity of the inhibitory projection neurons in the medial POA that innervate neurons in the caudal brain regions, including the dorsomedial hypothalamus and rostral medullary raphe, whose activation leads to sympathetic cold-defense responses (8, 27) (Fig. S7).
Complementing this model, the present findings suggest that the glutamatergic, skin warming-driven input from the LPBd activates inhibitory, descending projection neurons in the POA, either directly or through activation of local excitatory interneurons in the MnPO (Fig. S7). Such an excitatory input would enhance the descending inhibitory drive from the POA to the caudal brain regions controlling thermal effector activation, thereby leading to cutaneous vasodilation and to inhibition of thermogenesis through attenuated sympathetic outputs to CVCs and BAT, respectively (Fig. S7). In this model, the neural outputs to BAT and CVCs are determined by the integration of cutaneous warm and cool signals and central thermosensory signals, the latter sensed by warm-sensory POA neurons (3). This notion is consistent with our observation that skin warming-induced increases in the firing rates of warm-responsive LPB neurons and the resulting inhibitions of BAT SNA were in phase but did not always exhibit an exactly matching time course.
Recent findings on the efferent control of sympathetic CVC tone indicate that the effector signaling from the POA leading to temperature-dependent CVC control is mediated by the rostral medullary raphe including the rostral raphe pallidus (30), a site of sympathetic premotor neurons controlling skin blood vessels (31, 32). The absence of evidence for brain sites intermediate between the POA and the rostral medullary raphe that are required for the POA efferent signaling for temperature-dependent CVC control supports the significance of the direct projection from POA neurons to the rostral medullary raphe as the efferent pathway for febrile and thermoregulatory control of cutaneous vasoconstriction (30, 33, 34). The present results do not exclude the possibility that warm-responsive LPBd neurons have axonal branches that could bypass the POA and provide thermosensory signals directly to sites, such as the rostral medullary raphe, in the efferent pathways controlling thermoregulatory effectors, but this possibility seems unlikely, because there are very few projections from the LPB to the rostral medullary raphe (14), and antagonizing glutamate receptors in the MnPO resulted in a nearly complete blockade of heat-defensive responses to skin warming or stimulation of LPBd neurons. These findings emphasize the importance of the LPBd–POA warm signaling pathway in the mechanism that defends thermal homeostasis against elevated environmental temperature.
In addition to eliciting cutaneous vasodilation, skin warming also increased HR in the present study. This warming-induced tachycardia would promote heat dissipation from the body surface by increasing cardiac output during a period of cutaneous vasodilation. Our finding that antagonizing glutamate receptors in the LPBd, but not in the MnPO, blocked the skin warming-evoked HR increase indicates that LPBd neurons mediate skin warming-evoked tachycardia that is, however, independent of the LPBd–MnPO pathway. One of the possible mechanisms for the warming-evoked tachycardia is activation of cardiovascular neurons in the rostral ventrolateral medulla (RVLM), because neurons in the LPBd site where our NMDA injections evoked tachycardia project to the RVLM (35), and chemical stimulation of the LPB leads to activation of cardiovascular RVLM neurons (36).
The present findings demonstrate that autonomous heat-defense responses to elevated environmental temperature are not purely an outcome of a withdrawal of the cutaneous cool sensory signaling but are driven by the activation of the cutaneous warm sensory pathway to the POA. The best-known thermosensory pathway is the spinothalamocortical pathway, which mediates perception and discrimination of skin temperature (9). However, it is unlikely that this pathway is involved in the thermal afferent mechanism to elicit autonomous thermoregulatory responses, because skin cooling-evoked activation and rewarming-evoked deactivation of BAT thermogenesis were totally intact even after functional ablation of the spinothalamocortical pathway by lesioning the thalamic nuclei mediating the thermosensory signaling (8). This result clearly separates the thermal functions mediated by the spinothalamocortical pathway and by the spinal–LPB–POA pathways (Fig. S7), and the present study introduces an important functional role of the latter, thermoregulatory afferent pathway: cutaneous warm signaling for the defense of thermal homeostasis in heated environments.
Materials and Methods
Animals.
Male Sprague-Dawley rats (200–500 g; Charles River) were housed with ad libitum access to food and water in an air-conditioned room (22–23 °C) with a standard 12 h light/dark cycle. All procedures conform to the regulations detailed in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the Oregon Health & Science University.
CTb-Fos Study.
We followed our established protocol (8), which is detailed in SI Materials and Methods. Briefly, anesthetized rats received a CTb injection into a POA subregion. After acclimatization to a climate chamber for 3 days, the animals were exposed to 36 °C, 24 °C, or 4 °C for 4 h. Immediately after the exposure, the animals were transcardially perfused, and the brains were subjected to CTb and Fos immunohistochemistry as detailed in SI Materials and Methods.
In Vivo Physiology.
The basic procedures for the animal preparation and skin-temperature manipulation are detailed in SI Materials and Methods. Briefly, urethane-chloralose–anesthetized rats were paralyzed with D-tubocurarine and artificially ventilated with O2. The trunk was shaved and wrapped with a water jacket to control the temperature of the skin, and abdominal skin temperature was monitored. The left sural nerve was split, and the sympathetic CVC activity was recorded from an individual or a few axons. In some animals, postganglionic BAT SNA was recorded from the right interscapular BAT pad. Stereotaxic pressure-injection of drugs into the brain was performed in nanoliter volumes (nanoinjections): 60 nL/site of AP5/CNQX (5 mM each) into the LPB, 36 nL/site of NMDA (0.2 mM) into the LPB, and 100–200 nL in the MnPO. To mark the injection sites, fluorescent microspheres were injected at the same stereotaxic coordinates. Procedures for physiological data analyses are detailed in SI Materials and Methods.
Unit Recording.
In vivo electrophysiological recording from single neurons and postrecording juxtacellular labeling of them followed our established protocols (8), which are detailed in SI Materials and Methods.
Statistics.
All data are presented as the means ± SEM, and statistical results with a P value of < 0.05 were considered significant. Statistical significance was evaluated with a two-tailed paired or unpaired t test or a two-way ANOVA followed by a Bonferroni post hoc test. Correlation between unit firing rate and skin temperature was detected using Pearson's correlation test.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants NS40987 and DK57838 (to S.F.M.), by the Special Coordination Fund for Promoting Science and Technology (to K.N.) and Grants-in-Aid for Scientific Research (21890114 and 22689007) (to K.N.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by the Takeda Science Foundation and Kowa Life Science Foundation (K.N.). K.N. was a JSPS fellow for research abroad.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0913358107/-/DCSupplemental.
References
- 1.Luterbacher J, Dietrich D, Xoplaki E, Grosjean M, Wanner H. European seasonal and annual temperature variability, trends, and extremes since 1500. Science. 2004;303:1499–1503. doi: 10.1126/science.1093877. [DOI] [PubMed] [Google Scholar]
- 2.Hammel HT. Regulation of internal body temperature. Annu Rev Physiol. 1968;30:641–710. doi: 10.1146/annurev.ph.30.030168.003233. [DOI] [PubMed] [Google Scholar]
- 3.Boulant JA, Gonzalez RR. The effect of skin temperature on the hypothalamic control of heat loss and heat production. Brain Res. 1977;120:367–372. doi: 10.1016/0006-8993(77)90916-7. [DOI] [PubMed] [Google Scholar]
- 4.Nagashima K, Nakai S, Tanaka M, Kanosue K. Neuronal circuitries involved in thermoregulation. Auton Neurosci. 2000;85:18–25. doi: 10.1016/S1566-0702(00)00216-2. [DOI] [PubMed] [Google Scholar]
- 5.Romanovsky AA. Thermoregulation: Some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol. 2007;292:R37–R46. doi: 10.1152/ajpregu.00668.2006. [DOI] [PubMed] [Google Scholar]
- 6.Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93:773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huckaba CE, Downey JA, Darling RC. A feedback-feedforward mechanism describing the interaction of central and peripheral signals in human thermoregulation. Int J Biometeorol. 1971;15:141–145. doi: 10.1007/BF01803888. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci. 2008;11:62–71. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 10.Hensel H, Iggo A, Witt I. A quantitative study of sensitive cutaneous thermoreceptors with C afferent fibres. J Physiol. 1960;153:113–126. doi: 10.1113/jphysiol.1960.sp006522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrew D, Craig AD. Spinothalamic lamina I neurones selectively responsive to cutaneous warming in cats. J Physiol. 2001;537:489–495. doi: 10.1111/j.1469-7793.2001.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakurada S, Shido O, Fujikake K, Nagasaka T. Relationship between body core and peripheral temperatures at the onset of thermoregulatory responses in rats. Jpn J Physiol. 1993;43:659–667. doi: 10.2170/jjphysiol.43.659. [DOI] [PubMed] [Google Scholar]
- 13.Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: Metabolic mapping at the cellular level. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- 14.Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- 15.Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res Rev. 1984;7:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- 16.Bester H, Besson JM, Bernard JF. Organization of efferent projections from the parabrachial area to the hypothalamus: A Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1997;383:245–281. doi: 10.1002/(sici)1096-9861(19970707)383:3<245::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Bester H, Menendez L, Besson JM, Bernard JF. Spino (trigemino) parabrachiohypothalamic pathway: Electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1995;73:568–585. doi: 10.1152/jn.1995.73.2.568. [DOI] [PubMed] [Google Scholar]
- 18.Häbler HJ, Bartsch T, Jänig W. Rhythmicity in single fiber postganglionic activity supplying the rat tail. J Neurophysiol. 1999;81:2026–2036. doi: 10.1152/jn.1999.81.5.2026. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka M, McAllen RM. Functional topography of the dorsomedial hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2008;294:R477–R486. doi: 10.1152/ajpregu.00633.2007. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2007;292:R127–R136. doi: 10.1152/ajpregu.00427.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cechetto DF, Standaert DG, Saper CB. Spinal and trigeminal dorsal horn projections to the parabrachial nucleus in the rat. J Comp Neurol. 1985;240:153–160. doi: 10.1002/cne.902400205. [DOI] [PubMed] [Google Scholar]
- 22.Hylden JLK, Anton F, Nahin RL. Spinal lamina I projection neurons in the rat: Collateral innervation of parabrachial area and thalamus. Neuroscience. 1989;28:27–37. doi: 10.1016/0306-4522(89)90229-7. [DOI] [PubMed] [Google Scholar]
- 23.Broman J, Ottersen OP. Cervicothalamic tract terminals are enriched in glutamate-like immunoreactivity: An electron microscopic double-labeling study in the cat. J Neurosci. 1992;12:204–221. doi: 10.1523/JNEUROSCI.12-01-00204.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craig AD, Krout K, Andrew D. Quantitative response characteristics of thermoreceptive and nociceptive lamina I spinothalamic neurons in the cat. J Neurophysiol. 2001;86:1459–1480. doi: 10.1152/jn.2001.86.3.1459. [DOI] [PubMed] [Google Scholar]
- 25.Iriuchijima J, Zotterman Y. The specificity of afferent cutaneous C fibres in mammals. Acta Physiol Scand. 1960;49:267–278. doi: 10.1111/j.1748-1716.1960.tb01952.x. [DOI] [PubMed] [Google Scholar]
- 26.Jordt SE, McKemy DD, Julius D. Lessons from peppers and peppermint: The molecular logic of thermosensation. Curr Opin Neurobiol. 2003;13:487–492. doi: 10.1016/s0959-4388(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura K, Morrison SF. Preoptic mechanism for cold-defensive responses to skin cooling. J Physiol. 2008;586:2611–2620. doi: 10.1113/jphysiol.2008.152686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osaka T. Cold-induced thermogenesis mediated by GABA in the preoptic area of anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R306–R313. doi: 10.1152/ajpregu.00003.2004. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka M, McKinley MJ, McAllen RM. Roles of two preoptic cell groups in tonic and febrile control of rat tail sympathetic fibers. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1248–R1257. doi: 10.1152/ajpregu.91010.2008. [DOI] [PubMed] [Google Scholar]
- 30.Rathner JA, Madden CJ, Morrison SF. Central pathway for spontaneous and prostaglandin E2-evoked cutaneous vasoconstriction. Am J Physiol Regul Integr Comp Physiol. 2008;295:R343–R354. doi: 10.1152/ajpregu.00115.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith JE, Jansen ASP, Gilbey MP, Loewy AD. CNS cell groups projecting to sympathetic outflow of tail artery: Neural circuits involved in heat loss in the rat. Brain Res. 1998;786:153–164. doi: 10.1016/s0006-8993(97)01437-6. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura K, et al. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci. 2004;24:5370–5380. doi: 10.1523/JNEUROSCI.1219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura K, et al. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci. 2002;22:4600–4610. doi: 10.1523/JNEUROSCI.22-11-04600.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura Y, Nakamura K, Morrison SF. Different populations of prostaglandin EP3 receptor-expressing preoptic neurons project to two fever-mediating sympathoexcitatory brain regions. Neuroscience. 2009;161:614–620. doi: 10.1016/j.neuroscience.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chamberlin NL, Saper CB. Topographic organization of cardiovascular responses to electrical and glutamate microstimulation of the parabrachial nucleus in the rat. J Comp Neurol. 1992;326:245–262. doi: 10.1002/cne.903260207. [DOI] [PubMed] [Google Scholar]
- 36.Agarwal SK, Calaresu FR. Supramedullary inputs to cardiovascular neurons of rostral ventrolateral medulla in rats. Am J Physiol Regul Integr Comp Physiol. 1993;265:R111–R116. doi: 10.1152/ajpregu.1993.265.1.R111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.