Abstract
Lipoxins (Lxs) and aspirin-triggered epi-Lxs (15-epi-LxA4) act through the ALX/FPRL1 receptor to block leukocyte trafficking, dampen cytokine/chemokine synthesis, and enhance phagocytic clearance of apoptotic leukocytes—key requisites for inflammatory resolution. Although studies using primarily inbred rodents have highlighted resolution as an active event, little is known about the role resolution pathways play in controlling the duration/profile of inflammatory responses in humans. To examine this, we found two types of responders to cantharidin-induced skin blisters in male healthy volunteers: those with immediate leukocyte accumulation and cytokine/chemokine synthesis followed by early resolution and a second group whose inflammation increased gradually over time followed by delayed resolution. In early resolvers, blister 15-epi-LxA4 and leukocyte ALX were low, but increased as inflammation abated. In contrast, in delayed resolvers, 15-epi-LxA4 and ALX were high early in the response but waned as inflammation progressed. Elevating 15-epi-LxA4 in early resolvers using aspirin increased blister leukocyte ALX but reduced cytokines/chemokines as well as polymorphonuclear leukocyte and macrophage numbers. These findings show that two phenotypes exist in humans with respect to inflammation severity/longevity controlled by proresolution mediators, namely 15-epi-LxA4. These data have implications for understanding the etiology of chronic inflammation and future directions in antiinflammatory therapy.
Keywords: eicosanoids, inflammation, leukocytes
Increasing numbers of mediators are being identified that switch inflammation off, supporting the notion that inflammatory resolution is an active process (1–3). Of these proresolution factors, lipoxins (Lxs; e.g., LxA4/B4) and epilipoxins (e.g., 15-epi-LxA4/B4) are lipids with antiinflammatory and proresolution properties (4). By signaling through the ALX (FPRL1) receptor (5), Lxs reduce cytokine/chemokine synthesis and polymorphonuclear leukocyte (PMN) trafficking as well as enhance phagocytosis of apoptotic leukocytes, thereby facilitating multiple aspects of the resolution cascade.
Lxs are formed through three distinct transcellular biosynthetic pathways. The first involves platelet/leukocyte interactions in which leukocyte 5-lipoxygenase (LOX) converts arachidonic acid to the epoxide LTA4, which is released and further transformed by leukocyte-adherent platelets to LxA4 via the Lx synthase activity of platelet 12-LOX (6). A second biosynthetic route occurs at the mucosal surfaces by 15-LOX that inserts oxygen into arachidonic acid at carbon 15 to produce 15S-hydroxyleicosatetraenoic acid, which is taken up by PMNs and converted by 5-LOX to Lxs (7, 8). In a third pathway, aspirin triggers epimeric forms of Lxs as a result of acetylating the active site of inducible COX-2 in endothelial or epithelial cells. This results not in the inhibition of COX-2, but in the conversion of arachidonic acid to (15 R) hydroxyeicosatetraenoic acid, which is rapidly metabolized in a transcellular manner by leukocyte 5-LOX to 15-epi-LxA4 or B4 (9). Interestingly, epi-Lxs are also found in humans under aspirin-free conditions possibly derived from either a cytochrome P450 pathway and/or from an endogenous COX-2–acetylating agent capable of generating epi-Lxs in physiological and inflammatory settings (10, 11).
In addition to Lxs, there are a host of other soluble mediators and cells central to resolution whose expression/synthesis occurs at strategic checkpoints throughout inflammation to switch the response off and restore homeostasis (12–17). Although the sequential activation of these proresolution pathways has almost exclusively been elucidated using inbred rodents, little is known about their role in limiting inflammatory responses in humans. We recently found that cardioprotective doses of aspirin (75 mg; [ASAlow]) inhibits leukocyte trafficking into cantharidin-induced skin blisters in healthy human volunteers (18). A retrospective analyses of those data revealed that ASAlow worked in 60% of individuals in an inflammation-dependent manner, i.e., the more severe the inflammation in terms of leukocyte accumulation, the greater the antiinflammatory effects of ASAlow, with the remaining volunteers (40%) not responding to aspirin. The baseline temporal profile of cell trafficking in aspirin responders peaked in an immediate early fashion and resolved within a few days (hereafter called early resolvers; [Ervs]). Volunteers who were refractory to ASAlow had a gradual accumulation of cells that failed to clear the blister site, showing signs of delayed resolution (hereafter called delayed resolvers; [Drvs]). Further analysis showed that interindividual differences in the biosynthesis of endogenous 15-epi-LxA4 accounted for the differential inflammatory profiles in Drvs versus Ervs. Therefore, these data show that acute inflammatory responses in humans follow two distinct profiles controlled, at least in part, by proresolution pathways. Acquired dysregulation of these protective processes at the genetic level or by antiinflammatory agents with hitherto unappreciated resolution-toxic properties (19) may cause ongoing inflammation, tissue injury, or chronic inflammation.
Results
Aspirin Reveals Differential Inflammatory Responses in Humans.
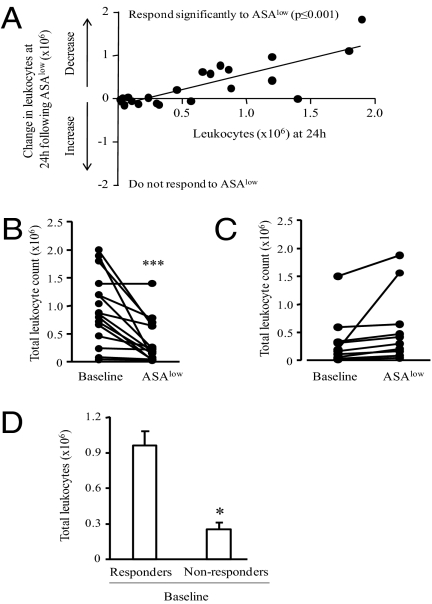
We previously found that ASAlow was antiinflammatory in 26 male healthy human volunteers in whom it dampened leukocyte trafficking into cantharidin-induced skin blisters (18). Further analysis of those data revealed that volunteers with the most severe inflammation in terms of blister leukocyte numbers exhibited the greatest antiinflammatory response to ASAlow. Fig. 1A shows the difference in intrablister total cell numbers before/after aspirin for each volunteer (y axis) against their corresponding baseline cell numbers on the x axis. Therefore, those above 0 on the y axis (16 volunteers) showed a reduction in cells after aspirin (i.e., responders) whereas those on or below 0 on the y axis showed no change or increased cell numbers, respectively (10 volunteers; i.e., nonresponders). Thus, there are two distinct groups of responders to ASAlow—those with significantly reduced inflammation after treatment (Fig. 1B) and a second group of 10 volunteers who did not respond to ASAlow (Fig. 1C). Moreover, there was a significant difference in baseline leukocyte numbers at 24 h between responders and nonresponders (Fig. 1D). These data suggest that, following tissue injury, some subjects display a robust inflammatory response conducive to the antiinflammatory effects of ASAlow and another group that displays a less severe initial response are refractory to ASAlow.
Fig. 1.
Aspirin reveals differential profiles of acute inflammatory responses in humans. Healthy male volunteers (n = 26) between the ages of 25 and 50 y had a cantharidin skin blister elicited on the ventral aspects of their forearm with baseline acute inflammatory responses established 24 h later. Each volunteer was then given ASAlow (75 mg) for 10 d, followed by creation of another blister. (A) The difference in blister total cell numbers before and after aspirin for each volunteer (y axis) against their corresponding baseline cell numbers on x axis (r = −0.81, P ≤ 0.001). Therefore, those above 0 on the y axis (n = 16) showed a reduction in cell after aspirin (i.e., responders) whereas those on or below 0 on the y axis showed no change or increased cell numbers, respectively (n = 10; nonresponders). Reduction in blister cell numbers after aspirin was highly significant in responders (B) but not in aspirin nonresponders (C), with differences in baseline inflammation severity between aspirin responders compared with nonresponders also significant (D). Data are presented as mean ± SEM; *P < 0.05 and ***P < 0.001.
Early Versus Delayed Resolution to Acute Inflammation.
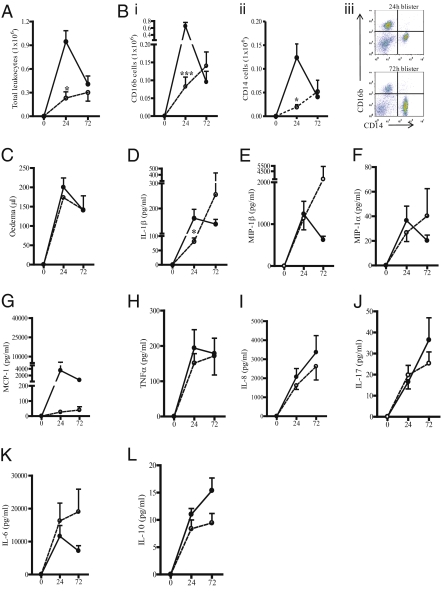
In blisters of volunteers who responded to ASAlow and who therefore possessed an Ervs phenotype, there was a peak in total cells at 24 h following cantharidin (solid line, Fig. 2A), with inflammation resolving by 72 h. In contrast, total cell numbers in cantharidin-induced blisters in the remaining 10 volunteers (Drvs; dotted line, Fig. 2A) was significantly lower at 24 h compared with Ervs but increased gradually up to 72 h (Fig. 2A). Profiles of CD16b-positive PMNs in Fig. 2B i and CD14-positive monocytes/macrophages (Fig. 2B ii) followed a similar profile to total cells peaking at 24 h in Ervs and declining thereafter but increasing progressively over time in Drvs. Representative FACS dot blots are in Fig. 2B iii, showing the change from predominantly CD16b-positive PMNs at 24 h to CD14-positive monocytes/macrophages at resolution using Ervs as an example. Unlike cells, edema accumulation and clearance followed similar profiles in Ervs and Drvs (Fig. 2C). Mirroring leukocyte kinetics, levels of IL-1β were significantly higher in Ervs versus Drvs at 24 h (Fig. 2D), with no such differences found with other cytokine and chemokines (Fig. 2 E–L). The results of these experiments reveal two types of responders to acute tissue injury in healthy humans with regard to leukocytes but not edema: the first displayed an early heightened innate immune-mediated response that resolved in an immediate manner, and the second showed a more tempered inflammatory profile that failed to resolve within the timeframe examined (72 h).
Fig. 2.
Dichotomy in the profile of acute inflammatory responses in humans. The inflammatory profile of cantharidin-elicited skin blisters in aspirin responders was charted over time (in h) and compared with nonresponders showing that blister total cell numbers (A), CD16b-positive PMNs (Bi), and CD14-positive monocytes/macrophages (Bii) in aspirin responders (solid line) peaked in an immediate early manner and resolved quicker than in aspirin nonresponders (dotted line); with (Biii) a representative FACS dot plot charting the change from primarily CD16b-positive PMNs at 24 h to CD14-positive monocytes/macrophages at resolution in Ervs. (C) Blister edema accumulation did not follow this trend. (D–L) Blister fluid exudate proinflammatory cytokines and chemokines with biologically relevant levels broadly mirrored inflammation in both groups. These profiles gave rise to us defining responses as early resolvers (Ervs) or delayed resolvers (Drvs). Data are presented as mean ± SEM; *P < 0.05 and ***P < 0.001 represent differences in inflammatory parameters between Ervs and Drvs at 24 h.
Differences in 15-epi-LxA4/ALX Expression Between Ervs and Drvs.
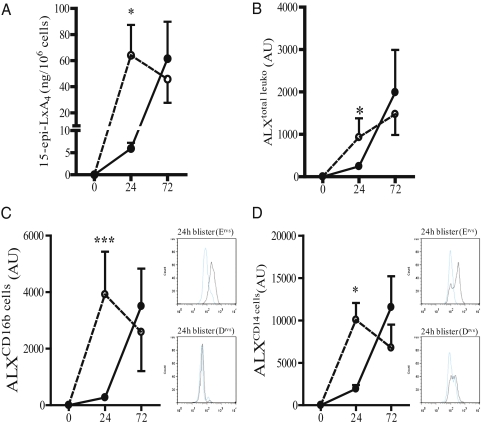
Inflammation severity is controlled by a diverse range of endogenous counter-regulatory signals. In this current study, by using cantharidin-induced skin blisters, we found no correlation between levels of antiinflammatory IL-10 and leukocyte numbers in Ervs and Drvs. Similarly, there was no association between leukocyte numbers and levels of prostaglandin E2, which exerts inhibitory effects on PMN trafficking via EP2/EP4 receptors by elevating cAMP (20). However, 15-epi-LxA4 is an endogenous lipid that dampens PMN trafficking (21), proinflammatory cytokine release (ref. 22 and Fig. S1), and facilitates macrophage phagocytosis of apoptotic PMNs (23) through its ALX receptor (5). Levels of blister fluid 15-epi-LxA4 (Fig. 3A) as well as ALX expression on total cells (Fig. 3B), CD16b-positive PMNs (Fig. 3C), and CD14-positive monocytes/macrophages (Fig. 3D) were significantly lower in Ervs (solid line, Fig. 3A) compared with Drvs (dotted line, Fig. 3A) at 24 h but were gradually elevated in Ervs as inflammation switched off. In contrast, blister fluid 15-epi-LxA4 (Fig. 3A) and leukocyte ALX expression (Fig. 3 B–D) were maximal in Drvs at 24 h and showed a trend toward a reduction by 72 h coincident with progressively increased cell influx. Representative FACS histograms in Fig. 3C illustrate increased ALX expression on CD16b-positive PMNs in Ervs compared with Drvs at 24 h, with similar comparative histograms in Fig. 3D illustrating increased ALX expression on CD14-positive monocytes/macrophages at 24 h. Thus, in groups of volunteers who have an enhanced early response to cantharidin, there are correspondingly low levels of 15epi-LxA4/ALX that increased in line with leukocyte clearance. In contrast, Drvs have significantly higher 15epi-LxA4/ALX than Ervs early in the response, but declined as cell numbers increased. Given the antiinflammatory/proresolution properties of epi-Lxs, these data suggest that the dichotomy in response to cantharidin could be caused at least in part by endogenous levels of 15epi-LxA4 and its receptor expression. The source of 15epi-LxA4 in humans not taking aspirin is unknown, but mice (not bearing inflammation) given celecoxib have reduced plasma 15epi-LxA4 whereas levels in animals given SK525A are unchanged (Fig. S2). These data exclude a role for cytochrome 450 in generating endogenous epilipoxins as proposed (11), but suggests the existence of an endogenous factor that acetylates COX-2, resulting in constitutive 15epi-LxA4 synthesis.
Fig. 3.
The 15-epi-LxA4 and its ALX receptor dictate inflammation severity and longevity in humans with acute inflammation. Of the endogenous antiinflammatory and proresolution factors measured over time (in h), significant differences were found only in levels of blister fluid 15-epi-LxA4 (A) as well as ALX expression on total blister leukocyte (B), CD16b-positive PMN (C), and CD14-positive monocytes/macrophages (D) between Ervs (solid line) and Drvs (dotted line). (C) Representative FACS histograms illustrate increased ALX expression on CD16b-positive PMNs in Ervs compared with Drvs with similar comparative histograms (D) illustrating increased ALX expression on CD14-positive monocytes/macrophages. Data are presented as mean ± SEM; *P < 0.05 and ***P < 0.001 represent differences in inflammatory parameters between Ervs and Drvs at 24 h. AU represents arbitrary units of ALX expression intensity determined by FACS.
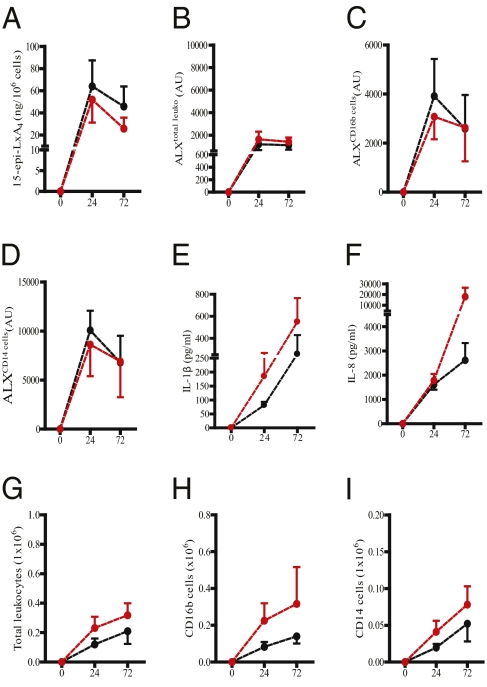
Modulating 15-epi-LxA4 and the Inflammatory Response.
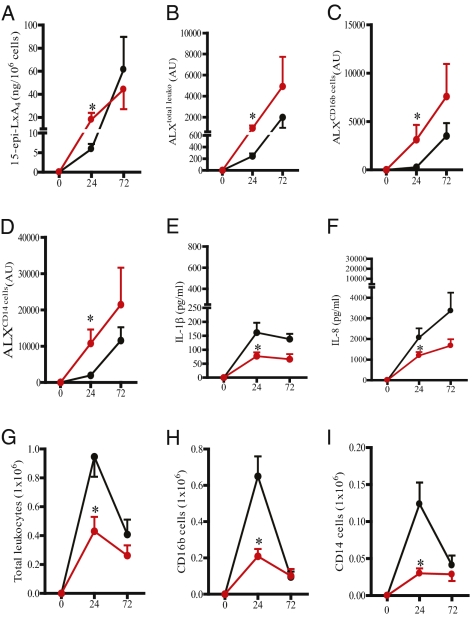
As 75 mg aspirin in healthy human volunteers triggers 15epi-LxA4 synthesis (10) as well as elevates ALX expression (18), we determined whether ASAlow altered 15epi-LxA4/ALX and hence inflammation in Ervs and Drvs. Ten days of prophylactic ingestion of 75 mg aspirin, taken orally once daily, in Ervs (black line, controls; red line, ASAlow; Fig. 4A) increased blister cell–free exudate levels of 15epi-LxA4 as well as total leukocyte ALX expression (Fig. 4 B–D) coincident with a significant reduction in IL-1β (Fig. 4E) and IL-8 (Fig. 4F) as well as cell numbers (Fig. 4 G–I) 24 h following cantharidin. Other cytokines and chemokines were not inhibited (Fig. S3 A–H). Equivalent treatment of Drvs (black line, controls; red line, ASAlow; Fig. 5A) had little effect on 15epi-LxA4, leukocyte ALX (Fig. 5 B–D), IL-1β/IL-8 (Fig. 5 E and F and Fig. S4 A–H), or cell numbers (Fig. 5 G–I). These data suggest that 15epi-LxA4/ALX are minimal in Ervs at 24 h and can be elevated above baseline resulting in a dampening of inflammation. However, in Drvs at the same early time point, 15epi-LxA4/ALX are maximal and cannot be further elevated. Moreover, it suggests that 15epi-LxA4 and its receptor are potent endogenous controllers of acute inflammatory responses in humans and their differential expression patterns dictate the severity and longevity of acute inflammatory responses.
Fig. 4.
Modulating 15-epi-LxA4 with ASAlow dampens inflammation in Ervs. ASAlow (75 mg/d for 10 d, data represented by red line) elevated 15-epi-LxA4 in the blister fluids of 16 volunteers (A) as well as ALX expression on total blister leukocyte (B), PMN (C), and monocytes (D) compared with controls (black line). This elevation in endogenous 15-epi-LxA4/ALX was associated with reduced blister fluid IL-β (E) and IL-8 (F) as well as blister total leukocyte (G), PMN (H), and monocyte numbers (I). Data are presented as mean ± SEM; *P < 0.05 represent differences after 24 h in cantharidin-induced skin blister inflammatory parameters in Ervs before and after aspirin. AU represents arbitrary units of ALX expression intensity determined by FACS.
Fig. 5.
ASAlow is without effect on Drvs. ASAlow (75 mg/d for 10 d; red dotted line) did not alter blister 15-epi-LxA4 or ALX expression (A) on total blister leukocyte (B), PMN (C), and monocytes (D) compared with controls (black dotted line) in 10 human volunteers with a delayed resolving phenotype. Also, there was no effect of ASAlow on blister fluid IL-β (E) and IL-8 (F) as well as blister total leukocyte (G), PMN (H), and monocyte numbers (I). Data are presented as mean ± SEM; *P < 0.05. AU represents arbitrary units of ALX expression intensity determined by FACS.
Discussion
At least two distinct inflammatory events can ensue following cantharidin-induced skin blisters in humans. The first is an immediate response typified by rapid cytokine/chemokine generation and leukocyte influx followed by early resolution (i.e., in Ervs). The second is a more tempered response characterized by a gradual increase in leukocyte accumulation as well as cytokine/chemokine generation. As inflammation in the latter group did not resolve within the timeframe examined, we called these delayed resolvers (Drvs). To gain mechanistic insight into the reasons for these differential responses we found that Ervs synthesize low quantities of 15-epi-LxA4 following injury with levels increasing as inflammation abates. In Drvs, synthesis of 15-epi-LxA4 is maximal early in the response but declines concomitant with delayed cell accumulation. Given that 15-epi-LxA4 possesses antiinflammatory and well as proresolution properties, we suggest that 15-epi-LxA4 acts as an internal braking signal tempering the severity and longevity of acute inflammatory responses in humans. In addition, this study shows that resolution is an active process in healthy individuals and that, unlike rodents, inflammatory responses in humans are at least dichotomous and resolution pathways dictate their longevity and eventual termination. Our findings exclude IL-10 and cAMP-elevating prostaglandin E2 as factors that could potentially govern the progression and resolution of this cantharidin-induced acute inflammatory response. Furthermore, we did not find any association between inflammation profiles and age or ethnicity. However, we cannot exclude the involvement of other factors, including phagocytosis-associated molecules such as TGFβ1 (24), apoptosis-inducing factors (e.g., TNF-related apoptosis-inducing ligand) (25), cytokine clearance systems (16, 26), or antiinflammatory signaling pathways (e.g., NF-κB p50/p50) (27).
The criteria for grouping individuals as responders or nonresponders to aspirin arose from our work (18) and that of others (10), which showed that COX-2 acetylation by aspirin generates epi-Lxs. The rationale was that the more severe the inflammatory response the greater the resultant COX-2 expression and therefore biochemical machinery available for aspirin to make proportionally more antiinflammatory epi-Lxs (28). Thus, aspirin acts as a type of “magic bullet” exerting its beneficial effects in an inflammation-dependent manner. On this basis, volunteers were grouped according to those who showed a response to aspirin (following cantharidin) and those who did not. Taking this further revealed that responders to aspirin showed a resolution of inflammation more readily than those who were refractory to aspirin. As mentioned earlier, under baseline conditions (i.e., without aspirin), the key determinant of this differential inflammatory profile is 15-epi-LxA4. Importantly, although aspirin was originally shown to trigger epi-Lxs (9), we (18) and others (10) found detectable levels of 15epi-LxA4 in healthy volunteers as well as mice not given aspirin. The source of this non–aspirin-generated epi-Lx is unknown, but it is possible that COX-2 is acetylated by an endogenous acetylating agent to produce 15R-hydroxy eicosatetraenoic acid. Indeed, in mice dosed with COX-2 inhibitors, levels of plasma 15-epi-LxA4 are significantly reduced (Fig. S2). Alternatively, epi-Lxs could be generated by a P450 pathway to produce 15R-hydroxy eicosatetraenoic acid (11). However, given that SKF525A [cytochrome P450 inhibitor selective for CYP2B6, CYP2C9, CYP2C19, CYP2D6, and CYP3A4/5 but not CYP1A2, CYP2A6, and CYP2E1 (29)] had no effect on plasma epi-Lxs (Fig. S2), we propose that epi-Lxs maybe generated by a cell-derived acetylating agent. Therefore, from the data presented here, we hypothesize that early in the inflammatory response of Ervs, acetylation of COX-2 is low but increases as inflammation resolves, triggering increases in blister fluid epi-Lx. In contrast, in Drvs, endogenous COX-2 acetylators generate epi-Lxs early in the response but decline in line with 15-epi-LxA4 synthesis, taking the “hand brake” off inflammation. This endogenous acetylator hypothesis would certainly explain why aspirin failed to trigger 15-epi-LxA4 in Drvs as the inflammatory milieu would be saturated and the addition of an exogenous acetylator (i.e., aspirin) would fail to further acetylate COX-2.
In our original study, ASAlow did not affect blister fluid cytokine levels in 26 volunteers despite triggering 15-epi-LxA4. This was surprising given that 15-epi-LxA4 inhibits cytokine and chemokine release from experimental tissue injury (30) and isolated cells stimulated in culture (31). Indeed, we found that 15-epi-LxA4 inhibited a range of cytokines/chemokines from LPS-stimulated human monocytes, monocyte-derived macrophages, and human umbilical vein endothelial cells (HUVECs), with the most broad-ranging suppressive effects being on monocytes (Fig. S1). However, the presence of a group of individuals who are refractory to ASAlow among those sensitive to ASAlow provided the erroneous impression of an overall null effect. Separating groups based on inflammation severity/longevity revealed that those with low baseline 15-epi-LxA4 and therefore high inflammation responded to ASAlow because it triggered 15-epi-LxA4/ALX and therefore dampened cytokines/chemokines and leukocyte trafficking. Drvs had low cytokine/chemokine levels as a result of maximum baseline 15-epi-LxA4/ALX. As the latter was not further elevated by ASAlow, these Drvs were ASAlow-refractory. This does not exclude the likelihood that Drvs may become responsive to ASAlow when baseline 15-epi-LxA4/ALX eventually wanes and cytokine/chemokine levels increase. However, we did not examine the long-term fate of inflammation/resolution in Drvs because of the inherent instability of their blisters. We were also unaware of how long inflammation lasts in these responders and what proresolution pathways are required to ultimately dampen their response.
This is one of the first reports showing that modulating a proresolution pathway dampens innate immune-mediated responses in healthy humans. These individuals did not take fish oil, nor did they have their diet supplemented with docosahexaenoic acid, a precursor that, when metabolized instead of arachidonic acid down the LOX/COX pathway in the presence of aspirin, results in the generation of lipids of the resolvin D series, which dampen PMN trafficking into and clear macrophages out of sites of inflammation (32). Given the broad antiinflammatory, proresolution, and antibacterial properties of resolvins (33), it would be important to examine the effects of fish oils in general, or docosahexaenoic acid in particular, with aspirin for their combined antiinflammatory, antibacterial, and cardioprotective effects in man. That notwithstanding, ASAlow has been associated with gastric bleeding, and its usefulness as a cardioprotective agent balanced against gastrointestinal side effects is of ongoing concern (34). Therefore, next generation epi-Lxs, which protect the gastric mucosa from injury (35), as well as resolvins, may replace parental aspirin as novel antiinflammatory/cardioprotective agents with reduced side effects.
In summary, we have shown that inflammation progresses at a different rate and with different degrees of severity in human skin blisters arising from differentially expressed proresolution pathways. And although this could be result from different secretion rates of proinflammatory signals (e.g., cytokines/chemokines, cell adhesion molecules), we propose that, as inflammation is tightly regulated to prevent it becoming “overexuberant,” the control of inflammation is tempered by endogenous checkpoint control systems including epi-Lxs. This system is differentially expressed in different populations, resulting in different degrees of inflammation severity and longevity. Arguably, this could impact the rate at which different populations neutralize bacterial infection, for instance, or the propensity to develop chronic inflammation/autoimmunity arising from a more sustained response. Indeed, there is an inverse correlation between levels of endogenous lipoxins and disease severity in severe asthma (36), Henoch–Schonlein purpura (37), and scleroderma lung disease (38). Our findings may explain, in general, why some individuals are refractory to conventional antiinflammatory agents and importantly emphasizes the need to tailor antiinflammatory treatment regimes to individuals depending on their rate/profile of inflammatory response. These data also highlight that, when developing antiinflammatory drugs, we need to be mindful of their potentially resolution-toxic properties, i.e., their ability to subvert the body's attempts to switch off inflammation.
Materials and Methods
Inflammatory Models and Drug Treatment.
Two blisters were elicited on the ventral aspect of the forearms of male healthy volunteers (aged 25–50 y) as previously described (39–42) by applying 10 μL of 0.1% Cantharone (Dormer Labs). Volunteers were of diverse ethnic backgrounds and were not taking NSAIDs or aspirin-containing medications for 2 weeks before commencement of the study. ASAlow (75 mg) was taken daily for 10 d before a second set of blisters was elicited on the contralateral forearm, with aspirin consumed for the duration of the response up to 72 h. Ethical approval was obtained from UCL Ethics (project identification no. 1309/001). Blister cell numbers were enumerated by hemocytometer. For plasma lipid measurements, animals were bred under standard conditions and maintained in a 12-h/12-h light/dark cycle at 22 ± 1 °C and given food and tap water ad libitum in accordance with United Kingdom Home Office regulations.
FACS and Lipid Mediators.
Anti-CD14, CD16b, and ALX (human) as well as isotype controls were from Serotec or BD Biosciences. Peripheral blood and blister-derived leukocytes were acquired by FACSCalibur (BD Biosciences) using appropriate compensation when necessary, and data were analyzed by CellQuest Pro. Thromboxane A2 in the form of stable thromboxane B2 was measured by enzyme immunoassay (GE Healthcare) and 15-epi-LxA4 was measured by ELISA (Neogen). Cytokines and chemokines were measured by Multiplex cytokine array analysis (Bio-Rad) using the manufacturer's protocols.
Leukocyte Isolation, in Vitro Cell Culturing, and Treatment Protocols.
Blood (80 mL) was collected in heparin from healthy male volunteers followed by red blood cell sedimentation using EloHAES. Remaining leukocyte-rich plasma was subjected to Percoll density gradient centrifugation to separate monocytes from platelets, PMNs, and lymphocytes. Monocyte fractions (1 × 106 cells) were incubated overnight and washed to remove nonadherent leukocytes. For differentiation into macrophages, monocytes were cultured in the presence of 2 ng/mL GM-CSF, which was replaced every 4 d for 12 d. Monocytes and macrophages were treated with 15-epi-LxA4 (0.003 μg/mL) in RPMI 1640 without/without LPS (1 μg/mL, 1 h before stimulation). HUVECs (passage 1; Promocell) prestimulated with LPS (1 μg/mL) for 1 h were treated with 0.3 μg/mL 15-epi-LxA4 (Calbiochem) for a further 24 h.
Statistical Analysis.
Data were analyzed using the two-tailed paired Student t test for normally distributed data, with P < 0.05 being considered significant. All analyses were paired Student t tests. Wilcoxon matched-pairs test was used to analyze data standardized to the number of cells accumulated within the blister (15-epi-LxA4/106 cells); because it did not follow a Gaussian distribution, P < 0.05 was considered significant. When comparing two groups with unequal variances, a two-tailed unpaired t test with the Welch correction was used. For animal studies and in vitro assays, data were analyzed by ANOVA followed by followed by the Bonferroni t test.
Supplementary Material
Acknowledgments
D.W.G. is a Wellcome Trust funded Senior Research Fellow. G.B. acknowledges support from University College London Hospital/University College London and the Department of Health National Institute for Health Research Biomedical Research Centers funding scheme. This work was funded by the United Kingdom Wellcome Trust and Medical Research Council (D.W.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.N.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/1000373107/DCSupplemental.
References
- 1.Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: New opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 2.Hallett JM, et al. Novel pharmacological strategies for driving inflammatory cell apoptosis and enhancing the resolution of inflammation. Trends Pharmacol Sci. 2008;29:250–257. doi: 10.1016/j.tips.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Serhan CN, Savill J. Resolution of inflammation: The beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 4.Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxin biosynthesis: An update and role in anti-inflammation and pro-resolution. Prostaglandins Other Lipid Mediat. 2002;68-69:433–455. doi: 10.1016/s0090-6980(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 5.Perretti M, et al. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat Med. 2002;8:1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romano M, Serhan CN. Lipoxin generation by permeabilized human platelets. Biochemistry. 1992;31:8269–8277. doi: 10.1021/bi00150a021. [DOI] [PubMed] [Google Scholar]
- 7.Edenius C, Haeggström J, Lindgren JA. Transcellular conversion of endogenous arachidonic acid to lipoxins in mixed human platelet-granulocyte suspensions. Biochem Biophys Res Commun. 1988;157:801–807. doi: 10.1016/s0006-291x(88)80320-6. [DOI] [PubMed] [Google Scholar]
- 8.Fiore S, Serhan CN. Formation of lipoxins and leukotrienes during receptor-mediated interactions of human platelets and recombinant human granulocyte/macrophage colony-stimulating factor-primed neutrophils. J Exp Med. 1990;172:1451–1457. doi: 10.1084/jem.172.5.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clària J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci USA. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiang N, Bermudez EA, Ridker PM, Hurwitz S, Serhan CN. Aspirin triggers antiinflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci USA. 2004;101:15178–15183. doi: 10.1073/pnas.0405445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clària J, Lee MH, Serhan CN. Aspirin-triggered lipoxins (15-epi-LX) are generated by the human lung adenocarcinoma cell line (A549)-neutrophil interactions and are potent inhibitors of cell proliferation. Mol Med. 1996;2:583–596. [PMC free article] [PubMed] [Google Scholar]
- 12.Rajakariar R, et al. Nonresolving inflammation in gp91phox-/- mice, a model of human chronic granulomatous disease, has lower adenosine and cyclic adenosine 5′-monophosphate. J Immunol. 2009;182:3262–3269. doi: 10.4049/jimmunol.0801739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bystrom J, et al. Resolution-phase macrophages possess a unique inflammatory phenotype that is controlled by cAMP. Blood. 2008;112:4117–4127. doi: 10.1182/blood-2007-12-129767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang Y, Yu S, Braley-Mullen H. Contrasting roles of IFN-gamma in murine models of autoimmune thyroid diseases. Thyroid. 2007;17:989–994. doi: 10.1089/thy.2007.0261. [DOI] [PubMed] [Google Scholar]
- 15.Hurst SM, et al. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–714. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- 16.Jamieson T, et al. The chemokine receptor D6 limits the inflammatory response in vivo. Nat Immunol. 2005;6:403–411. doi: 10.1038/ni1182. [DOI] [PubMed] [Google Scholar]
- 17.Rajakariar R, et al. Novel biphasic role for lymphocytes revealed during resolving inflammation. Blood. 2008;111:4184–4192. doi: 10.1182/blood-2007-08-108936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris T, et al. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol. 2009;183:2089–2096. doi: 10.4049/jimmunol.0900477. [DOI] [PubMed] [Google Scholar]
- 19.Serhan CN, et al. Resolution of inflammation: State of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wise H. The inhibitory effect of prostaglandin E2 on rat neutrophil aggregation. J Leukoc Biol. 1996;60:480–486. doi: 10.1002/jlb.60.4.480. [DOI] [PubMed] [Google Scholar]
- 21.Takano T, Clish CB, Gronert K, Petasis N, Serhan CN. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J Clin Invest. 1998;101:819–826. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goh J, et al. Lipoxin A(4) and aspirin-triggered 15-epi-lipoxin A(4) antagonize TNF-alpha-stimulated neutrophil-enterocyte interactions in vitro and attenuate TNF-alpha-induced chemokine release and colonocyte apoptosis in human intestinal mucosa ex vivo. J Immunol. 2001;167:2772–2780. doi: 10.4049/jimmunol.167.5.2772. [DOI] [PubMed] [Google Scholar]
- 23.Godson C, et al. Cutting edge: Lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- 24.Fadok VA, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang Y, Sharp GC, Yagita H, Braley-Mullen H. A critical role for TRAIL in resolution of granulomatous experimental autoimmune thyroiditis. J Pathol. 2008;216:505–513. doi: 10.1002/path.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ariel A, et al. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat Immunol. 2006;7:1209–1216. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-kappaB in the resolution of inflammation. Nat Med. 2001;7:1291–1297. doi: 10.1038/nm1201-1291. [DOI] [PubMed] [Google Scholar]
- 28.Paul-Clark MJ, Van Cao T, Moradi-Bidhendi N, Cooper D, Gilroy DW. 15-epi-lipoxin A4-mediated induction of nitric oxide explains how aspirin inhibits acute inflammation. J Exp Med. 2004;200:69–78. doi: 10.1084/jem.20040566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franklin MR, Hathaway LB. 2-Diethylaminoethyl-2,2-diphenylvalerate-HCl (SKF525A) revisited: Comparative cytochrome P450 inhibition in human liver microsomes by SKF525A, its metabolites, and SKF-acid and SKF-alcohol. Drug Metab Dispos. 2008;36:2539–2546. doi: 10.1124/dmd.108.023549. [DOI] [PubMed] [Google Scholar]
- 30.Leonard MO, et al. 15-Epi-16-(para-fluorophenoxy)-lipoxin A(4)-methyl ester, a synthetic analogue of 15-epi-lipoxin A(4), is protective in experimental ischemic acute renal failure. J Am Soc Nephrol. 2002;13:1657–1662. doi: 10.1097/01.asn.0000015795.74094.91. [DOI] [PubMed] [Google Scholar]
- 31.Baker N, O'Meara SJ, Scannell M, Maderna P, Godson C. Lipoxin A4: Anti-inflammatory and anti-angiogenic impact on endothelial cells. J Immunol. 2009;182:3819–3826. doi: 10.4049/jimmunol.0803175. [DOI] [PubMed] [Google Scholar]
- 32.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spite M, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taha AS, McCloskey C, Prasad R, Bezlyak V. Famotidine for the prevention of peptic ulcers and oesophagitis in patients taking low-dose aspirin (FAMOUS): A phase III, randomised, double-blind, placebo-controlled trial. Lancet. 2009;374:119–125. doi: 10.1016/S0140-6736(09)61246-0. [DOI] [PubMed] [Google Scholar]
- 35.Fiorucci S, et al. Cyclooxygenase-2-derived lipoxin A4 increases gastric resistance to aspirin-induced damage. Gastroenterology. 2002;123:1598–1606. doi: 10.1053/gast.2002.36558. [DOI] [PubMed] [Google Scholar]
- 36.Levy BD, et al. Severe Asthma Research Program, National Heart, Lung, and Blood Institute Diminished lipoxin biosynthesis in severe asthma. Am J Respir Crit Care Med. 2005;172:824–830. doi: 10.1164/rccm.200410-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu SH, Liao PY, Yin PL, Zhang YM, Dong L. Inverse temporal changes of lipoxin A4 and leukotrienes in children with Henoch-Schönlein purpura. Prostaglandins Leukot Essent Fatty Acids. 2009;80:177–183. doi: 10.1016/j.plefa.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 38.Kowal-Bielecka O, et al. Cyclooxygenase- and lipoxygenase-derived eicosanoids in bronchoalveolar lavage fluid from patients with scleroderma lung disease: An imbalance between proinflammatory and antiinflammatory lipid mediators. Arthritis Rheum. 2005;52:3783–3791. doi: 10.1002/art.21432. [DOI] [PubMed] [Google Scholar]
- 39.Day RM, Harbord M, Forbes A, Segal AW. Cantharidin blisters: A technique for investigating leukocyte trafficking and cytokine production at sites of inflammation in humans. J Immunol Methods. 2001;257:213–220. doi: 10.1016/s0022-1759(01)00467-7. [DOI] [PubMed] [Google Scholar]
- 40.Harbord MW, et al. Impaired neutrophil chemotaxis in Crohn's disease relates to reduced production of chemokines and can be augmented by granulocyte-colony stimulating factor. Aliment Pharmacol Ther. 2006;24:651–660. doi: 10.1111/j.1365-2036.2006.03016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Philippidis P, et al. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: Antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res. 2004;94:119–126. doi: 10.1161/01.RES.0000109414.78907.F9. [DOI] [PubMed] [Google Scholar]
- 42.Piérard-Franchimont C, Piérard GE. Cantharidin-induced acantholysis. Am J Dermatopathol. 1988;10:419–423. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.