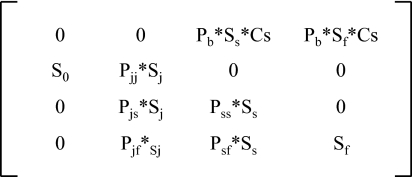Abstract
Many proximate causes of global amphibian declines have been well documented, but the role that climate change has played and will play in this crisis remains ambiguous for many species. Breeding phenology and disease outbreaks have been associated with warming temperatures, but, to date, few studies have evaluated effects of climate change on individual vital rates and subsequent population dynamics of amphibians. We evaluated relationships among local climate variables, annual survival and fecundity, and population growth rates from a 9-year demographic study of Columbia spotted frogs (Rana luteiventris) in the Bitterroot Mountains of Montana. We documented an increase in survival and breeding probability as severity of winter decreased. Therefore, a warming climate with less severe winters is likely to promote population viability in this montane frog population. More generally, amphibians and other ectotherms inhabiting alpine or boreal habitats at or near their thermal ecological limits may benefit from the milder winters provided by a warming climate as long as suitable habitats remain intact. A more thorough understanding of how climate change is expected to benefit or harm amphibian populations at different latitudes and elevations is essential for determining the best strategies to conserve viable populations and allow for gene flow and shifts in geographic range.
Keywords: amphibian, climate change, demography, Rana luteiventris, snowpack
Amphibian populations are declining around the globe at an alarming rate (1–3), and climate change now figures prominently as a potential interactive driver of some of these declines (4–6). A shift to earlier breeding phenology has been documented in a number of species (7–11), but this shift is not universal across species and has not been tied to population-level consequences. Other work has associated climatic conditions with disease-related declines in the Neotropics (4, 5), yet no mechanisms have been definitively linked to these correlations (12, 13). Reading et al. (14) showed a decrease in adult female body condition and survival in a population of common toads (Bufo bufo) that corresponded with an increase in average annual temperatures. However, these demographic changes were not explicitly linked to changes in population size over time. Kiesecker et al. (15) showed that disease, UV-B, and climate change could interact to increase embryo mortality in western toad (Bufo boreas) populations, yet these populations have not declined. This increased premetamorphic mortality may not be sufficient to cause otherwise increasing populations to decline in the long term. Alternatively, it may be that conditions in short-term or laboratory-based studies are not always representative of long-term patterns in natural populations. To determine mechanisms of population change, we first need to know which vital rates are affected by changes in climate, and then how these changes affect population dynamics.
The effects of climate change on growth and survival are particularly relevant for amphibian species in high elevation temperate ecosystems, where individuals are exposed to extreme and variable temperatures (e.g., ref. 16). In these environments, amphibians are active at a wider range of temperatures than closely related species inhabiting lower elevations (17). In general, juveniles and adults in high elevation temperate environments must reproduce and acquire resources over much shorter growing seasons than low elevation individuals. Similarly, tadpoles must be able to develop and metamorphose over a short season or survive long cold winters. In these environments, longer growing seasons due to climate warming may allow more time for adults, juveniles, and tadpoles to grow and acquire resources. Winter severity may decrease, reducing winterkill due to freezing or hypoxia, which can be a major cause of mortality in these systems (18, 19). However, reduced precipitation and a warm environment could result in less water in these high-elevation landscapes, which may negatively impact amphibian species that rely on ephemeral pools for reproduction and foraging. Additionally, amphibian populations and species that are adapted to a colder thermal regime in alpine systems may have physiological constraints that prevent adaptation to an increase in temperature (20). With climate change models for the western United States predicting a reduction in snowpack, as well as increased variability in precipitation levels (21, 22), understanding the role of these climate variables in the growth and survival of high-elevation populations of amphibians is critical to predicting future impacts of climate change on amphibians.
We evaluated relationships between annual vital rate estimates, estimates of asymptotic population growth rate, and local climate variables in a high elevation population of a temperate pond-breeding frog species, the Columbia spotted frog (Rana luteiventris). To evaluate these relationships, we used both mark-recapture and demographic analyses. A longstanding principle of demography is that not all vital rates are equally important for population dynamics. For example, large changes in annual recruitment, which are commonly documented in amphibians, tend to have less impact on population growth rates than relatively small changes in postmetamorphic survival rates (23). We examined variation in survival, growth, and recruitment in relation to summer and winter climate variables. We then used matrix models to calculate asymptotic growth rate (λ) for each set of annual vital rates (24–26) and related λ to these climate variables by using linear regression. Our results advance our understanding of how climate change may affect amphibian populations, and have implications for the conservation of other temperate amphibian species occupying alpine and boreal habitats.
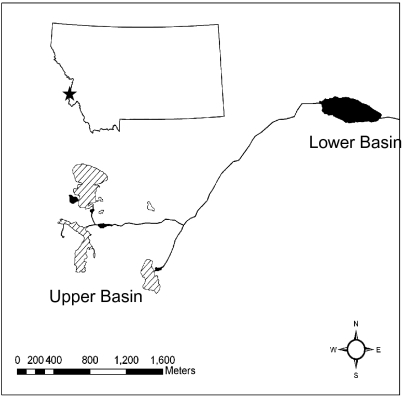
We conducted our study in the Little Rock Creek Basin, found in the Selway-Bitterroot Wilderness ≈16 km south of Hamilton, MT. This drainage is composed of two glacial cirques. Our analyses were focused on the upper basin (2,200 m), which is delineated by glacial headwalls (Fig. 1). Although this upper basin contains multiple breeding and foraging sites, they function as one population (27). The basin contains requisite habitats for R. luteiventris overwintering, breeding, and summer foraging, and is fishless (Fig. 1). We have not tested for the presence of the amphibian chytrid fungus Batrachochytrium dendrobatidis (Bd) in this population, but Bd is widespread in Montana and has been detected in amphibians within a few miles of this watershed (28). However, no mortality events were noted in this watershed, no local or broad-scale declines have been detected for R. luteiventris in western Montana during the course of this study (28), and R. luteiventris is known to have skin peptides that are highly resistant to Bd (29). Therefore, we believe that common culprits for amphibian declines at high elevation sites (i.e., introduced fish and disease; refs. 30 and 31) do not complicate our analyses. We monitored all life stages of R. luteiventris from 2000 to 2008 and related these demographic data to climate data collected at a nearby weather station. Climate variables included (i) peak snow-water equivalency (hereafter “SWE”, a common measure of snowpack), (ii) winter length, (iii) end of winter Julian date (hereafter “last day of winter”), (iv) summer length, and (v) growing degree days (see Methods for complete description of climate variables).
Fig. 1.
The Little Rock Creek drainage, Bitterroot Mountains, Montana. All analyses were based on data collected in the upper basin. Habitat used by R. luteiventris includes the following: (i) permanent water bodies with no emergent vegetation used for summer foraging and overwintering (hatch marks); (ii) permanent ponds and lakes with emergent vegetation used for breeding, foraging, and overwintering (solid); and (iii) ephemeral ponds with emergent vegetation used for breeding and foraging (open).
Results
Climate Variables.
During the 9 years of our study, SWE ranged from 67 to 135 cm, winter lengths varied by 41 days, last day of winter varied by 33 days, summer length ranged from 84 to 120 days, and growing degree days ranged from 431 to 648 days. There were no trends in any of the climate variables over the study period. The range of SWE falls within the historical range of peak SWE at this station (46–171 cm), and average peak SWE for the length of our study is identical to the historical average (101 cm). Therefore, the range and magnitude of variability in climate variables seen in our study captures what has been recorded historically. Winter variables were correlated with each other (R2 > 0.62), as were summer variables (R2 = 0.66), but winter variables were not correlated with summer variables.
Relationships Between Climate and Vital Rates.
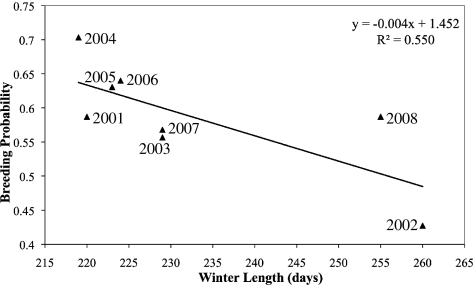
Breeding probability was inversely related to the length of the previous winter (P = 0.002; Fig. 2). There was no relationship between breeding probability and either of the summer climate variables. There was no relationship between survival to 1 yr of age and any of the winter or summer climate variables.
Fig. 2.
Linear regression of female breeding probability with length of previous winter. Longer winters were associated with lower breeding probabilities in the following spring (P = 0.002).
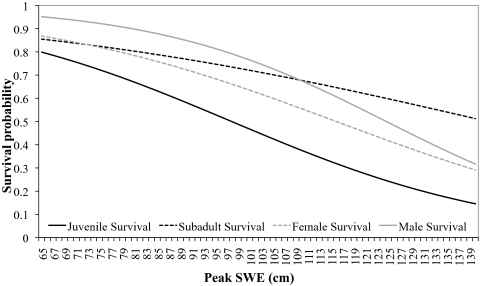
Survival and transition probabilities depended on winter severity. Capture-recapture data for 4,279 individual frogs captured over 9 years were analyzed by using program MARK (32), with climate variables included as group covariates in various combinations. Model selection results for the capture probability models indicated that probability of initial capture (p) and probability of recapture (c) varied both across years and among life stages. Therefore, we used this capture probability structure in all subsequent transition and survival models. Capture probabilities ranged from 4 to 50%, depending on the year and the life stage. Models with SWE and last day of winter were far better supported than any other candidate models (AIC weight = 0.999, model likelihood = 1.0, 93 parameters). In these models, an increase in SWE was associated with a decrease in survival for juvenile and adult stage classes (Fig. 3). Juvenile frogs showed the strongest relationship with variation in SWE (β = −0.042 ± 0.005 SE), but the effect was also significant in adult females (β = −0.037 ± 0.011 SE) and adult males (β = −0.051 ± 0.012 SE). There was no relationship between SWE and survival for subadult females (β = −0.023 ± 0.018 SE).
Fig. 3.
Relationship between stage-specific survival rates in R. luteiventris and SWE for four age/sex classes: juvenile, subadult female, adult male, and adult female. The lines are the predicted survival curves for different values of SWE from the top model.
SWE was inversely related to transition from juvenile to subadult female or male (β = −0.029 ± 0.007 SE) and transition from juvenile to adult female (β = −0.081 ± 0.024 SE). There was no relationship with transition from subadult to adult female (β = 0.015 ± 0.015 SE).
Demographic Analysis.
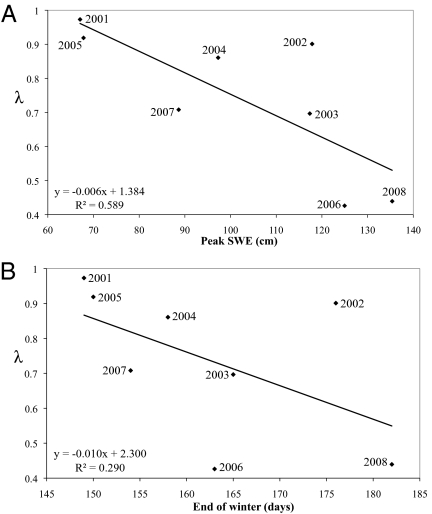
We used survival and transition parameter estimates from a time-varying capture-recapture model to calculate annual vital rates (Table S1) to parameterize female-based, postbirth pulse Leftkovich matrix models for each year of the study (Fig. 4). Across these matrices, estimates of λ were higher in years with lower SWE (P = 0.023; Fig. 5A), tended to be lower in years with a later last day of winter (P = 0.185; Fig. 5B), and were not related to winter length (P = 0.611).
Fig. 4.
Female-based, postbirth pulse matrix model for R. luteiventris. Each year, average values for survival, growth, and fecundity were used to calculate the asymptotic population growth rate. Average clutch size was 406 female eggs for all years based on measurements of clutch size.
Fig. 5.
Linear regression of annual asymptotic λ values and winter variables, including SWE (P = 0.021) (A) and end of winter Julian date (P = 0.185) (B).
Discussion
Parameters that describe winter severity were negatively correlated with survival, transition, and breeding probabilities in this high elevation R. luteiventris population. These relationships resulted in higher estimated λ in years with earlier ending winters and lower snowpack. Climate change predictions for the Rocky Mountains suggest that snowpack will continue to decrease and snowmelt will occur at an increasingly earlier date (33). Because effects of decreased winter severity were uniformly positive, our results indicate that this climate trend will increase population viability of R. luteiventris in this mountain system. Researchers have typically assumed that if climate change affects amphibian species, the outcome will be negative. Rarely do we allow ourselves to consider that global warming may confer benefits to some species. Contrary to much of what has been discussed in the literature (e.g., refs. 6 and 34, but see ref. 35), these results suggest that under certain circumstances, a warming climate may be helpful to some amphibian populations, particularly those that live in harsh conditions at the edge of their thermal tolerances. A reduced winter severity could benefit high and low elevation populations of R. luteiventris alike, although failures in recruitment due to drying of breeding habitat may overwhelm positive effects of warmer winters in some populations, especially at lower elevations and latitudes.
There are several reasons that we might expect R. luteiventris juvenile and adult survival in these systems to increase with decreased snowpack. At high elevations, this species spends up to 8 or 9 months overwintering in lakes and permanent ponds, where its metabolism slows considerably. Hypoxic conditions have been shown to be an important source of mortality for overwintering amphibian populations in high elevation systems (18). Winters with above-average precipitation had thicker ice covering on lakes used by mountain yellow-legged frogs (R. muscosa) for overwintering, resulting in an increase in the rate of oxygen depletion (18). Radio-tagged R. luteiventris overwintering in low elevation ponds in eastern Oregon all remained in shallow water within 1 m of shore, but they moved to microhabitats with higher water temperatures or dissolved oxygen concentrations (36). Juvenile frogs in our system may be more likely to overwinter in shallow lakes than adult frogs, because we find them in higher concentrations at these water bodies during summer surveys and they may not be able to locate larger water bodies. Overwintering in shallow waters may expose juveniles to more hypoxic conditions in the winter than adult frogs and likely explains the strong negative associations among SWE and juvenile survival and transition rates.
Years with higher snowpack tended to have snow on the ground for a longer time period and later into the spring. As a result, it is also possible that high winter severity depletes the fat reserves of frogs and leads to greater mortality rates. The later the last day of winter, the longer frogs remain in overwintering sites, and the later insect prey emerge. This delay in foraging and depletion of fat reserves may contribute to the negative relationship between SWE and transition probability to larger age classes, which we observed for juveniles. The depletion of fat reserves may be less important for subadult and adult frogs and may explain why we do not see a relationship between their transition rates and winter severity variables.
In all years of our study, asymptotic estimates of λ were less than one, suggesting a declining population. This agrees with our observation that this population has generally declined over the 9 years of the study, from 997 frogs in 2000 to 700 frogs in 2008. A decreasing population growth rate during most years is consistent with population growth patterns measured in other amphibian populations (37, 38). However, in contrast to our asymptotic estimates of λ, population size fluctuated considerably among years, with some large increases in population size between years. Therefore, our asymptotic analyses do not capture the range of stochastic fluctuations observed in the field. For example, it may be that population dynamics are partly driven by intermittent pulses of recruitment that were not captured in our 9-year study. As such, we cannot discern whether a warming climate will actually lead to a viable population in the long term. However, this caveat does not change the result that we observed uniformly positive effects of climate warming.
The results of this study highlight the need for amphibian decline research to focus on environmental variables affecting postmetamorphic survival parameters and those affecting recruitment and larval survival (23). Although repeated failures in recruitment due to drought may critically affect the persistence of some populations, changes in juvenile and adult survival could have a greater effect on population growth and viability in many amphibian species. Although we saw high annual fluctuations in the number of egg masses laid and successful metamorphs produced in this system (Table S1), differences in juvenile and adult survival rates, particularly survival of juvenile frogs, drove the differences we observed in λ across years. In our population, juveniles and adults have greater variance in vital rates among years, as well as higher elasticities in those rates (Table S2). Vital rates for R. luteiventris juveniles and adults are therefore disproportionately important to the dynamics of this population. Long-term monitoring of amphibian populations has typically focused on variation in recruitment or breeding population size (e.g., ref. 37). Past research has focused on the effect of weather variability on production of juveniles, which can depend heavily on rainfall in some regions (39). However, it is less clear to what extent this measured variation in annual recruitment affects long-term population dynamics in these systems, because recruitment dynamics are rarely placed in a population-level context (but see refs. 23 and 40).
Although there was variation in annual recruitment, we never witnessed complete failures in recruitment, even in the driest years. Every year, individuals breed in both ephemeral and permanent water bodies. On cool and wet years, most successful recruitment comes from ephemeral ponds, which persist throughout the growing season, whereas tadpoles from colder permanent ponds do not have enough time to reach metamorphosis. However, in hot, dry years the majority of recruitment comes from permanent ponds, because ephemeral ponds dry up before animals are able to successfully metamorphose. Therefore, it may be that frogs in our study system have the ability to compensate recruitment in dry years through the diversity of breeding sites available across this glacial basin. The presence of a diversity of unimpacted breeding sites in these types of systems may be important for amphibian populations to persist in the face of climate change. In high-elevation systems where amphibians are negatively impacted by introduced fish (e.g., ref. 31), reduction of hydroperiod in ephemeral, fishless breeding sites may be much more important to species persistence. Because this system is fishless, amphibians are able to breed and forage in a greater diversity of habitats than in areas where fish and other predators restrict populations to ephemeral ponds and wetlands.
Our analyses suggest that for amphibians and other ectotherms living at temperatures below their physiological optima, such as those found at high elevations or latitudes, global warming may have neutral or positive impacts, at least under some climate change scenarios (41). However, outcomes will surely depend on how quickly and to what extent the climate changes. It is possible that changes in temperature and precipitation may only have benefits to a certain point, after which habitat will be negatively affected, possibly by changing community structure or reducing heterogeneity of breeding sites. At the extreme, if ongoing climate change eventually makes high elevation and latitude sites unsuitable, species may have no other refugia to move to (42). Both the positive and negative impacts of climate change on species will need to be reassessed as climate change progresses to determine whether such a tipping point will be reached.
We have shown how individual amphibian vital rates and asymptotic population growth rates vary with summer and winter climate variables in a high elevation population of the Columbia spotted frog. These results unambiguously demonstrate that earlier ending winters with lower snowpack in this system lead to higher survival rates, higher probabilities of breeding, and higher population viability. Most research on amphibian declines assumes that climate change will have negative impacts on already vulnerable species, yet we show that this may not be the case for alpine and boreal amphibian populations currently persisting in harsh environments. This provides a unique perspective to the role of climate change in amphibian declines in temperate ecosystems. Previous shifts in climate have had dramatic effects on the distribution, genetics, and ecology of numerous temperate amphibian species (43–45). The impacts of ongoing climate change will vary across the globe, and this is likely to increase the viability of some species while being detrimental to others. To best conserve viable populations, promote gene flow, and allow for shifts in geographic range in the face of climate change, developing a more comprehensive understanding of how climate change is expected to benefit or harm amphibian populations at different latitudes and elevations is imperative. Ultimately, research that simultaneously evaluates the effects of climate change on multiple vital rates is key to disentangling which population processes may be affected by a warming climate.
Materials and Methods
Population Surveys.
We collected demographic data from 2000 to 2008. We conducted systematic searches for egg masses in late spring. We searched all shallow water environments for egg masses over several weeks and recorded counts of egg masses at each water body. We calculated multiyear estimates and variances in clutch size for R. luteiventris at most of the breeding ponds by using volumetric displacement (46–48). During the egg mass surveys, we also measured the snout-vent length of females present at the breeding site to determine the range of size of breeding females. These data were used to distinguish breeding adult females from subadult females in our demographic analyses.
We used a robust design capture-mark-recapture method to monitor juvenile and adult frogs (49). For this method, we captured animals for multiple consecutive secondary sessions (days) within the primary sampling period (year). Across the secondary sessions, we assumed that the population was closed to immigration, emigration, births, and deaths. This closure allowed population size, corrected for capture probability, to be estimated. Between primary sessions, the population was open to gains and losses, and survival was estimated by using the Cormack-Jolly-Seber model (50–52). We monitored three female life stages: juveniles were frogs that were too young to be sexed, subadults were frogs that were larger than the size at which secondary sex characteristics of males were present but smaller than the smallest documented breeding female, and adults were frogs large enough to breed. During each primary sampling period, we systematically surveyed all of the ponds and lake shores in the basin each year and captured animals by hand or net. We individually marked animals by clipping unique combinations of toes by using an alphanumeric coding system (53) and weighed and measured snout-vent length. We also recorded the general location of all new and recaptured animals at each session.
Climate Data.
Climate data were from the Twin Lakes SNOTEL site (www.wcc.nrcs.usda.gov/snow), located 18 km northwest of the upper Little Rock Creek basin at 1,950 m elevation. This SNOTEL site is located within the same mountain range and has similar aspect and elevation to our study area. We used data on SWE and temperature as annual covariates. SWE is the depth of water that would theoretically result if one were to melt the entire snowpack instantaneously and is commonly used as a measure of snowpack (e.g., ref. 21). We used the peak SWE value recorded for each year in our analyses. We calculated the end of winter Julian date as the last day that SWE was recorded in the spring. Then we calculated the number of consecutive days with snow on the ground (winter length) and the number of consecutive days without snow on the ground (summer length). Finally, we calculated the cumulative number of days throughout the summer that the temperature was >10 °C (www.ipm.ucdavis.edu/WEATHER/ddconcepts.html), which represented growing degree days. The value of 10 °C was used as a relative physiological zero for the species, temperatures below which we would not expect to see growth. Although it is unlikely that this temperature is the true physiological zero, which is unknown, this cutoff allows for comparison of relative growing season length across years. We used winter length, last day of winter, and SWE data as indices of local winter severity, and we used growing degree days and summer length as indices of summer intensity.
We tested for relationships among all climate variables over the 9 years of the study by using principal components analysis. The first two axes explained 89% of the variance. Axis 1 (56% of variance) was correlated with winter severity (r > 0.85 for all winter variables; r < 0.53 for all summer variables). Axis 2 (33% of variance) was correlated with summer variables (r > 0.75 for all summer variables; r < 0.40 for all winter variables). Therefore, we can clearly distinguish winter from summer variables but cannot statistically separate individual winter or summer variables. However, we chose to analyze all variables individually to discuss hypothetical mechanisms associated with each variable.
Breeding Probability and Prejuvenile Analysis.
We estimated breeding probability as the number of egg masses deposited each year divided by the number of adult females that year (closed population estimate from the mark-recapture data). We estimated survival from eggs to 1 year for each year by dividing the number of 1-year-old frogs by the number of eggs laid the previous spring (average number of eggs per mass multiplied by the number of egg masses deposited). We used an average of 812 eggs per mass (assumed to be equivalent to 406 female embryos given a 1:1 sex ratio) from our clutch size estimates for all years of the analysis. To estimate the number of 1-year-old frogs, we isolated the capture histories for all animals 34 mm snout-vent length and smaller each year and then calculated the abundance of those animals by using Huggins closed population models in program MARK (32).
We used weighted linear regressions to examine the dependence of breeding probability and first-year survival on the five climate variables described above. Additionally, we examined relationships between number of egg masses deposited and climate variables.
Post-Metamorph Analysis.
We analyzed capture data of juveniles, subadults, and adults in program MARK by using closed robust design, multistate mark-recapture models (54, 55). The “states” in our models were frog life stages: juvenile, subadult female, adult female, and adult male frogs. These models provided parameter estimates and variances for age- and sex-specific survival, transition rates between life stages, and population size for each year (56, 57). Survival, transitions, and the covariates were our primary parameters of interest, but we first evaluated models of capture probability (p) and recapture probability given initial capture (c) to avoid bias and imprecision in the survival estimates (58). We evaluated the following capture probability models: (i) p and c were constant across years but varied among age classes, (ii) p and c varied across years but did not vary among age classes, (iii) p and c varied across years and differed between juvenile and adult age classes, but not within adult age classes, and (iv) p and c varied across years and among all age classes. For these models, survival and transition rates were held constant.
We modeled age-specific survival and transition probabilities to examine their variation in relation to snowpack, winter length, end of winter, summer length, and growing degree-days. First, we examined how each of these variables related to survival in each age class individually. Then, we examined whether additive models containing multiple climate variables improved the fit. For each model structure, we estimated models where (i) the effect of covariates was the same for each age class and (ii) the effect of covariates was different for each age class, resulting in a total of 16 basic models (Table 1). Once we tested these models, we also examined two additional models where (i) male survival was equal to female survival and (ii) all adult age classes had the same survival. For these models, we kept transition probabilities constant. Once we had modeled survival, we examined how climate variables affected growth by using the structure from the best survival model. We examined the same suite of variables for growth as we did for survival. In addition to the 16 models described above, we estimated models where probability of transitioning from juvenile to subadult was equal to transitioning to male (Table 1). We evaluated all models for relative support and ranked them by using Akaike's Information Criterion (AICc) (59, 60).
Table 1.
Model structures for assessing how climate variables relate to survival and growth parameters
| Winter variables | Summer variables |
| Stage + (SWE) | Stage + (summer length) |
| Stage + (end of winter) | Stage + (growing degree days) |
| Stage + (winter length) | Stage + (summer length + growing degree days) |
| Stage + (SWE + winter length) | Stage × (summer length) |
| Stage + (SWE + end of winter) | Stage × (growing degree days) |
| Stage × (SWE) | Stage × (summer length + growing degree days) |
| Stage × (end of winter) | |
| Stage × (winter length) | |
| Stage × (SWE + winter length) | |
| Stage × (SWE + end of winter) |
For all models, we let p and c vary by both age class and time. We first estimated survival probabilities, keeping growth probabilities constant, and then estimated growth probabilities by using the best model from the survival analysis. For transition models, we examined scenarios where all transitions were different and where probability of juvenile to male transition was equal to juvenile to subadult transition.
Demographic Analysis.
We determined the demographic consequences of changes in climate variables on the population growth rate using matrix models (19). We constructed female-based postbirth pulse stage-structured matrix models by using the average vital rates estimated for each year (Table S1 and Fig. 4). Survival and transition parameters came from the best time-dependent model ranked by using AICc. We tested four time-dependent models where survival varied by both year and life stage: (i) survival varies in the same way for each life stage in each year; (ii) juvenile survival varies differently from subadult, male, and female survival, which vary in the same way each year; (iii) male and female survival vary in the same way, but both juvenile and subadult survival vary differently in each year; and (iv) all life stages varied differently from each other in each year. The best model varied transitions for each life stage and year, but transition probability from juvenile to subadult was equal to that for juvenile to male. Capture and recapture probabilities varied by year and life stage for all models. Other vital rates were calculated as described above. For each year, we calculated asymptotic λ, the rate at which a population would increase if vital rates remained constant over time. This metric represents an integrative measure of population performance. We then used linear regression of the asymptotic lambda values for each year against values of climate variables for each year to see whether this integrative metric showed similar patterns to individual vital rates.
Supplementary Material
Acknowledgments
We thank the many field assistants who helped collect the demographic data used in this study and K. Griffin for assistance with the mark-recapture analyses. We thank E. E. Crone, L. Eby, P. S. Corn, L. S. Mills, W. H. Lowe, and two anonymous reviewers for helpful comments and insights on analyses and drafts of the manuscript. Fieldwork was partly supported with funding from the US Geological Survey's Amphibian Research and Monitoring Initiative through cooperative agreements among the US Geological Survey, the University of Montana, and the Montana Natural Heritage Program. Research was also partly supported by US Forest Service Region 1 Inventory and Monitoring grants (to B.A.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0912945107/-/DCSupplemental.
References
- 1.Alford RA, Richards SJ. Global amphibian declines: A problem in applied ecology. Annu Rev Ecol Syst. 1999;30:133–165. [Google Scholar]
- 2.Houlahan JE, et al. Quantitative evidence for global amphibian population declines. Nature. 2000;404:752–755. doi: 10.1038/35008052. [DOI] [PubMed] [Google Scholar]
- 3.Stuart SN, et al. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- 4.Pounds JA, Crump ML. Amphibian declines and climate disturbance: The case of the golden toad and the harlequin frog. Conserv Biol. 1994;8:72–85. [Google Scholar]
- 5.Pounds JA, et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature. 2006;439:161–167. doi: 10.1038/nature04246. [DOI] [PubMed] [Google Scholar]
- 6.Corn PS. Climate change and amphibians. Anim Biodivers Conserv. 2005;28:59–67. [Google Scholar]
- 7.Beebee TJC. Amphibian breeding and climate change. Nature. 1995;374:219–220. [Google Scholar]
- 8.Forchhammer MC, Post E, Stenseth NC. Breeding phenology and climate. Nature. 1998;391:29–30. [Google Scholar]
- 9.Blaustein AR, et al. Amphibian breeding and climate change. Conserv Biol. 2001;15:1804–1809. [Google Scholar]
- 10.Corn PS. Amphibian breeding and climate change: The importance of snow in the mountains. Conserv Biol. 2003;17:622–625. [Google Scholar]
- 11.Reading CJ. The effect of winter temperatures on the timing of breeding activity in the common toad Bufo bufo. Oecologia. 1998;117:469–475. doi: 10.1007/s004420050682. [DOI] [PubMed] [Google Scholar]
- 12.Rohr JR, et al. Evaluating the links between climate, disease spread, and amphibian declines. Proc Natl Acad Sci USA. 2008;105:17436–17441. doi: 10.1073/pnas.0806368105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lips KR, Diffendorfer J, Mendelson JR, III, Sears MW. Riding the wave: Reconciling the roles of disease and climate change in amphibian decline. PLoS Biol. 2008;6:e72. doi: 10.1371/journal.pbio.0060072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reading CJ. Linking global warming to amphibian declines through its effects on female body condition and survivorship. Oecologia. 2007;151:125–131. doi: 10.1007/s00442-006-0558-1. [DOI] [PubMed] [Google Scholar]
- 15.Kiesecker JM, Blaustein AR, Belden LK. Complex causes of amphibian population declines. Nature. 2001;410:681–684. doi: 10.1038/35070552. [DOI] [PubMed] [Google Scholar]
- 16.Carey C. Factors affecting body temperatures of toads. Oecologia. 1978;35:197–219. doi: 10.1007/BF00344732. [DOI] [PubMed] [Google Scholar]
- 17.Wells KD. The ecology and behavior of amphibians. Chicago: Univ Chicago Press; 2007. [Google Scholar]
- 18.Bradford DF. Winterkill, oxygen relations, and energy metabolism of a submerged dormant amphibian, Rana muscosa. Ecology. 1983;64:1171–1183. [Google Scholar]
- 19.Tattersall GJ, Boutelier RG. Balancing hypoxia and hypothermia in cold-submerged frogs. J Exp Biol. 1997;200:1031–1038. doi: 10.1242/jeb.200.6.1031. [DOI] [PubMed] [Google Scholar]
- 20.Bernardo J, Spotila JR. Physiological constraints on organismal response to global warming: Mechanistic insights from clinally varying populations and implications for assessing endangerment. Biol Lett. 2006;2:135–139. doi: 10.1098/rsbl.2005.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamlet AF, Mote PW, Clark MP, Lettenmaier DP. Effects of temperature and precipitation variability on snowpack trends in the western United States. J Clim. 2005;18:4545–4561. [Google Scholar]
- 22.MacCracken M, et al. In: Climate change impacts on the United States: the potential consequences of climate variability and change. Team NAS, editor. Cambridge, UK: Cambridge Univ Press; 2001. pp. 13–71. [Google Scholar]
- 23.Biek R, Funk WC, Maxell BA, Mills LS. What is missing in amphibian decline research: insights from ecological sensitivity analysis. Conserv Biol. 2002;16:728–734. [Google Scholar]
- 24.Caswell H. Matrix population models–construction, analysis, and interpretation. Sunderland, MA: Sinauer Associates; 2001. [Google Scholar]
- 25.Leftkovitch LP. The study of population growth in organisms grouped by stages. Biometrics. 1965;21:1–18. [Google Scholar]
- 26.Leslie PH. On the use of matrices in certain population mathematics. Biometrika. 1945;33:183–212. doi: 10.1093/biomet/33.3.183. [DOI] [PubMed] [Google Scholar]
- 27.Funk WC, et al. Population structure of Columbia spotted frogs (Rana luteiventris) is strongly affected by landscape. Mol Ecol. 2005;14:483–496. doi: 10.1111/j.1365-294X.2005.02426.x. [DOI] [PubMed] [Google Scholar]
- 28.Maxell BA. State-wide assessment of status, predicted distribution, and landscape-level habitat suitability of amphibians and reptiles in Montana. Missoula: PhD dissertation (University of Montana; 2009. [Google Scholar]
- 29.Rollins-Smith LA, et al. Activity of antimicrobial skin peptides from ranid frogs against Batrachochytrium dendrobatidis, the chytrid fungus associated with global amphibian declines. Dev Comp Immunol. 2002;26:471–479. doi: 10.1016/s0145-305x(01)00088-x. [DOI] [PubMed] [Google Scholar]
- 30.Muths E, Corn PS, Pessier AP, Green DE. Evidence for disease-related amphibian decline in Colorado. Biol Conserv. 2003;110:357–365. [Google Scholar]
- 31.Knapp RA, Matthews KR. Non-native fish introductions and the decline of the mountain yellow-legged frog from within protected areas. Conserv Biol. 2000;14:428–438. [Google Scholar]
- 32.White GC, Burnham KP. Program MARK: Survival estimation from populations of marked animals. Bird Study. 1999;46(Suppl):120–138. [Google Scholar]
- 33.Adam JC, Hamlet AF, Lettenmaier DP. Implications of global climate change for snowmelt hydrology in the twenty-first century. Hydrol Process. 2009;23:962–972. [Google Scholar]
- 34.McMenamin SK, Hadly EA, Wright CK. Climatic change and wetland desiccation cause amphibian decline in Yellowstone National Park. Proc Natl Acad Sci USA. 2008;105:16988–16993. doi: 10.1073/pnas.0809090105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scherer RD, Muths E, Lambert BA. Effects of weather on survival in populations of boreal toads in Colorado. J Herpetol. 2008;42:508–517. [Google Scholar]
- 36.Bull EL, Hayes MP. Overwintering of Columbia spotted frogs in northeastern Oregon. Northwest Sci. 2002;76:141–147. [Google Scholar]
- 37.Semlitsch RD, Scott DE, Pechmann JHK, Gibbons JW. In: Long-Term Studies of Vertebrate Communities. Cody ML, Smallwood JA, editors. San Diego: Academic; 1996. pp. 217–248. [Google Scholar]
- 38.Pechmann JHK, et al. Declining amphibian populations: The problem of separating human impacts from natural fluctuations. Science. 1991;253:892–895. doi: 10.1126/science.253.5022.892. [DOI] [PubMed] [Google Scholar]
- 39.Daszak P, et al. Amphibian population declines at Savannah River Site are linked to climate, not chytridiomycosis. Ecology. 2005;86:3232–3237. [Google Scholar]
- 40.Vonesh JR, De La Cruz O. Complex life cycles and density dependence: assessing the contribution of egg mortality to amphibian declines. Oecologia. 2002;133:325–333. doi: 10.1007/s00442-002-1039-9. [DOI] [PubMed] [Google Scholar]
- 41.Deutsch CA, et al. Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA. 2008;105:6668–6672. doi: 10.1073/pnas.0709472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wake DB, Vredenburg VT. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc Natl Acad Sci USA. 2008;105:11466–11473. doi: 10.1073/pnas.0801921105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Funk WC, et al. Range-wide phylogeographic analysis of the spotted frog complex (Rana luteiventris and Rana pretiosa) in northwestern North America. Mol Phylogenet Evol. 2008;49:198–210. doi: 10.1016/j.ympev.2008.05.037. [DOI] [PubMed] [Google Scholar]
- 44.Goebel AM, Ranker TA, Corn PS, Olmstead RG. Mitochondrial DNA evolution in the Anaxyrus boreas species group. Mol Phylogenet Evol. 2009;50:209–225. doi: 10.1016/j.ympev.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 45.Vieites DR, Min M-S, Wake DB. Rapid diversification and dispersal during periods of global warming by plethodontid salamanders. Proc Natl Acad Sci USA. 2007;104:19903–19907. doi: 10.1073/pnas.0705056104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morris RL, Tanner WW. The ecology of the western spotted frog, Rana pretiosa pretiosa Baird and Girard: A life history study. Great Basin Nat. 1969;29:45–81. [Google Scholar]
- 47.Corn PS, Livo LJ. Leopard frog and wood frog reproduction in Colorado and Wyoming. Northwest Nat. 1989;70:1–9. [Google Scholar]
- 48.Werner JK, Weaselhead J, Plummer T. The accuracy of estimating eggs in anuran egg masses using weight or volume measurements. Herpetol Rev. 1999;30:30–31. [Google Scholar]
- 49.Pollock KH. A capture-recapture design robust to unequal probability of capture. J Wildl Manage. 1982;46:752–757. [Google Scholar]
- 50.Cormack RM. Estimates of survival from the sighting of marked animals. Biometrika. 1964;51:429–438. [Google Scholar]
- 51.Jolly GM. Explicit estimates from capture-recapture data with both death and immigration stochastic model. Biometrika. 1965;52:225–247. [PubMed] [Google Scholar]
- 52.Seber GAF. A note on the multiple recapture census. Biometrika. 1965;52:249–259. [PubMed] [Google Scholar]
- 53.Waichman AV. An alphanumeric code for toe clipping amphibians and reptiles. Herpetol Rev. 1992;23:19–21. [Google Scholar]
- 54.Hestbeck JB, Nichols JD, Malecki RA. Estimates of movement and site fidelity using mark-resight data of wintering Canada geese. Ecology. 1991;72:523–533. [Google Scholar]
- 55.White GC, Kendall BE, Barker RJ. Multistate survival models and their extensions in Program MARK. J Wildl Manage. 2006;70:1521–1529. [Google Scholar]
- 56.Morris WF, Doak DF. Quantitative Conservation Biology. Sunderland, MA: Sinauer Associates; 2002. [Google Scholar]
- 57.Kendall BE. Estimating the magnitude of environmental stochasticity in survivorship data. Ecol Appl. 1998;8:184–193. [Google Scholar]
- 58.Lebreton JD, Burnham KP, Clobert J, Anderson DR. Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecol Monogr. 1992;62:67–118. [Google Scholar]
- 59.Akaike H. In: Second International Symposium on Information Theory. Csaki F, Petrov BN, editors. Budapest: Akademiai Kiado; 1973. [Google Scholar]
- 60.Burnham KP, Anderson DR. Model Selection and Inference: A Practical Information-Theoretic Approach. New York: Springer; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.