Summary
The mechanism of formation of haemozoin, a detoxification by-product of several blood-feeding organisms including malaria parasites, has been a subject of debate; however, recent studies suggest that neutral lipids may serve as a catalyst. In this study, a model system consisting of an emulsion of neutral lipid particles was employed to investigate the formation of β-haematin, the synthetic counterpart of haemozoin, at the lipid-water interface. A solution of monoglyceride, either monostearoylglycerol (MSG) or monopalmitoylglycerol (MPG), dissolved in acetone and methanol was introduced to an aqueous surface. Fluorescence, confocal and transmission electron microscopic (TEM) imaging and dynamic light scattering analysis of samples obtained from beneath the surface confirmed the presence of homogeneous lipid particles existing in two major populations: one in the low micrometer size range and the other in the hundred nanometre range. The introduction of haem (Fe(III)PPIX) to this lipid particle system under biomimetic conditions (37 °C, pH 4.8) produced β-haematin with apparent first order kinetics and an average half life of 0.5 min. TEM of monoglycerides (MSG or MPG) extruded through a 200 nm filter with haem produced β-haematin crystals aligned and parallel to the lipid/water interface. These TEM data, together with a model system replacing the lipid with an aqueous organic solvent interface using either methyl laurate or docosane demonstrated that the OH and C=O groups are apparently necessary for efficient nucleation. This suggests that β-haematin crystallizes via epitaxial nucleation at the lipid-water interface through interaction of Fe(III)PPIX with the polar head group. Once nucleated, the crystal grows parallel to the interface until growth is terminated by the curvature of the lipid particle. The hydrophobic nature of the mature crystal favours an interior transport resulting in crystals aligned parallel to the lipid-water interface and each other, strikingly similar to that seen in malaria parasites.
INTRODUCTION
Nature provides numerous examples of organic-inorganic interactions that produce precisely controlled biominerals in which structural morphologies are reproducible in size, shape and orientation1. While considerable advances have been made in understanding how the organic components of organisms (proteins2 and lipids)3 direct the formation for some biominerals, such as calcium carbonate4, in many cases their role remains unclear. This is especially true in malaria parasites of the genus Plasmodium, where an unusual type of biomineral occurs in the form of haemozoin or malaria pigment5, 6, a crystalline haem product. During the pathogenic blood stage in the host, the malaria parasite releases free haem as a consequence of haemoglobin degradation. To circumvent the toxicity of free haem, it sequesters it in the form of haemozoin7. This process is inhibited by chloroquine and other quinoline and related antimalarials5. Therefore, understanding the molecular mechanism behind its formation may help in the elucidation of new strategies for the design of antimalarials.
Haemozoin is a microcrystalline cyclic dimer of ferriprotoporphyrin IX (Fe(III)PPIX) in which the propionate side chain of one protoporphyrin coordinates to the iron(III) centre of the other8. The dimers hydrogen bond to their neighbours via the second propionic acid group, forming extended chains through the crystal. Haemozoin is chemically and structurally identical to a synthetic haematin product called β-haematin. The mechanism of haemozoin formation is still not well understood. Initially it was proposed that an enzyme, a so-called haem polymerase, was responsible for its formation9. However, this putative enzyme has never been isolated. Subsequent proposals were that the process is spontaneous10 or autocatalytic11. Recent thinking is that the mechanism of haemozoin formation is a form of biomineralization and is brought about by lipids,12–16 proteins17, 18, or both19. Definitive evidence remains lacking.
Lipids have been implicated in the formation of haemozoin. Bendrat and colleagues initially speculated that this process is mediated by lipids when they observed that an acetonitrile extract of P. falciparum promoted the formation of β-haematin16. Recently, convincing evidence for the lipid model has begun to accumulate, demonstrating that this is likely to be the basis of the biological process. Of significance, Sullivan and colleagues imaged haemozoin enveloped by neutral lipid nanospheres within the digestive vacuoles of P. falciparum12. The association of haemozoin with neutral lipid droplets was also observed in the helminth Schistosoma mansoni20. Recently, biologically realistic half-lives of about 5 minutes at 37 °C were reported for β-haematin formation in a lipid-water interface model14, suggesting that the interface may play an essential role during haem crystallization. The question remains, how does the lipid-water interface facilitate haemozoin formation? In an attempt to address this question, we have examined the organization of lipids and performed pH and temperature studies on the rate of β-haematin formation using a lipid-water interface model. Here we show that the lipid forms an emulsion, with β-haematin formation occurring at the surface of the lipid particles. We also provide a kinetic study of the process of β-haematin formation in this system. A detailed understanding of β-haematin formation using the lipid-water interface system may provide more insight into the mechanism of haemozoin nucleation and growth processes in nature.
EXPERIMENTAL
Materials
All materials used were of analytical grade or of the highest grade of purity available from commercial suppliers. Stock solutions made from citric acid purchased from Merck were used to prepare citrate buffer (50 mM) and pH adjusted to 4.8 using sodium hydroxide (NaOH) slurry. A stock solution (3.2 mM) of haematin was formed by dissolving 2 mg of haematin in 0.4 mL NaOH (0.1 M) followed by the addition of 0.6 mL of 1:9 acetone/methanol. The following lipids were used in this study: monoglyceride lipids rac 1-monopalmitoylglycerol (MPG), rac 1-monostearoylglycerol (MSG), rac 1-monomyristoylglycerol (MMG) and rac 1-monooleoylglycerol (MOG); diglycerides 1,3-dioleoylglycerol (DOG), 1,3-dipalmitoylglycerol (DPG) and 1,3-dilinoleoylglycerol (DLG); triglycerides trioleoylglycerol (TOG) and trimyristoylglycerol (TMG); and cholesterol (CHL). All lipids used in this study were obtained from Sigma-Aldrich South Africa. 3.3 mM solutions of lipids were prepared in 1:9 acetone/ methanol solvent. In order to carry out a reaction, 200 µL of lipid solution was pre-mixed with 2 µL of haematin solution and was layered on the surface of the citrate buffer (5 mL) which was pre-incubated at 37 °C.
Characterization of neutral lipid particles
When a solution of neutral lipid is deposited onto an aqueous surface, a monolayer of lipid is formed at the surface21. The selectively fluorescent, hydrophobic phenoxazone dye, Nile Red22 was used to characterize the organization of lipid molecules dispersed in the solution beneath this surface monolayer. 200 µL of stock MPG solution was layered on top of a 5 mL solution of citrate buffer (50 mM, 37 °C) drop-wise using a microsyringe. After 5 min. incubation, 2 µL of a 500 µg/mL Nile Red stock solution was introduced to the surface. Samples were then withdrawn from beneath the surface and placed on a glass slide for imaging by a Nikon Epifluorescent microscope and a Zeiss LSM 510 meta inverted confocal microscope. Confocal imaging was performed using a 488 nm argon laser with 505–550 nm band pass filter. Confocal orthogonal projection was produced using Zeiss LSM Image Browser software. Another 2 µL of the sample was stained with a 2 % solution of uranyl acetate for imaging using a JEM 1200EXII TEM operated at 120 kV. The size distribution of the lipid emulsions were measured using two Malvern particle sizing systems: The Malvern Zetasizer with a lower limit of 0.1 nm and an upper limit of 6 µm and the Malvern Mastersizer with a lower limit of 50 nm and upper limit of 800 µm. 1 mL of MPG stock solution was deposited onto surface of citric acid buffer (50 mL, 50 mM, pH 4.8) and 0.5 mL samples extracted from below the surface were used for Zetasizer measurements while 2 mL samples were used for the Mastersizer. Three measurements were taken for each sample at 0, 20, 40, and 60 minutes after the lipids were deposited to determine the stability of the size distribution of the lipid particles in these emulsions with time.
Localization of haem
Haematin was dissolved in 0.1 M NaOH and then mixed with an acetone/methanol (1:9 v/v) solution to form a 2 mg/mL stock solution (3.2 mM) of Fe(III)PPIX (from here on generically referred to as haem). 3.3 mM lipid solutions of MPG and MSG were prepared as described above, except that the ratio of acetone (acetone/methanol) was increased for MSG to ensure that the lipid had dissolved completely. Then 2 µL of haem solution and 200 µL of lipid were mixed. This premixed solution was introduced, as previously described, onto the surface of citric acid buffer solution (5 mL, 37 °C, pH 4.8) in a 15 mL conical tube (Falcon, diameter = 46.87 mm). To determine the localization of haem with respect to the lipid particles, samples were taken immediately from beneath the layered surface and prepared for transmission electron microscopy imaging using a LEO 912 transmission electron microscope. Additional samples were withdrawn after ten minutes of incubation at 37 °C. Iron distribution was measured from the latter sample on objects that resembled β-haematin crystals using TEM electron spectroscopic imaging (ESI) for elemental analysis of iron.
Characterization of β-haematin
A scaled up version of the above experiment was performed 24 times using MPG and MSG for product characterization purposes. The β-haematin formed in the lipid-water system was characterized by Fourier transformed infrared (FTIR) spectroscopy of undried material as Nujol mulls. Powder X-ray diffraction (XRD) of the extensively washed (5% pyridine solution) and dried product, and transmission electron microscopy imaging of samples removed directly from MSG-lipid interface were also performed. XRD was carried out using Cu Kα radiation (λ=1.541 Å), with data collection on a Philips PW1050/80 vertical goniometer in the 2θ range 5 – 40° using an Al sample holder. TEM images were obtained using the LEO 912. Haem incubated in the aqueous medium alone served as control.
The role of OH and C=O groups in nucleating β-haematin
Experiments at pentanol and octanol/aqueous interfaces were carried out exactly as described previously14 using 10 mL of the solvent. The experiment with toluene was conducted in the same way except that toluene was substituted for pentanol or octanol.
In the case of docosane, a 1.9 mg/mL stock solution was prepared by dissolving 1.9 mg in 1.0 mL of a solution containing 1:3:6 acetone:chloroform:methanol (v/v), while for methyl laurate a 2.61 mg/mL stock solution was prepared to volume in a 1.0 mL volumetric flask by dissolving 3 µL of neat methyl laurate (0.869 g/mL) in 1:9 acetone:methanol solution. 2.0 mg haematin was dissolved in 1.2 mL of 0.1 M NaOH in a 10 mL glass vial. After complete dissolution of the solid, 1.8 mL of the 1:9 acetone:methanol solution was added and the new solution was thoroughly mixed to give a final concentration of 0.67 mg/mL. This solution was prepared fresh for each individual experiment.
Experiments were carried out in Schott Duran crystallising dishes with an internal diameter of 5 cm in the case of pentanol, octanol and toluene and 9 cm in the case of docosane and methyl laurate. 50.0 mL of citrate buffer was measured into each vessel and incubated with a pentanol, octanol or toluene layer on top of the aqueous solution. In the case of the other two, the aqueous layer was pre-incubated at 37°C while docosane, methyl laurate and haem solutions were prepared. On average this process took 15 – 20 minutes. These organic liquids are all largely insoluble in aqueous medium and the solvent system used to dissolve docosane showed a tendency to precipitate haem at high concentrations. Thus it was reasoned that premixing any of them with the haem/NaOH solution would not produce a sensible result and so the interface was prepared with no premixing. The non-aqueous layer was spread over the surface of the citrate buffer. Subsequently, 0.5 mL (0.33 mg) of haem solution was added drop-wise to the pre-incubated interface using a syringe with a needle diameter of 0.5 mm. Once haem had been delivered to the interface, the experiments were left to incubate for 30 minutes. Control experiments in the presence and absence of MMG were carried out at the same time. At the end of the incubation period the surface was agitated to force all the haem products (both unconverted haem and β-haematin) to precipitate into the acidic bulk medium. The total volume of solvents and precipitate (~ 50.5 mL) was transferred to an 85 mL Nalgene centrifuge tube and centrifuged at 10,000 rpm for 10 minutes. The clear supernatant was discarded and the pellet kept for further analysis. 1.0 mL of a pyridine:water:acetone solution (1:17.9:5.5 pH 7.5) was added to wash the pellet. In the case of methyl laurate, the supernatant remaining after a second centrifugation was subsequently diluted (40:1960 µL) and the absorbance measured at 405 nm to quantify the unreacted haematin. The remaining pellet from docosane and methyl laurate experiments was analysed as a nujol mull by FT-IR.
β-haematin formation via methods of extrusion
A premixed solution of MPG (1 mL, 3.3mM) and haematin (10 µL, 3.2mM)) was adjusted to pH 7.0 with 0.01 M hydrochloric acid (HCl). Citric acid buffer (~300 µL, 50 mM, pH 7.0) was carefully added to this solution, to avoid precipitation of the lipid solution. MPG/haem vesicles were formed by extrusion through Nucleopore track-etch polycarbonate membranes (Avanti Polar Lipids Inc.) 19 times at temperature above the Tc. The newly formed haem-MPG lipid emulsions were introduced into an aqueous solution buffered at pH 4.8 containing 50 mM citrate. The mixture was incubated at 37 °C for about 10 minutes and TEM imaging of 3 µL of samples was taken using the LEO 912 electron microscope. This experiment was repeated for MSG.
Kinetic studies of β-haematin formation
Kinetics for the following lipids were examined: monoglycerides MPG, MSG, and MOG; diglycerides DOG, DPG, and DLG; triglycerides TOG and TMG; and CHL. The experimental setup was as described above. Briefly, premixed solution was formed using 2 µL of haem and 200 µL of lipid stock solutions and was layered onto citric acid buffer. The mixture was incubated at 37 °C for 0, 1, 3, 5, 15, 30, 45 and 60 minutes. Each time point was performed in triplicate. The reaction was quenched with the addition of 1 mL of an aqueous solution containing 30% pyridine, 10% HEPES buffer (2.0 M, pH 7.5), and 40% acetone (v/v). The sample was immediately centrifuged for 10 minutes at 4,000 rpm. The percentage of unconverted haem was determined by performing colorimetric measurements at 405 nm on the supernatant of quenched and centrifuged samples. This is a modification of an assay published by Ncokazi and Egan23, 24.
The effect of temperature on kinetics of β-haematin formation
For temperature analysis of β-haematin formation in the lipid-water system, measurements of percentage of haem conversion were conducted at 25, 30, 40, and 50 °C. A premixed solution of 200 µL MSG and 2 µL haem stock solution was deposited onto a citric acid buffer (50 mM, pH 4.8) sub-phase at designated temperatures. The reaction was quenched and colorimetric measurements were performed as described above at various time points.
The effect of pH on kinetics of β-haematin formation
In order to examine the effect of pH on β-haematin formation at the lipid-water interface, the pH of the aqueous solution on which the lipid solution was deposited was adjusted to 0.5, 1.0, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 4.8, 5.0, 5.5, 6.0, 6.5 and 7.0 at 37 °C. The method is as described previously. After 1 hr of incubation the reaction was quenched with pyridine solution and the β-haematin percentage yield was recorded by means of colorimetric measurements at 405 nm.
RESULTS
Characterization of neutral lipid particles
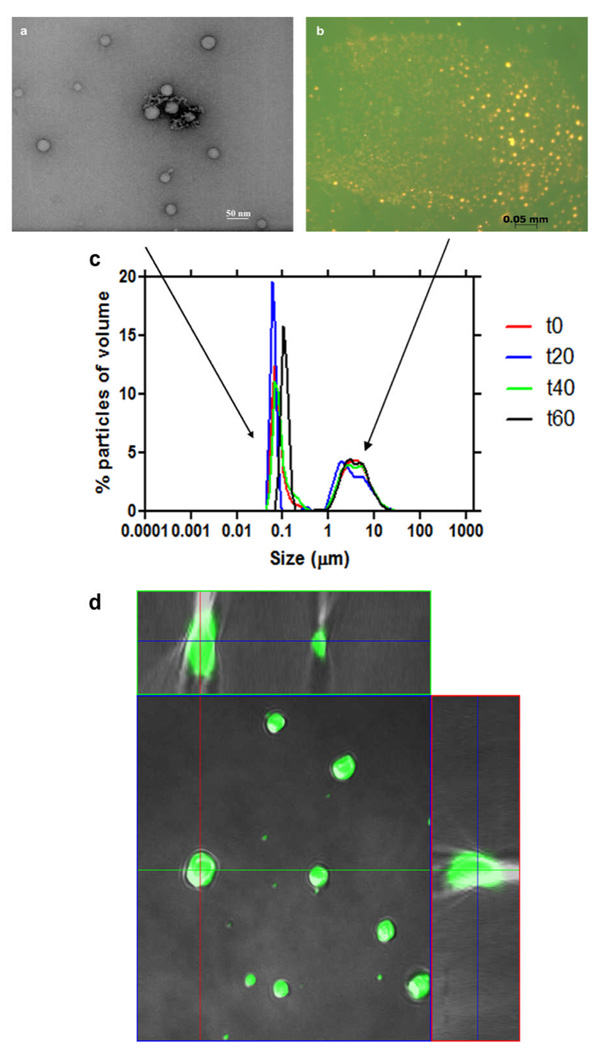
Studies of lipid mediation of β-haematin formation have demonstrated rapid haem conversion when haematin was introduced to a solution of rac 1-monomyristoylglyerol (MMG) that was deposited onto an aqueous subphase14. By contrast, previous crystallization studies using only a monolayer of the phospholipid dipalmitoylphosphatidyl-choline (DPPC) reported the insufficiency of the lipid monolayer to mediate β-haematin formation, even after 24 h of incubation25. Instead, when an aged bulk solution of lipid was substituted, β-haematin crystals were detected. Together, these data point to an unknown lipid mediated haem conversion process that exists beneath the surface monolayer which produces most if not all of the β-haematin. Little is known about the organization of these lipids beneath the surface. To investigate the distribution of neutral lipids in this system, transmission electron microscopy (TEM) and fluorescence imaging were performed on samples extracted from just beneath the surface. Imaging data suggest that lipids spontaneously organize into a lipid emulsion beneath the surface (Fig. 1a, b). Fluorescence imaging of samples stained with the lipophilic stain, Nile Red, and a water soluble dye, fluorescein, showed lipid particles in the emulsion layer in the micrometer size range (Fig. 1b), while TEM imaging showed structures in the nanometre size range (Fig. 1a). Confocal microscopy of the samples confirmed that the lipid particles contain a homogeneous core (Fig. 1d) and are not hollow liposomes. Measurements of the particle size distribution in these lipid emulsions revealed two populations. A large population in the 50 to 200 nm diameter range (Fig. 1c) and a smaller population of lipid particles in the 1 to 10 µm range. These emulsions were stable over time, as little fluctuation in size distribution was observed in four independent measurements at 20 min intervals over a period of an hour.
Fig. 1.
Characterization of monopalmitoyl glycerol in acetone/methanol solution after deposition onto an aqueous surface. Samples were extracted from just beneath the surface. TEM images show lipid particles in the nanometre size range (a), while fluorescence imaging of samples stained with the lipophilic stain, Nile Red (orange), and the water soluble dye, fluorescein (green), show particles in the micrometer size range (b). Measurements of lipid particle size distribution reveal two populations, one in the range 50 – 200 nm and the other 1 – 10 µm. This size distribution remained stable over a period of an hour (c). Confocal orthogonal projection confirms that lipid particles are not hollow, but rather continuous throughout their interiors (d).
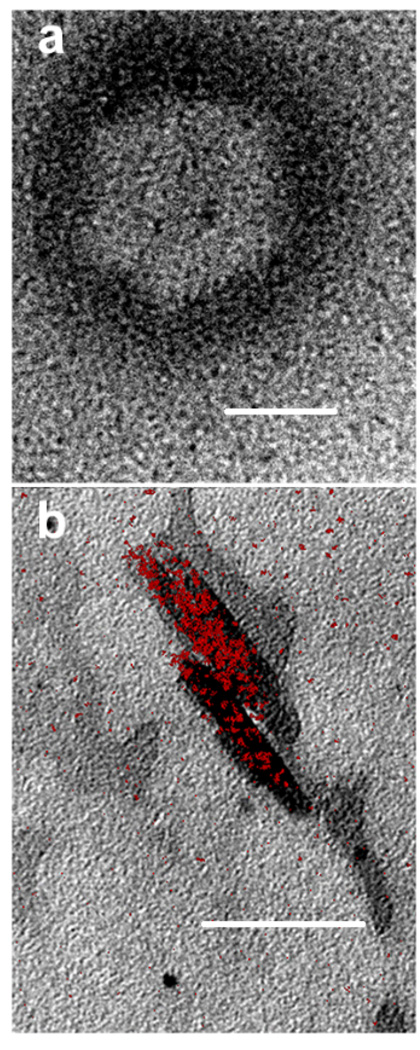
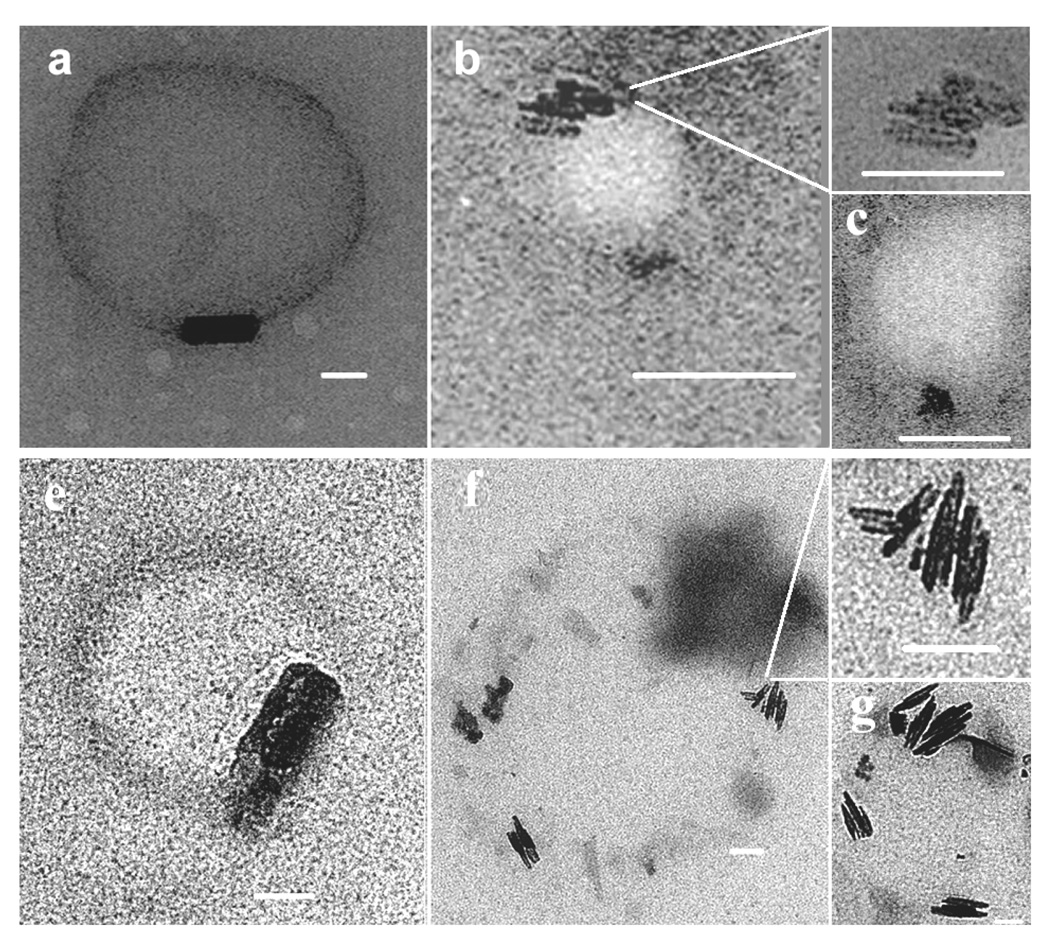
Localization of haem and product characterization
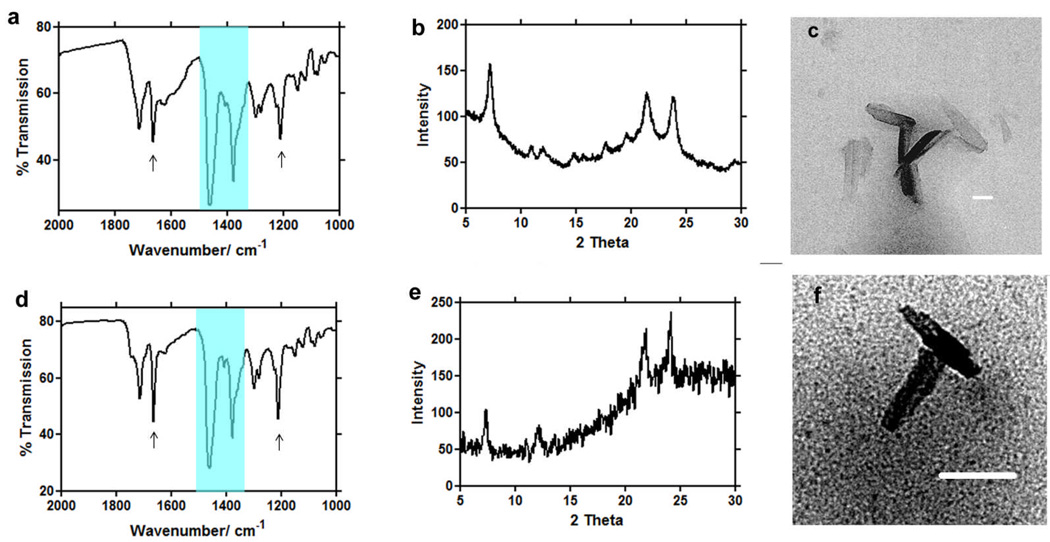
TEM imaging showed that when haematin was premixed with MPG and introduced onto an aqueous surface, electron dense material, probably haem, rapidly localized around the surface of the lipid particles, apparently at or near the lipid-water interface (Fig. 2a). Images of samples incubated for 10 minutes showed crystals resembling β-haematin (Fig. 2b). TEM/ESI of iron distribution in these samples confirmed the presence of iron in these putative β-haematin crystals (Fig. 2b). Morphologically, these crystals resemble haemozoin in their crystal habit (Fig. 3c, f). Infrared spectra and powder diffraction data of the products formed using MSG and MPG clearly demonstrates the production of β-haematin with percentage of product conversion of 75 ± 2 and 65 ± 4, respectively (Fig. 4a). Prominent IR peaks at 1662 cm−1 and 1210 cm−1 are readily discernible indicating significant formation of β-haematin (Fig. 3a, d). These findings are confirmed by XRD (Fig. 3b, e) showing the characteristic diffraction peaks of β-haematin. The diffraction pattern of the product formed with MSG in particular, definitively demonstrates the presence of β-haematin. In contrast to current results, previous studies by Sullivan and co-workers reported MSG as ineffective in promoting β-haematin12. This result may be owing to its low solubility. By employing a 1:9 acetone/methanol mixture pre-incubated at 37 °C, MSG was effectively solubilized. Though this does not directly mimic the physiological process, it is necessary in order to introduce the lipid and haematin to the interface in a soluble form so as to promote β-haematin crystallization, probably promoting lipid droplet formation and preventing precipitation of unconverted haem.
Fig. 2.
TEM showing haem localization at or near the lipid-water interface. When haematin was premixed with MPG and introduced onto an aqueous surface and immediately imaged using TEM, electron dense material, presumably haematin, was localized at or near the lipid-water interface (a). Elemental imaging of iron using TEM with electron spectroscopic imaging (ESI) conducted on samples reacted for 10 min. confirmed the presence of β-haematin crystals (b). Regions highlighted in red denote the presence of Fe. Scale bar = 50 nm.
Fig. 3.
Infrared (IR), X-ray diffraction (XRD), and transmission electron microscopy (TEM) evidence for the formation of β-haematin at the lipid-water interface using MSG (a, b, c), and MPG (d, e, f). Two large Nujol peaks (a, d) obscure the region between 1550 and 1320 cm−1 but do not interfere with the characteristic β-haematin IR peaks at 1660 and 1210 cm−1. Both products formed under mediation of MSG (a) and MPG (d) show IR characteristics of β-haematin. XRD of products obtained in presence of MSG (b) and MPG (e) show patterns characteristic of β-haematin39. TEM images of these products closely resemble haemozoin in their crystal habit (c, f). Scale bar = 50nm.
Fig. 4.
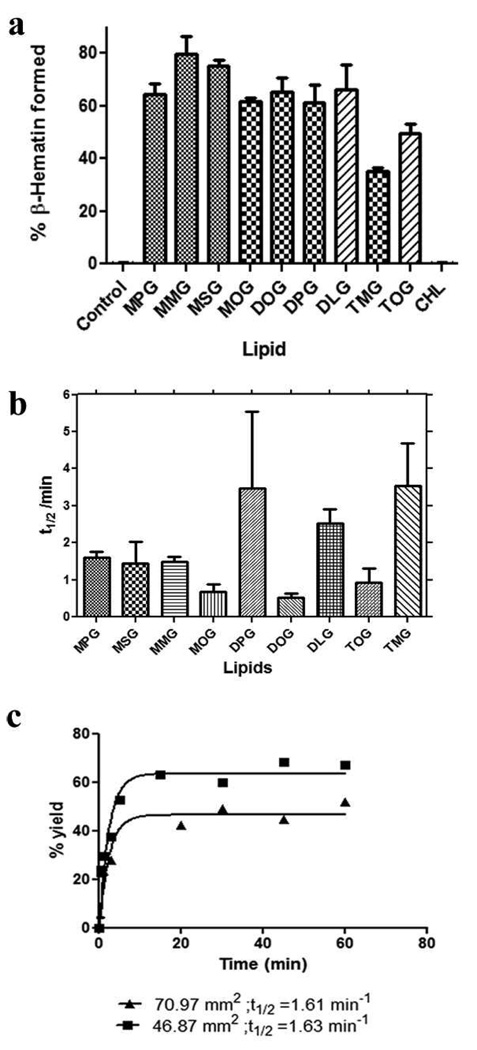
β-Haematin formation using various neutral lipids. The percentage of β-haematin formation was measured for premixed solutions of haematin and lipid deposited onto an aqueous solution. Mono- and diglycerides exhibited about the same efficiency in mediating β-haematin formation, while the triglyceride mediated reaction resulted in less product. The control reaction in which haem was incubated in the aqueous medium alone produced no product (a). No statistically significant difference was observed in the rate of β-haematin formation for these lipids, except for MOG and DOG which are significantly faster than the others (b). This rate did not change when the surface area of the experimental vessel was increased for MSG (c); however, a reduction in the percent yield was observed in reactions performed in a vessel with a 71 mm2 surface area (t1/2 = 1.63 min; 47% yield) compared with that with a 47 mm2 surface area (t1/2 = 1.61 min; 63.8 % yield).
β-Haematin nucleation and extension
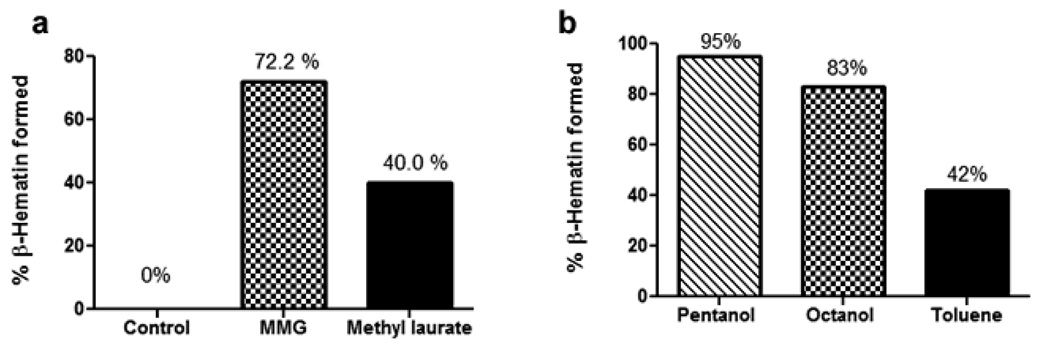
To examine the role of the hydrophilic head group during nucleation, percentage haem conversion was examined in the presence of various mediators. Octanol and pentanol resemble neutral mono- and diglyceride lipids in having OH head groups and aliphatic chains. Docosane, CH3(CH2)20CH3, and methyl laurate, (CH3)-O-C(=O)-(CH2)10CH3, lack OH groups while methyl laurate, but not docosane, contains C=O groups. Toluene can π-stack with haem, but lacks either chains or polar head groups. These solvents were therefore proposed as good substitutes for lipids in order to probe the role of the terminal OH groups, C=O groups and π-electron containing groups in nucleating β-haematin.
In the presence of the hydroxyl (-OH) group, such as in the case of octanol and pentanol, a percentage of β-haematin formation comparable to that mediated by monoglyceride was found, 83% and 95%, respectively (Fig. 5b). This percentage was reduced by nearly half (40%) when the ester methyl laurate was substituted (Fig. 5a). However, under the mediation of a long chain alkane, docosane, which mimics the alkyl chain of the lipid, but lacks the hydrophilic head group, no product was detected. Interestingly, toluene as a mediator produced 42% conversion to β-haematin (Fig. 5a).
Fig. 5.
Comparison of efficiency of lipids, solvents and amphiphiles in forming β-haematin at the interface with aqueous solution (pH 4.8, 37 °C). Comparison of MMG, methyl laurate and a control in which haem is spread on the surface of the aqueous medium in the absence of an amphiphilic molecule, measured at 30 min (a). Comparison of organic solvents pentanol, octanol and toluene in a two-phase system with haem introduced at the interface, measured at 30 min (b).
TEM imaging of extruded samples of both MPG/haem and MSG/haem solutions showed β-haematin formed near the outer region of the lipid particles (Fig. 6). In all observations, crystal size did not exceed dimensions of associated lipid particles. Clusters of β-haematin crystals were observed localized near and aligned parallel to the interface and each other (Fig. 6b, c, f, g). In several images, the β-haematin crystals were located along the lipid-water interface of the lipid particles (Fig. 6a, e).
Fig. 6.
TEM of β-haematin formed at the MSG/water (a – c) and MPG/water (e – g) interface. A lipid and haematin solution was extruded through a fixed membrane filter and then introduced into an aqueous solution containing 50 mM citric acid (pH 4.8, 37 °C). Samples were taken for TEM images after 10 min of incubation. β-haematin crystals were observed aligned along the lipid-water interface for both extruded samples of MSG (top row) and MPG (bottom row). Clusters of β-haematin crystals were oriented parallel to each other and to the lipid-water interface (MSG, b, c; MPG, f, g).
Kinetic studies of β-haematin formation
A premixed lipid-haematin solution was introduced onto the aqueous sub-phase. After the designated incubation period, the percentage of β-haematin formation was measured using a pyridine-based assay. This assay has been described and validated elsewhere23, 24. Briefly, the assay is based on the ability of 5% (v/v) aqueous pyridine solution (pH 7.5) to react with unconverted haem, but not β-haematin, resulting in the formation of a low spin pyridine-haem complex with a distinctive absorbance at 405 nm and a characteristic orange-pink colour. When the percent yield for the 60 minute time point was compared, similar percentages of haem conversion were measured for mono- and diglycerides while less of the product was formed using triglycerides (Fig. 4a). Interestingly, cholesterol was found to be inefficient at mediating β-haematin formation. Though there are observed differences between mono- and diglycerides on the one hand and triglycerides on the other in the percentage of haem conversion, the rates of product formation mediated by these lipids were not greatly affected by the lipid. Overall, the reaction was completed within five minutes and a significant quantity of product was formed in less than one minute (Fig. 4b). Interestingly, unsaturated glycerides promoted β-haematin formation more rapidly than saturated glycerides. Nearly a two fold increase in rate was observed for formation of β-haematin with unsaturated glycerides (MOG, DOG, and TOG) compared to the rate calculated from saturated glycerides. In the absence of the lipid particles, no product was formed. Reaction rates were also unaffected by the cross-sectional area of the vessel in which the reaction was conducted (Fig. 4c).
Temperature and pH dependence of β-haematin formation
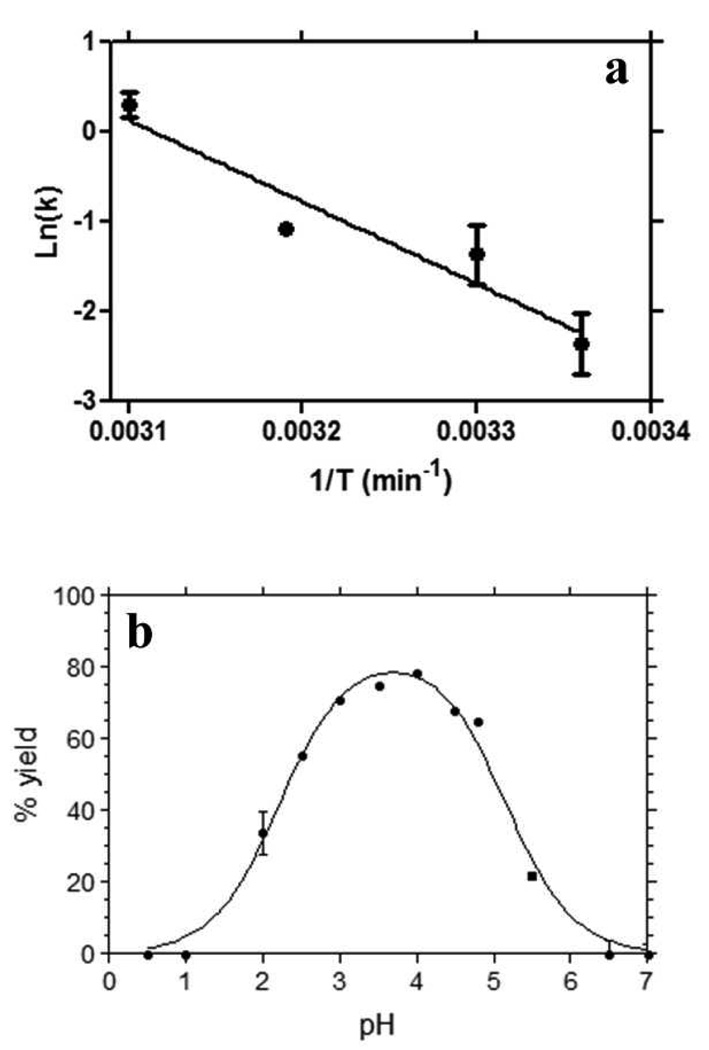
The dependence of the rate of β-haematin formation on temperature in the MSG/water emulsion exhibited Arrhenius behaviour with an activation energy of 76 ± 16 kJ/mol (Fig. 7a). The activation energy obtained in the lipid water system is a little lower than that for β-haematin formation in 4.5 M acetate buffer and 50 mM benzoic acid buffer (84 ± 4 kJ/mol), but the difference is not statistically significant. The pH dependence of the percentage of β-haematin formation at the lipid-water interface was also determined after 1 h incubation at 37 °C. It follows a bell-shaped curve (Fig. 7b) with maximal production at pH 4. As the reaction moves away from this optimal pH, the percentage of haem conversion is reduced. No product was detected below pH 2 or above 6.
Fig. 7.
Temperature and pH dependence of β-haematin formation at the lipid-water interface. The Arrhenius plot (a) was constructed from rate constants (k) measured in an MSG interface system at pH 4.8 and temperatures ranging between 25 and 50 °C. The slope gives an activation energy of 76 ± 16 kJ/mol. The effect of pH (b) was measured at 37 °C and using an MSG interface system. The solid line fitted through the pH data is a best fit to an equation representing two pKa values with, pKa1 = 2.3 ± 0.01 and pKa2 = 5.2 ± 0.01.
DISCUSSION
In a recent study using grazing incidence X-ray diffraction (GIXD) and X-ray reflectivity (XR) measurements, de Villiers et al. showed that when sufficient MMG in methanol/acetone solution to form a multi-layer was introduced onto the surface of an aqueous solution, only a monolayer of lipid is formed at the surface.21 Experimental evidence presented here incontrovertibly demonstrates that the remaining lipid forms an emulsion in the sub-phase beneath this monolayer. Furthermore, the sizes of the lipid particles in this emulsion fall into two populations, one in the low micrometer range and the other in the range 50 – 200 nm. The lipid particles are not micelles or hollow liposomes, but rather have a “solid” core (i.e. are homogeneous through their interiors) as shown by confocal microscopy of the population of larger particles. These lipid particles, which form spontaneously as shown in Scheme 1, are strikingly similar in appearance to neutral lipid bodies reported associated with the digestive vacuole of P. falciparum and the gut lumen of S. mansoni and which, at least in the case of the former organism have been shown to consist largely of mono-, di- and triglycerides.12, 13, 20, 26, 27 Indeed, they appear to be essentially synthetic counterparts of these lipid bodies.
Scheme 1.
Evolution of the lipid emulsion following deposition of a solution on the aqueous surface.
When the lipid emulsion is formed in the presence of soluble haem from a mixed acetone/methanol solution of lipid and haematin, TEM evidence suggests that the haem accumulates near the surface of the lipid particles. Such accumulation of the amphiphilic haem molecule at the surface of lipid bodies in P. falciparum and S. mansoni has previously been proposed,12, 27, 28 but this represents the first direct evidence of localization of free haem at the interface of lipid particles and water. Subsequent formation of β-haematin has been unequivocally demonstrated in this work by FTIR spectroscopy and powder XRD. TEM also demonstrates the formation of crystals identical in habit to haemozoin. Experiments in which lipids were extruded together with haematin revealed β-haematin crystals positioned at the lipid-water interface and oriented parallel to the interface and to each other. These images closely resemble observations in P. falciparum reported by Coppens and Vielemeyer and Pisciotta et al.,12, 26 while images of early stages of haemozoin formation in S. mansoni also appear to be similar upon close inspection.27 The observations in the current study provide direct evidence that crystal nucleation occurs at the lipid-water interface and that crystal elongation occurs parallel to this interface. Based on the work of Buller et al. which relates the shape of β-haematin crystals to the {h,k,l} indices of the crystal,29 it appears likely that crystal extension occurs parallel to the c-axis until its growth is limited by the curvature of the lipid particle. Thus, the slowest growing {100} face is in contact with the lipid surface and the fastest growing {001} face is approximately perpendicular to the interface. This arrangement is in agreement with the findings of the recent GIXD and XR study of β-haematin formation in a premixed MMG/haematin system which showed that the {100} face of the crystals is perfectly aligned with the surface of the solution.21 However, in the absence of electron diffraction data in the present study, this remains somewhat speculative.
The lipids used in this study all contain carbonyl (C=O) head groups, while mono- and diglycerides also contain hydroxyl (OH) head groups. These lipids pack together by virtue of interactions between their polar head groups.30 As each additional hydroxyl group is esterified in going from mono- to di- to triglycerides the polarity of the head group is reduced.31 In Langmuir-Blodgett films in which the alkyl chains are positioned towards the air and the head groups interact with the aqueous solution, lateral compression of diglycerides reports a collapse pressure of 25 – 35 mN/m, significantly higher than that of triglycerides (12 – 14 mN/m). This indicates that the former anchor more strongly in the water phase than triglycerides.32 The higher polarity of mono- and diglycerides is likely to contribute to their efficiency in directing crystal nucleation. These molecules likely self assemble with their polar head groups facing the aqueous layer, presenting OH groups and lone pair electrons which can interact with haem. This is supported by observations with other amphiphiles which indicate that oxygen containing head groups (OH or –(C=O)-O-) play a crucial role in β-haematin formation, since pentanol, octanol and methyl laurate all promote its formation, while no β-haematin was formed in the presence of docosane. These observations support the proposal of Leiserowitz and colleagues that haemozoin formation occurs at lipid-water interfaces through epitaxial growth.25 Indeed, the role of amphiphilic molecules orienting nucleation of inorganic crystals through structural complementarity and/or lattice epitaxy between head groups of the amphiphile and the later arrangement within the crystal is well established in other systems.33 However, it is also the case that decreasing surface tension plays a role in crystal nucleation, as has been demonstrated by Huy et al. for β-haematin formation in acidic aqueous alcohol solutions using water miscible alcohols.34 The activities of the various amphiphilic solvents used in this study and of the lipids could thus alternatively simply reflect their ability to lower surface tension and warrants future investigation.
The dependence of yield on pH follows a bell shaped curve, with near optimal formation at the physiological pH of the malaria parasite digestive vacuole (4.8 – 5.2).35 This pH dependence can be ascribed to the protonation state of β-haematin itself, in which one propionic acid group is un-ionised, while the other is ionised. At low pH (< 2) both propionic acid groups are un-ionised and β-haematin formation is abolished, while at high pH (> 6) both are ionised and β-haematin formation is similarly abolished.
Turning to the kinetics of β-haematin formation, the neutral lipid particle system is competent for the detoxification of free haem at a biologically realistic rate under physiological conditions. Indeed, the observed rates are the fastest so far reported for β-haematin formation with half-lives of about 0.5 min at 37 °C for the monoglycerides MPG, MMG and MSG and only slightly slower for the di- and triglycerides (DLG, DPG and TMG). If haemoglobin degradation occurs at a constant rate, lipid-water interface mediated haemozoin formation would be more than sufficient to manage the entire flux during the intraerythrocytic stage. The rate of formation is about an order of magnitude faster than that previously reported with the lipid-water system.14 In this previous study, the haem and lipid solutions were separately introduced to the aqueous interface, whereas in the current work they were premixed. This demonstrates that the method by which haem is introduced to the interface strongly influences the kinetics, and also suggests that the rate of diffusion or transport of haem molecules to the lipid particles contributes to the efficacy of the biological process. The reaction rates for the different lipids do not vary greatly, but the unsaturated glycerides (MOG, DOG, TOG) promote crystal formation significantly more rapidly than the saturated glycerides. It is possible that unsaturated lipid particles favour initial interaction of haem and lipid via π-stacking.36 It is interesting in this regard that while the alkane docosane does not support β-haematin formation, the aromatic solvent toluene does, albeit less efficiently than octanol and pentanol. This lends support to the notion that a π-system may further promote β-haematin formation at the interface. It is noteworthy that the unsaturated fatty acid linoleic acid has been reported to increase 40% in parasitized erythrocytes relative to control,37 raising the question of whether it serves as a promoter of haemozoin formation.
Finally, the activation energy for β-haematin formation brought about by MSG lipid particles (76 ± 16 kJ/mol) is not significantly different from that reported in acetate or benzoate buffer (84 ± 4 kJ/mol).6, 24 Thus the very much faster rate observed in the lipid system arises from a much bigger pre-exponential factor, consistent with the idea that the interface is involved mainly in preorganizing haem and hence facilitating interactions between haem molecules. When adjusted for concentration of lipid per unit total volume, the concentration of lipid in the lipid-interface system is 384 times less than that of benzoic acid and 34,615 times less than acetic acid. Thus, the lipid particle/water interface is far more active in promoting β-haematin formation than the other two systems and meets the requirement for physiologically relevant rates of formation. Its biological counterpart, the neutral lipid body-water interface, is thus likely to be the site of haemozoin formation in vivo.
CONCLUSION
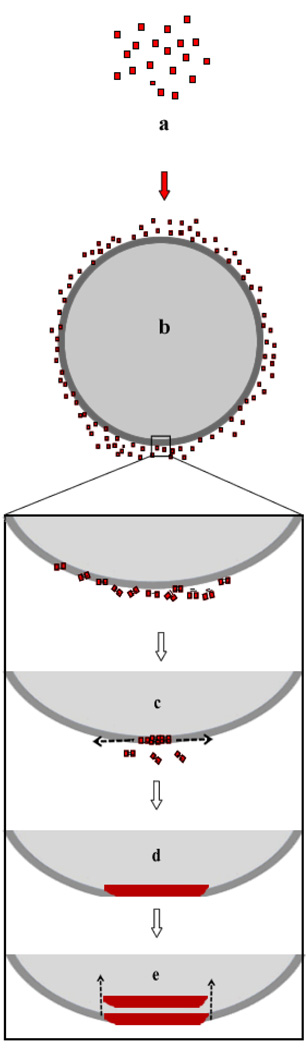
The precise mechanism of formation of haemozoin remains an unanswered question. Paradigms from the field of biomineralization suggest that the process involves the supramolecular preorganization of an organic matrix (e.g. lipids or proteins) that provides a foundation for the second order assembly for inorganic species1, 38. In malaria, a growing body of evidence suggests that a lipid rich environment is essential for the formation of haemozoin12–16. However, previous experimental data for β-haematin formation under physiological conditions could not account for the speed of haemozoin formation in vivo or provide details of the nucleation and crystal extension process. In the present study, lipid particles were synthetically produced to serve as a model for the neutral lipid bodies that exist within the digestive food vacuole of P. falciparum and to shed light on their possible roles during haemozoin formation in nature. Experimental evidence provided here supports the hypothesis that the nucleation and extension of β-haematin is localized at the interface of neutral lipid bodies and water and that these bodies are responsible for the reproducibility of the structural morphology and size of haemozoin crystals observed in nature. Based on these observations, β-haematin formation schematically represented in Fig 8 is proposed. This model accounts for the orientation and formation of β-haematin crystals with respect to these particles. In particular, the model stems from the observation made during these experiments, which revealed the presence of (i) haem localization and nucleation at the lipid-water interface, (ii) crystal extension along the interface, (iii) orientation of mature crystals and (iv), crystal sizes that appear to be limited by the size of the lipid particles. Finally, it should be noted that it is likely that the transport and delivery of haem to the lipid droplets in vivo will differ in several respects. The extent of haem accumulation may be much lower, since in the current experimental setup the entire quantity of haem was introduced to the lipid system at once, whereas in vivo haemoglobin degradation results in the continuous release of haem. In addition, it is possible that proteins may play a role in the delivery of haem to the lipid droplets.
Fig. 8.
The proposed mechanism of haemozoin formation in vivo. Free haem (a) released as a result of haemoglobin degradation accumulates at the interface of the neutral lipid body within the digestive food vacuole of P. falciparum (b). Nucleation occurs at the interface and elongation of the crystal extends along the interface (c) until curvature of the lipid body prevents further elongation of the crystal (d). The hydrophobic nature of the mature crystal (e) favours its transport into the lipid interior leading to a stack of aligned crystals.
ACKNOWLEDGEMENTS
TJE and DWW acknowledge support from the NIH (1R01AI083145-01). DWW and TJE recognise support from the Vanderbilt International Office. For preliminary funding the following are acknowledged: ANH, who conducted a significant portion of the work at the University of Cape Town, acknowledges the Vanderbilt/Fisk Nanosciences IGERT (NSF 0333392); TJE acknowledges part support by the National Research Foundation under Grant No. 2061833, the Medical Research Council of South Africa and the University of Cape Town (any opinion, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Research Foundation or of the Medical Research Council); DWW would like to thank the U.S. Department of Defence grant W81XWH-07-C-0092 for support of this work. Finally, DWW would like to thank MF Richards for critical reading of this manuscript.
REFERENCES
- 1.Mann S. Nature. 1993;365:499. [Google Scholar]
- 2.Addadi L, Weiner S, Geva M. Z. Kardiol. 2001;90 Suppl. 3:92. doi: 10.1007/s003920170049. [DOI] [PubMed] [Google Scholar]
- 3.Addadi L, Rubin N, Scheffer L, Ziblat R. Acc. Chem. Res. 2008;41:254. doi: 10.1021/ar700153u. [DOI] [PubMed] [Google Scholar]
- 4.Somerdijk NAJM, de With G. Chem. Rev. 2008;108:4499. doi: 10.1021/cr078259o. [DOI] [PubMed] [Google Scholar]
- 5.Ziegler J, Linck R, Wright DW. Curr. Med. Chem. 2001;8:171. doi: 10.2174/0929867013373840. [DOI] [PubMed] [Google Scholar]
- 6.Egan TJ, Mavuso WW, Ncokazi KK. Biochemistry. 2001;40:204. doi: 10.1021/bi0013501. [DOI] [PubMed] [Google Scholar]
- 7.Egan TJ, Combrinck JM, Egan J, Hearne GR, Marques HM, Ntenteni S, Sewell BT, Smith PJ, Taylor D, van Schalkwyk DA, Walden JC. Biochem. J. 2002;365:343. doi: 10.1042/BJ20020793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pagola S, Stephens PW, Bohle DS, Kosar AD, Madsen SK. Nature. 2000;404:307. doi: 10.1038/35005132. [DOI] [PubMed] [Google Scholar]
- 9.Slater AFG, Cerami A. Nature. 1992;355:167. doi: 10.1038/355167a0. [DOI] [PubMed] [Google Scholar]
- 10.Egan TJ, Ross DC, Adams PA. FEBS Lett. 1994;352:54. doi: 10.1016/0014-5793(94)00921-x. [DOI] [PubMed] [Google Scholar]
- 11.Dorn A, Stoffel R, Matile H, Bubendorf A, Ridley RG. Nature. 1995;374:269. doi: 10.1038/374269a0. [DOI] [PubMed] [Google Scholar]
- 12.Pisciotta JM, Coppens I, Tripathi AK, Scholl PF, Shuman J, Bajad S, Shulaev V, Sullivan DJ. Biochem. J. 2007;402:197. doi: 10.1042/BJ20060986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson KE, Klonis N, Ferguson DJP, Adisa A, Dogovski C, Tilley L. Mol. Microbiol. 2004;54:109. doi: 10.1111/j.1365-2958.2004.04284.x. [DOI] [PubMed] [Google Scholar]
- 14.Egan TJ, Chen JY-J, de Villiers KA, Mabotha TE, Naidoo KJ, Ncokazi KK, Langford SJ, McNaughton D, Pandiancherri S, Wood BR. FEBS Lett. 2006;580:5105. doi: 10.1016/j.febslet.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 15.Fitch CD, Cai G-Z, Chen Y-F, Shoemaker JD. Biochim. Biophys. Acta. 1999;1454:31. doi: 10.1016/s0925-4439(99)00017-4. [DOI] [PubMed] [Google Scholar]
- 16.Bendrat K, Berger BJ, Cerami A. Nature. 1995;378:138. doi: 10.1038/378138a0. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan DJ, Gluzman IY, Goldberg DE. Science. 1996;271:219. doi: 10.1126/science.271.5246.219. [DOI] [PubMed] [Google Scholar]
- 18.Jani D, Nagarkatti R, Beatty W, Angel R, Slebodnick C, Andersen J, Kumar S, Rathore D. PLOS Pathogens. 2008;4 doi: 10.1371/journal.ppat.1000053. e1000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandey AV, Babbarwal VK, Okoyeh JN, Joshi RM, Puri SK, Singh RL, Chauhan VS. Biochem. Biophys. Res. Commun. 2003;308:736. doi: 10.1016/s0006-291x(03)01465-7. [DOI] [PubMed] [Google Scholar]
- 20.Soares JBRC, Lara FA, Cunha PRBB, Atella GC, Maya-Monteiro CM, d'Avila JCP, Menezes D, Vannier-Santos M, Oliveira PL, Egan TJ, Oliveira MF. FEBS Lett. 2007;581:1742. doi: 10.1016/j.febslet.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 21.de Villiers KA, Osipova M, Mabotha TE, Solomonov I, Feldman Y, Kjaer K, Weissbuch I, Egan TJ, Leiserowitz L. Cryst. Growth Des. 2009;9:626. [Google Scholar]
- 22.Greenspan P, Mayer EP, Fowler SD. J. Cell Biol. 1985;100:965. doi: 10.1083/jcb.100.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ncokazi KK, Egan TJ. Anal. Biochem. 2005;338:306. doi: 10.1016/j.ab.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Egan TJ, Tshivhase MG. Dalton Trans. 2006:5024. doi: 10.1039/b610866k. [DOI] [PubMed] [Google Scholar]
- 25.Solomonov I, Osipova M, Feldman Y, Baehtz C, Kjaer K, Robinson IK, Webster GT, McNaughton D, Wood BR, Weissbuch I, Leiserowitz L. J. Am. Chem. Soc. 2007;129:2615. doi: 10.1021/ja0674183. [DOI] [PubMed] [Google Scholar]
- 26.Coppens I, Vielemeyer O. Int. J. Parasitol. 2005;35:597. doi: 10.1016/j.ijpara.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira MF, Kycia S, Gonzales A, Kosar AD, Bohle DS, Hempelmann E, Menezes D, Vannier-Santos M, Oliveira PL, Ferreira ST. FEBS Lett. 2005;579:6010. doi: 10.1016/j.febslet.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 28.Pisciotta JM, Sullivan DJ. Parasitol. Int. 2008;57:89. doi: 10.1016/j.parint.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buller R, Peterson ML, Almarsson Ö, Leiserowitz L. Cryst. Growth Des. 2002;2:553. [Google Scholar]
- 30.Kaneko F, Yano J, Sato K. Curr. Opin. Struct. Biol. 1998;8:417. doi: 10.1016/s0959-440x(98)80117-6. [DOI] [PubMed] [Google Scholar]
- 31.Mattson FH, Volpenhein RA. J. Lipid Res. 1962;3:281. [Google Scholar]
- 32.Small DM. The physical chemistry of lipids. Plenum Press; 1986. [Google Scholar]
- 33.Weissbuch I, Lahav M, Leiserowitz L. Cryst. Growth Des. 2003;3:125. [Google Scholar]
- 34.Huy NT, Maeda A, Uyen DT, Trang DTX, Sasai M, Shiono T, Oida T, Harada S, Kamei K. Acta Trop. 2007;101:130. doi: 10.1016/j.actatropica.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Hayward R, Saliba KJ, Kirk K. J. Cell Sci. 2006;119:1016. doi: 10.1242/jcs.02795. [DOI] [PubMed] [Google Scholar]
- 36.Yu Y, Smith JM, Flaschenriem CJ, Holland PL. Inorg. Chem. 2006;45:5742. doi: 10.1021/ic052136+. [DOI] [PubMed] [Google Scholar]
- 37.Beaumelle BD, Vial HJ. In Vitro Cell. Dev. Biol. 1988;24:711. doi: 10.1007/BF02623610. [DOI] [PubMed] [Google Scholar]
- 38.Carter MD, Hoang AN, Wright DW. 2008 [Google Scholar]
- 39.Bohle DS, Dinnebier RE, Madsen SK, Stephens PW. J. Biol. Chem. 1997;272:713. doi: 10.1074/jbc.272.2.713. [DOI] [PubMed] [Google Scholar]