Abstract
Research relating stress to health has progressed from anecdotal evidence in the 1930s and 1940s to complex multivariate models that identify underlying longitudinal mechanisms. Enduring questions that have guided our research are: How does the early life environment affect health outcomes into adulthood? How is the latent damage stored and what processes are set into motion that link early life stress to health disorders in the later years? An emerging perspective focuses on the accumulation of interacting dysregulations in multiple physiological systems that compromise the systems’ abilities to respond flexibly to stressful circumstances. Our research explores: the antecedents of these processes, including genetic predispositions, the harshness of the early environment, and their interaction; the mediating roles of neural regulation in the brain and psychological and social resources; and health-related outcomes, such as metabolic functioning and inflammatory processes.
Keywords: environment, genes, health
How stress affects health has long occupied scientists’ attention (1). Although many definitions of stress exist, conventionally stress is defined as a negative emotional experience accompanied by predictable biochemical, physiological, cognitive, and behavioral changes that are directed either toward altering the stressful event or accommodating to its effects (2). During the last several decades of stress research, the field has advanced from anecdotal evidence to current multivariate, integrative, longitudinal models that drive research efforts. These advances can be characterized in four phases.
Early phase 1 research linking stress to health was dominated by case histories and relatively simplistic personality profiles. For example, anecdotal accounts of sudden death feature people whose lives are suddenly upended by a major tragic event and who rapidly succumb to death, often from acute cardiovascular events (3). Personality profiles, developed by Dunbar and Alexander in the 1940s, attempted to link specific personality profiles to specific illnesses. More recently, the concept of a cancer-prone personality has attracted popular attention. Although these profiles are now considered oversimplifications (4), individual differences continue to be implicated in the unfolding pathways from stress to health (5).
Phase 2 studies of stress and health were demonstration studies that provided better evidence for the relationship between stress and health but little clarity on the underlying mechanisms. For example, work by Holmes and Rahe (6) reported that when people must accommodate to stressors in the environment, their likelihood of subsequent illness is increased, although the size of these lagged correlational relationships was typically quite modest. Another example is social support (7): although this vast literature conclusively demonstrates that people (and animals) who lack social companionship have higher rates of illness and a higher risk of early death, until recently, much of this research merely demonstrated these links without identifying pathways by which these relations might occur.
In phase 3 research, concern with underlying mechanisms that might tie stress to health began to emerge. This phase is marked by studies that link a particular psychological or social variable to an underlying physiological parameter with potential health implications. For example, studies of medical students facing examinations and kindergarten children beginning school reveal changes in immune functioning potentially prognostic for adverse health outcomes, including changes in numbers of total t-lymphocytes, natural killer cell cytotoxicity, and lymphocyte responsivity to mitogenic stimulation (e.g., 8, 9). These early studies represented breakthroughs in the understanding of stress and health because they begin to identify the biological systems that stress affects and how they are affected.
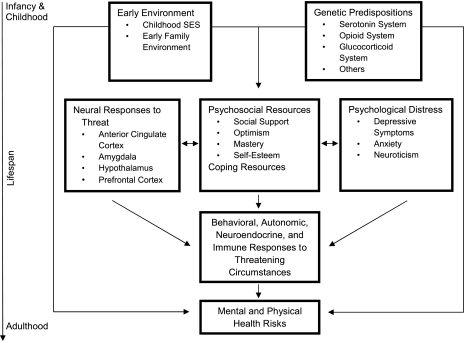
Phase 4 studies, which characterize the current state of the field, are guided by complex multivariate models that focus on underlying mechanisms over time. For example, the model that guides our laboratory’s research is pictured in Fig. 1. We examine the early environment and genetic predispositions as joint determinants of neural responses to stress, psychological and social resources for combating stress, chronic negative affect, and ways of coping with stress. These, in turn, have effects on autonomic, neuroendocrine, and immune responses to stress, among other systems. The cumulative adverse biological effects of chronic or recurring stress responses, in conjunction with genetic risks and those acquired in the early environment, in turn, lead to mental and physical health risks (10). I next briefly review some of our laboratory’s findings that address these pathways.
Fig. 1.
Stress and health across the lifespan.
Developmental Origins of Links Between Stress and Health
Both animal (11) and human studies (12) clearly indicate that the early environment affects biological functioning and health outcomes, not only in early life but throughout adulthood into old age, controlling for other risk factors. Research most clearly demonstrating this relationship includes evidence that low childhood socioeconomic status (SES) predicts adult health outcomes, controlling for adult SES (13) and evidence that a harsh early family environment marked by abuse, conflict, cold nonnurturant parenting, or neglect predicts adverse health outcomes (12). In the case of low childhood SES, chronic exposure to such stressors as financial hardship, threat of violence, violence exposure, family turmoil, and instability in parental employment may confer an underlying risk profile that remains in latent form until adulthood, when early onset chronic diseases may begin to appear (13). In addition, the lower one's childhood SES, the more one is exposed to risks in the environment, such as a poor diet leading to obesity or exposure to pathogens leading to asthma, which confer risk for health disorders across the lifespan. Similarly, manifold evidence now ties family environments characterized by adverse conditions to adult health risks. For example, in a study of adults enrolled in Kaiser Permanente, a large California health maintenance organization, questionnaire responses to items assessing abuse and dysfunction in the early family environment predicted multiple health disorders in adulthood in dose–response fashion, including ischemic heart disease, any cancer, depression, and stroke (14).
Nonetheless, it is not immediately clear why conditions in the early environment would affect health risks decades later, particularly as whatever risk factors are conferred by the early environment appear to be stored in latent form. Thus, the tasks of research in this area are, first, to characterize that latent form and, second, to identify whether and what processes might be set into effect as a result of exposure to a harsh early environment that would sustain health risks over the long term. Our work has focused on these issues. We examine alterations in biological stress regulatory systems; alterations in the neural regulation of stress responses; and expression of genes related to stress responses, both as latent indicators of impending health risks and as indicators of psychological and biological stress-reactive processes that may accelerate those risks. Neural, physiological, and genetic mechanisms are, of course, not mutually exclusive, but rather represent different facets of underlying interactive pathways, as will be seen.
Effects of Stress on Biological Stress Regulatory Systems.
The two major stress systems of the body are the sympathetic nervous system and the hypothalamic pituitary-adrenal (HPA) axis. Sympathetic arousal stimulates the adrenal medulla, which, in turn, leads to the secretion of the catecholamines, epinephrine and norepinephrine, leading to stress-related changes in blood pressure, heart rate, and constriction of peripheral blood vessels, among other changes. With respect to HPA axis functioning, the hypothalamus releases corticotropin-releasing factor (CRH), which stimulates the pituitary gland to secrete adrenocorticotropic hormone (ACTH), which in turn stimulates the adrenal cortex to release glucocorticoids, such as cortisol. Although activation of these systems in response to stress is functional because it mobilizes the organism to confront or flee from a stressor (the fight-or-flight response), repeated activation of these stress systems can ultimately compromise their functioning (10). Moreover, when this repeated engagement of stress systems occurs early in life, the developing systems themselves may be adversely affected.
Researchers have pointed out that the fight-or-flight response served early humans well, but, at the present time, rarely do stressful events require the kinds of physiological mobilization these systems confer. Job interviews, work stress, and interpersonal problems, for example, rarely require literal fight or flight. Consequently, in the present day, people often experience the effects of sudden elevations of circulating stress hormones that, in important respects, do not serve the functions around which they originally developed. Over the long term, excessive discharge of epinephrine and norepinephrine can lead to the suppression of cellular immune functioning; produce hemodynamic changes, such as increased pressure in heart rate; provoke variations in normal heart rhythms; and produce neurochemical imbalances that may contribute to the development of psychiatric disorders. Chronic or recurring exposure to catecholamines may also affect lipid levels and free-fatty acids, which are important in the development of atherosclerosis. Corticosteroids have immunosuppressive effects, which can compromise the functioning of the immune system. Prolonged cortisol secretion, for example, has been related to destruction of neurons in the hippocampus and to metabolic and immune changes potentially prognostic for developing chronic illnesses (10).
Our laboratory has explored whether there are identifiable stress-related alterations in biological stress regulatory systems that result from an adverse early environment. In one study (15), we enrolled young adults whose early environments had been assessed through questionnaires and interviews. We then exposed them to stress tasks in the laboratory. While participants were performing the stressful tasks (counting backward by 13s from 9,095 rapidly), we assessed their cortisol responses (indicative of hypothalamic-pituitary-adrenal functioning), heart rate, and blood pressure.
A harsh early environment was associated with an elevated flat cortisol trajectory across the stress tasks, suggesting that HPA axis functioning may have been compromised by recurring or chronic early life stress exposure. Normally, one would expect to see low cortisol levels prestress, an increase in response to stress, and a return to baseline during recovery, and so an elevated, flat cortisol trajectory suggests that the HPA axis may have lost some of its resiliency (10). Among men only, those from the harshest early environments also showed elevated heart rate and blood pressure, changes that may be prognostic for the long-term development of hypertension or heart disease. The fact that these changes were observed only in men is consistent with research suggesting that males may be more vulnerable to stress effects of the early environment, particularly those that affect cardiovascular risk (16).
However, it should be noted that assessment of family environment involves reconstruction by adult participants and thus may engage certain biases. Most problematic is the potential for a negative emotional overlay to influence both the reconstruction of early environment as well as downstream physiological and emotional processes. We have taken several steps in our research to ensure that a reporting bias does not account for the assessment of early environment. The instrument on which our assessment is based has demonstrated a dose–response relationship to a broad array of diagnosed mental and physical health outcomes (14), and a response bias is highly unlikely to yield such effects. Moreover, in our investigations, we formally evaluate alternative statistical models that give psychosocial functioning and negative affect causal priority to see if either explains the reconstruction of childhood events. In all cases, the alternative model is a weak fit to the data.
Studies such as these (15), then, indicate that in response to early life stress, the functioning of stress-related biological symptoms may be compromised in ways suggesting that they are losing their resiliency. McEwen has characterized these accumulated risks as “allostatic load:” As physiological systems fluctuate to meet the demands posed by a stressful environment, physiological costs of chronic exposure to recurring or heightened neural or neuroendocrine responses to repeated or chronic stress accumulate, resulting in interacting dysregulations in multiple physiological systems (10).
Neural Regulation of Stress Responses.
The links between early life stress, alterations in biological stress regulatory systems, and health outcomes likely depend on neural regulation of stress responses in the brain. Accordingly, our work has addressed the question, does early life stress affect how people respond to stress at the neural level?
Until recently, researchers knew relatively little about the effects of the early environment on neural functioning, but the behavioral costs have been recognized for decades (12). Children from harsh early environments show higher levels of avoidant coping, which means that they try not to deal with stressors if it is possible to avoid them. But they show overly aggressive responses to stressors that cannot be avoided. Offspring from harsh early environments also show ineffective coping that does not reduce their stress (12). We sought to determine if there is evidence at the neural level for these processes. Because early life stress has been tied to stronger biological responses to stress, a viable hypothesis is that a harsh early environment will be associated with greater amygdala responsivity to threat cues. Because a nurturant early environment has been tied to better regulation of stress responses, a viable hypothesis is that a supportive family environment will be tied to greater cortical responses to threat and consequent lesser amygdala reactivity; activity in the amygdala and the right ventrolateral prefrontal cortex (RVLPFC) have been found to be negatively related in response to threat cues (17).
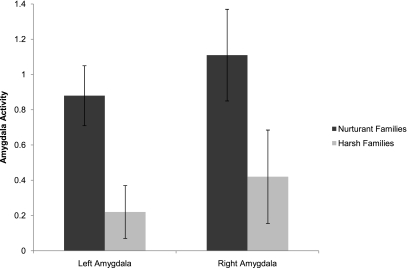
In a study using functional magnetic resonance imaging (fMRI) (18), participants responded to cues suggestive of threat (faces conveying negative emotions) with instructions either: to simply observe the faces (a task that usually engages amygdala activation), to label what the pictured emotion was (a task that typically activates the RVLPFC), or a control task (identifying the gender of the pictured face). When only observing faces conveying negative emotions, those who grew up in harsh families had lower amygdala activity than those from nurturant families, suggesting that people from the harsh families were tuning out or avoiding the stimuli. However, in the labeling task, a task that cannot be avoided, those from harsh families had higher amygdala activation than those from more nurturant families. Most important was a pattern of right ventrolateral prefrontal activity and amygdala activity. In people from nurturant families, activation of the right ventrolateral prefrontal cortex was associated with reduced amygdala activity. But among participants from harsh families, we observed a strong positive correlation, suggesting that, although these people were recruiting RVLPFC for managing the threat cues suggested by the emotional faces, their amygdala activity was not correspondingly reduced.
Significantly, these patterns of neural reactivity map closely onto the behavioral evidence described above. That is, these patterns suggest that people from harsh families shut out threatening cues with which they do not need to engage, but when they are forced by task demands to engage, their amygdala responses are stronger (see Fig. 2), and they are unable to recruit the prefrontal cortex effectively for regulating emotional responses to threatening cues. Importantly, then, this evidence links early family environment to patterns of regulating stress responses in the brain that correspond to behavioral observations made by developmental health researchers.
Fig. 2.
Neural activity in the left and right amygdala for people from nurturant and harsh families.
Genetic Contributions to Stress Responses.
Genes and their expression also influence responses to stress and the downstream pathways that affect biological functioning and ultimately health. Although scientists and laypeople alike once believed that the effects of genes on health and behavior are direct and immutable, scientists now recognize that there is substantial regulation of genetic expression by the environment, resulting in gene–environment interactions. Several laboratories that explore such epigenetic processes have productively focused on genes in the glucocorticoid system (19–21). Our laboratory has especially explored genes in the serotonin and the opioid systems (22, 23). As an example, I describe a study from our laboratory that focused on the serotonin transporter gene (5-HTTLPR) (24). This polymorphism has two predominant alleles: a short (s) form and a long (l) form. Previous research has suggested that being homozygous for the short allele (s/s) may confer risk for depression (25).
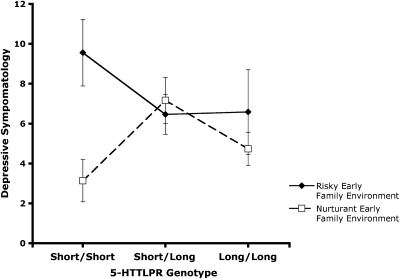
Our research, however, suggests a robust gene–environment interaction. Rather than being a risk factor for depression, s/s may instead reflect enhanced sensitivity to the environment, conferring a risk for depression in highly stressful environments but promoting thriving in nurturant, supportive environments. Consistent with this hypothesis, we (24) found that people homozygous for the short allele have significantly greater risk for depression if they have experienced a harsh early family environment, but significantly less risk for depression if they come from a supportive early environment, compared to people who are s/l or l/l (see Fig. 3). This pattern was replicated with an assessment of the current environment as well. That is, a current stressful environment confers enhanced risk for depression among those homozygous for the s allele, but a current supportive environment confers less risk for depression than is true of people who are s/l or l/l. Subsequent analyses of these data revealed that the s/s allelic pattern distinctively confers sensitivity to the social aspects of the environment and not to nonsocial threats (26). As such, a guiding hypothesis is that the serotonin system is implicated in the substantial effects of both the positive and the threatening aspects of the social environment on genetic expression, physiological functioning, and health (22).
Fig. 3.
Relationship of the 5-HTTLPR and the quality of the early family environment to depressive symptomatology. [Reprinted from Biological Psychiatry, vol. 60, Taylor SE, et al., Early family environment, current adversity, the serotonin transporter polymorphism, and depressive symptomatology, 672, Copyright (2006), with permission from Elsevier; http://journals.elsevierhealth.com/periodicals/bps.]
Overall, our research on the impact of the early environment on health identifies several interrelated mechanisms that may help to explain why the early environment affects adult health. An early adverse environment affects developing biological stress regulatory systems in ways that may compromise the resilience of these systems, ultimately conferring health risks over time. An early environment influences developing neural mechanisms by which stress responses are regulated in the brain. Reflecting this neural activity, people from harsh early environments cope poorly by responding ineffectively to cues suggesting threat. And the early environment can affect the expression of genes related to stress responses.
Psychosocial Resources as Mediators of the Stress-Health Relationship
A pivotal set of variables that much of our research has addressed concerns what is broadly termed psychosocial resources. These include social support from others and a set of positive beliefs, namely optimism, mastery, and self-esteem, that have been found to confer protection against psychological and physiological damage from stress (27, 28). The origins of psychosocial resources reside, in part, in the early environment (12), in genetic predispositions, and in social processes that select people into environments (such as SES). But the unfolding environment across the lifespan also plays an important role in whether and how people deploy these psychosocial resources. Some of our earliest work (27) revealed that in highly stressful times, people often respond with enhanced, even illusory, optimism, a sense of personal control or mastery, and self-enhancement, mustering these coping resources to ward off the threats that stress may pose. Our recent work has examined the pathways by which these positive resources may confer biological benefits that ultimately translate into health-protective benefits.
Social Support, Stress, and Health.
Social support is defined as information that one is loved and cared for, esteemed and valued, and part of a social network of communication and mutual obligations (7). In animal studies, social “support” is assessed in terms of whether an animal is socially isolated and housed alone or whether it has cagemates. In both animal and human studies, social support and social contact are significant and reliable predictors of health outcomes and mortality, with effect sizes in humans on par with smoking and lipids (29).
One possible reason why social support has these benefits is that people who experience their environments as socially supportive may react to stress with less biological reactivity. To chart a potential pathway by which social support confers biological benefits, we completed a three-part study (30). Participants were paged multiple times a day over a 9-day period and asked to rate how supportive their most recent social interaction was. At a second point in time, their brains were scanned while they participated in a virtual social task in the scanner, during which they were gradually excluded from the social interaction; this paradigm has previously been found to provoke significant social distress that is correlated with activity in the dorsal anterior cingulate cortex (dACC) (31), a brain region associated with monitoring threat. At a third time, participants completed stressful tasks in the laboratory, including difficult arithmetic calculations and preparing and delivering a speech to an unresponsive audience [a standardized laboratory stress paradigm called the Trier Social Stress Task (TSST)] (32).
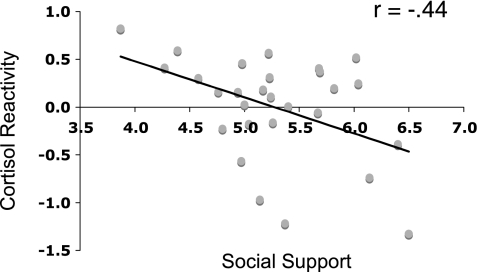
The results of the investigation showed that people who had reported more experiences of social support over the preceding 9 days had lower dorsal anterior cingulate cortex activity in response to the virtual social exclusion task and also exhibited lower cortisol responses to the TSST, suggesting lower levels of experienced stress. Individual differences in dACC during the social exclusion task mediated the relation between experienced social support and cortisol reactivity (30) (Fig. 4). Thus, it appears that social support may influence health, at least in part, by modulating neural reactivity to stress and consequent neuroendocrine stress responses.
Fig. 4.
Relationship between daily social support and cortisol reactivity during the Trier Social Stress Task (TSST).
Positive Beliefs, Stress, and Health.
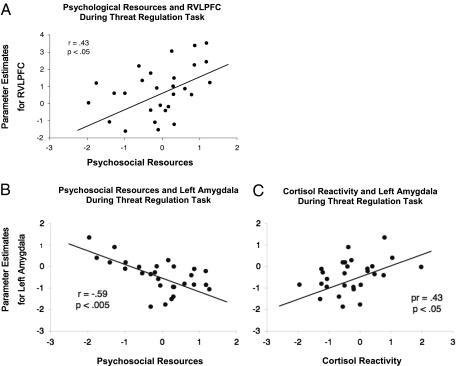
Taylor et al. (33) also explored why positive beliefs associated with optimism, self-esteem, and mastery lead to a lesser biological toll in response to stress. We conducted a similar three-part investigation during which we measured these positive beliefs at time 1; scanned participants’ brains while they responded to threatening cues (faces conveying negative emotions) in the scanner at time 2; and assessed biological stress responses during laboratory stressors at time 3. We found that people who held these positive beliefs showed greater RVLPFC activity and lower amygdala activity in response to threat cues, suggesting that they were coping effectively with the threat. These positive beliefs were also associated with lower cortisol and systolic blood pressure responses to the stress tasks. Lower amygdala activity mediated the relation between positive beliefs and low cortisol reactivity (Fig. 5). Thus, positive beliefs associated with optimism, a sense of personal control, and self-esteem appear to help people manage stress by virtue of cortical regulation of amygdala activity and consequent lower downstream neuroendocrine responses.
Fig. 5.
(A) RVLPFC activity during labeling of emotions as a function of psychosocial resources; (B) Left amygdala activation during threat task as a function of psychosocial resources; (C) Relation of left amygdala during threat task to cortisol reactivity during TSST.
Are There Relationships to Health?
Thus far, this brief overview of our research program has focused primarily on subclinical outcomes suggestive of potential health risks, but more direct ties to health-related outcomes can be drawn as well. To address more proximal health-related outcomes, we have collaborated with Coronary Artery Risk Development in Young Adults (CARDIA), a 15-year, four-site investigation of risk factors for coronary artery disease in more than 3,500 African American and white respondents. In three investigations, we tested whether our model (Fig. 1) can predict health-related outcomes.
In a first study, we used structural equation modeling to identify whether the model in Fig. 1 predicts metabolic functioning (e.g., cholesterol, insulin levels, glucose, triglycerides) (34). Metabolic functioning is a particularly important intermediate outcome between stress-related physiological compromise and health outcomes because it is prognostic for heart disease, hypertension, and Type II diabetes, among other chronic health disorders. The analyses showed that our model was a good fit to the metabolic functioning data in the CARDIA sample, implicating low childhood SES and early family environment effects on metabolic functioning that were mediated in part by poor social support and chronic negative affect.
In a second study, we explored whether the model could explain variability in C-reactive protein (35), an indicator of inflammation. Inflammatory processes are believed to be an important previously unacknowledged contributor to heart disease and to depression, among other disorders. Indeed, the high degree of comorbidity seen between heart disease and depression may be underpinned, at least in part, by inflammatory processes, as may be indexed by C-reactive protein. As predicted, the model fit the C-reactive protein evidence in the CARDIA sample. Low childhood SES and a harsh family environment were associated with elevated C-reactive protein, mediated in part by psychosocial resources and also by obesity; higher body mass index was a particularly significant predictor of elevated C-reactive protein. This evidence is important not only because it ties variables in our model to an important health-relevant outcome, but also because it may speak to at least one fundamental comorbidity between mental and physical health disorders, namely that between depression and heart disease.
In a third investigation, we explored whether the model could explain blood pressure and changes in blood pressure in the CARDIA sample over time (36). Again, our model explained significant variance and was a good fit to the data in CARDIA for year 15 blood pressure, as well as for increases in blood pressure over the 15-year duration of the study. Of note, the model explained increases in blood pressure over time in all four of the subgroups examined, namely, male and female African-American and white participants.
Taken together, then, these studies indicate that there are indeed links from the early environment to psychosocial resources, to biological functioning, and ultimately to health-related outcomes. As such, they provide support for a general model of the effects of stress on health outcomes across the lifespan.
Toward Phase 5 Models
Examining the course of stress research since the 1930s reveals enormous progress in the sophistication and complexity of the models that guide research linking stress to health. As additional empirical progress is made, what will phase 5 models look like? The phase 4 model that currently guides our program of research is crude. It implicitly ignores direct connections from genetic predispositions to the neural and endocrine levels and between genetic factors and specific health outcomes. It also ignores feedback loops; for example, stress hormones, inflammatory processes, and environmental factors feed back to genetic expression. Ultimately, a model such as that pictured in Fig. 1 will give way to a phase 5 family of more specific models that integrate specific experiential, genetic, psychosocial, and biological precursors of specific health disorders. These models will lend empirical vision to the stress research of the future and provide clinical guidance in the treatment of health disorders.
Acknowledgments
Preparation of this article was supported by the National Institute of Aging (Grant AG030309).
Footnotes
The author declares no conflict of interest.
References
- 1.Cannon W. The Wisdom of the Body. New York: Norton; 1932. [Google Scholar]
- 2.Baum A. Stress, intrusive imagery, and chronic distress. Health Psychol. 1990;9:653–675. doi: 10.1037//0278-6133.9.6.653. [DOI] [PubMed] [Google Scholar]
- 3.Engel GL. A life setting conducive to illness. The giving-up—given-up comples. Ann Intern Med. 1968;69:293–300. doi: 10.7326/0003-4819-69-2-293. [DOI] [PubMed] [Google Scholar]
- 4.Price MA, et al. The role of psychosocial factors in the development of breast carcinoma: Part I. The cancer prone personality. Cancer. 2001;91:679–685. [PubMed] [Google Scholar]
- 5.Antoni MH, Lutgendorf S. Psychosocial factors and disease progression in cancer. Curr Dir Psychol Sci. 2007;16:42–46. [Google Scholar]
- 6.Holmes TH, Rahe RH. The social readjustment rating scale. J Psychosom Res. 1967;11:213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- 7.Taylor SE. In: Oxford Handbook of Health Psychology, Social Support: A review. Friedman HS, editor. New York, NY: Oxford Univ Press; 2010. [Google Scholar]
- 8.Boyce WT, et al. Adrenocortical and behavioral predictors of immune responses to starting school. Pediatr Res. 1995;38:1009–1017. doi: 10.1203/00006450-199512000-00030. [DOI] [PubMed] [Google Scholar]
- 9.Glaser R, Rice J, Speicher CE, Stout JC, Kiecolt-Glaser JK. Stress depresses interferon production by leukocytes concomitant with a decrease in natural killer cell activity. Behav Neurosci. 1986;100:675–678. doi: 10.1037//0735-7044.100.5.675. [DOI] [PubMed] [Google Scholar]
- 10.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 11.Liu D, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 12.Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychol Bull. 2002;128:330–366. [PubMed] [Google Scholar]
- 13.Chen E, Matthews KA, Boyce WT. Socioeconomic differences in children's health: How and why do these relationships change with age? Psychol Bull. 2002;128:295–329. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- 14.Felitti VJ, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 15.Taylor SE, Lerner JS, Sage RM, Lehman BJ, Seeman TE. Early environment, emotions, responses to stress, and health. J Pers. 2004;72:1365–1393. doi: 10.1111/j.1467-6494.2004.00300.x. [DOI] [PubMed] [Google Scholar]
- 16.Allen MT, Matthews KA, Sherman FS. Cardiovascular reactivity to stress and left ventricular mass in youth. Hypertension. 1997;30:782–787. doi: 10.1161/01.hyp.30.4.782. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman MD, et al. Putting feelings into words: Affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 18.Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural responses to emotional stimuli are associated with childhood family stress. Biol Psychiatry. 2006;60:296–301. doi: 10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 19.Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: Life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller GE, et al. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weaver IC, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 22.Way BM, Taylor SE. Social influences on health: Is serotonin a critical mediator? Psychosom Med. 2010;72:107–112. doi: 10.1097/PSY.0b013e3181ce6a7d. [DOI] [PubMed] [Google Scholar]
- 23.Way BM, Taylor SE, Eisenberger NI. Variation in the mu-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proc Natl Acad Sci USA. 2009;106:15079–15084. doi: 10.1073/pnas.0812612106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor SE, et al. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biol Psychiatry. 2006;60:671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 25.Caspi A, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 26.Way BM, Taylor SE. The serotonin transporter promoter polymorphism is associated with cortisol response to psychosocial stress. Biol Psychiatry. 2010;67:487–492. doi: 10.1016/j.biopsych.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor SE, Brown JD. Illusion and well-being: A social psychological perspective on mental health. Psychol Bull. 1988;103:193–210. [PubMed] [Google Scholar]
- 28.Taylor SE, Lerner JS, Sherman DK, Sage RM, McDowell NK. Are self-enhancing cognitions associated with healthy or unhealthy biological profiles? J Pers Soc Psychol. 2003;85:605–615. doi: 10.1037/0022-3514.85.4.605. [DOI] [PubMed] [Google Scholar]
- 29.House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 30.Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage. 2007;35:1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 32.Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 33.Taylor SE, et al. Neural bases of moderation of cortisol stress responses by psychosocial resources. J Pers Soc Psychol. 2008;95:197–211. doi: 10.1037/0022-3514.95.1.197. [DOI] [PubMed] [Google Scholar]
- 34.Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relation of childhood socioeconomic status and family environment to adult metabolic functioning in the CARDIA study. Psychosom Med. 2005;67:846–854. doi: 10.1097/01.psy.0000188443.48405.eb. [DOI] [PubMed] [Google Scholar]
- 35.Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biol Psychiatry. 2006;60:819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 36.Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to blood pressure in the CARDIA study. Health Psychol. 2009;28:338–346. doi: 10.1037/a0013785. [DOI] [PMC free article] [PubMed] [Google Scholar]