Abstract
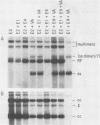
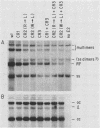
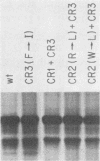
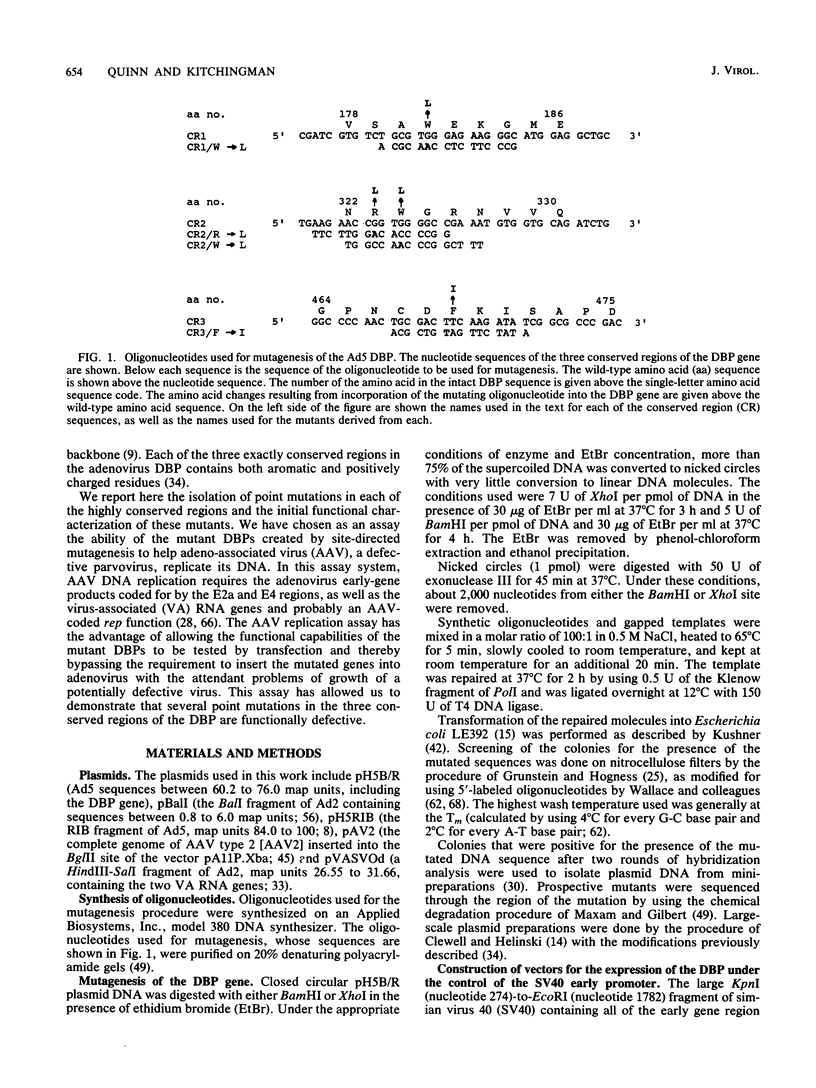
We generated four point mutations in the DNA-binding protein (DBP) gene of adenovirus type 5 by oligonucleotide-directed site-specific mutagenesis. The sites mutated were in the three conserved regions (CR; amino acids 178-186 [CR1], 322-330 [CR2], and 464-475 [CR3]) identified previously by comparative sequence analysis (G. R. Kitchingman, Virology 146:90-101, 1985). The mutations resulted in changes in amino acids 181 (Trp to Leu), 323 (Arg to Leu), 324 (Trp to Leu), and 469 (Phe to Ile). The mutated DBP genes were put under the control of the simian virus 40 early promoter and analyzed by transfection for their ability to help adeno-associated virus replicate its DNA in COS-1 monkey cells. Mutations in the aromatic amino acids 324 and 469 reduced the amount of AAV DNA replication approximately 10-fold, while the mutation in Arg 323 produced a reduction of approximately fourfold. The Trp-to-Leu mutation in amino acid 181 had no effect on AAV DNA replication. The decreased helper activity of the 323, 324, and 469 mutations was not caused by any effect of the mutation on the stability of the DBP. These results suggest that CR2 and CR3 are involved in AAV helper activity, specifically in AAV DNA replication. The relevance of these findings to the identification of residues important for the functions of DBP in adenovirus infection is discussed.
Full text
PDF
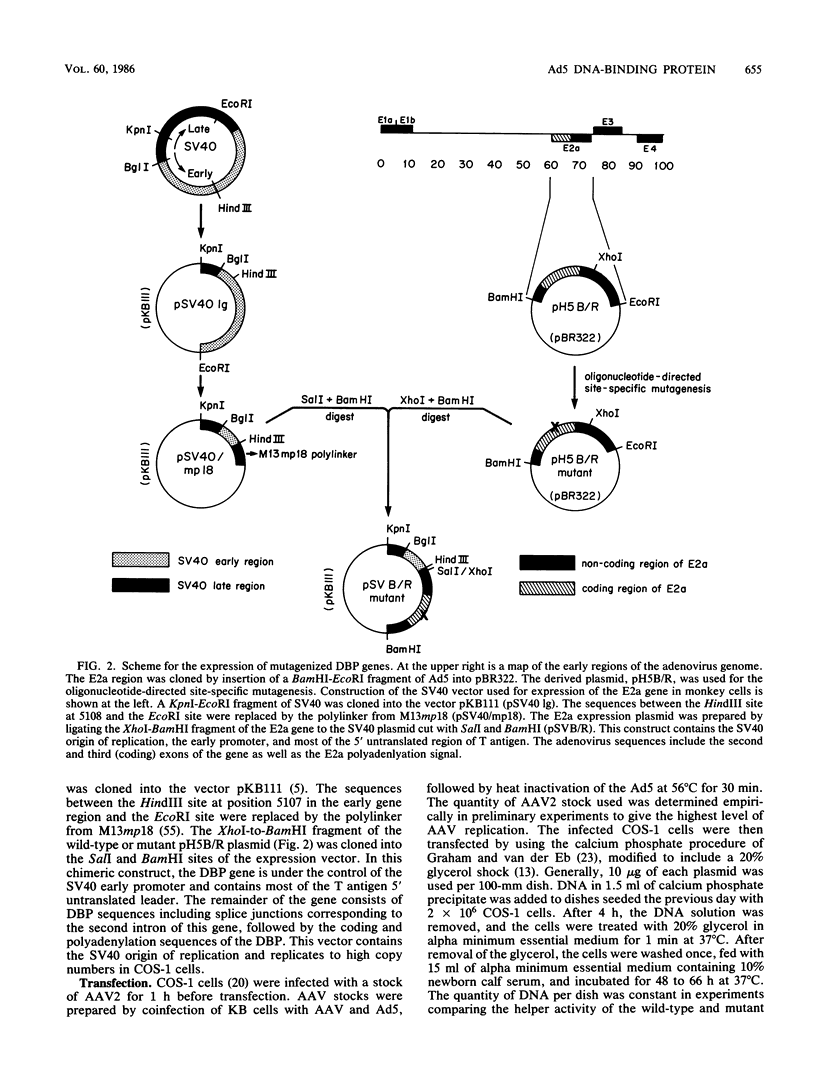






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. P., Klessig D. F. Altered mRNA splicing in monkey cells abortively infected with human adenovirus may be responsible for inefficient synthesis of the virion fiber polypeptide. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4023–4027. doi: 10.1073/pnas.81.13.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariga H., Klein H., Levine A. J., Horwitz M. S. A cleavage product of the adenovirus DNA binding protein is active in DNA replication in vitro. Virology. 1980 Feb;101(1):307–310. doi: 10.1016/0042-6822(80)90510-3. [DOI] [PubMed] [Google Scholar]
- Babich A., Nevins J. R. The stability of early adenovirus mRNA is controlled by the viral 72 kd DNA-binding protein. Cell. 1981 Nov;26(3 Pt 1):371–379. doi: 10.1016/0092-8674(81)90206-3. [DOI] [PubMed] [Google Scholar]
- Baldy M. W. The UV sensitivity of some early-function temperature-sensitive mutants of phage T4. Virology. 1970 Feb;40(2):272–287. doi: 10.1016/0042-6822(70)90403-4. [DOI] [PubMed] [Google Scholar]
- Beckingham K. A plasmid cloning vector for Kpnl-cleaved DNA. Plasmid. 1980 Nov;4(3):354–356. doi: 10.1016/0147-619x(80)90074-8. [DOI] [PubMed] [Google Scholar]
- Berger H., Warren A. J., Fry K. E. Variations in genetic recombination due to amber mutations in T4D bacteriophage. J Virol. 1969 Feb;3(2):171–175. doi: 10.1128/jvi.3.2.171-175.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Lee F., Harrison T., Williams J., Sharp P. A. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell. 1979 Aug;17(4):935–944. doi: 10.1016/0092-8674(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Berkner K. L., Sharp P. A. Generation of adenovirus by transfection of plasmids. Nucleic Acids Res. 1983 Sep 10;11(17):6003–6020. doi: 10.1093/nar/11.17.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayer G. D., McPherson A. A model for intracellular complexation between gene-5 protein and bacteriophage fd DNA. Eur J Biochem. 1985 Jul 15;150(2):287–296. doi: 10.1111/j.1432-1033.1985.tb09019.x. [DOI] [PubMed] [Google Scholar]
- Carter T. H., Blanton R. A. Autoregulation of adenovirus type 5 early gene expression II. Effect of temperature-sensitive early mutations on virus RNA accumulation. J Virol. 1978 Nov;28(2):450–456. doi: 10.1128/jvi.28.2.450-456.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T. H., Blanton R. A. Possible role of the 72,000 dalton DNA-binding protein in regulation of adenovirus type 5 early gene expression. J Virol. 1978 Feb;25(2):664–674. doi: 10.1128/jvi.25.2.664-674.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Sharp P. A. SV40 DNA transfection of cells in suspension: analysis of efficiency of transcription and translation of T-antigen. Gene. 1981 Mar;13(2):197–202. doi: 10.1016/0378-1119(81)90008-1. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist L. W., Madden M. J., Schiop-Stanley P., Vande Woude G. F. Cloning of herpes simplex type 1 DNA fragments in a bacteriophage lambda vector. Science. 1979 Feb 9;203(4380):541–544. doi: 10.1126/science.216076. [DOI] [PubMed] [Google Scholar]
- Ezoe H., Fatt R. B., Mak S. Degradation of intracellular DNA in KB cells infected with cyt mutants of human adenovirus type 12. J Virol. 1981 Oct;40(1):20–27. doi: 10.1128/jvi.40.1.20-27.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Friefeld B. R., Krevolin M. D., Horwitz M. S. Effects of the adenovirus H5ts125 and H5ts107 DNA binding proteins on DNA replication in vitro. Virology. 1983 Jan 30;124(2):380–389. doi: 10.1016/0042-6822(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Ginsberg H. S., Lundholm U., Linné T. Adenovirus DNA-binding protein in cells infected with wild-type 5 adenovirus and two DNA-minus, temperature-sensitive mutants, H5ts125 and H5ts149. J Virol. 1977 Jul;23(1):142–151. doi: 10.1128/jvi.23.1.142-151.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Goding C., Jalinot P., Zajchowski D., Boeuf H., Kédinger C. Sequence-specific trans-activation of the adenovirus EIIa early promoter by the viral EIV transcription unit. EMBO J. 1985 Jun;4(6):1523–1528. doi: 10.1002/j.1460-2075.1985.tb03812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godowski P. J., Knipe D. M. Mutations in the major DNA-binding protein gene of herpes simplex virus type 1 result in increased levels of viral gene expression. J Virol. 1983 Sep;47(3):478–486. doi: 10.1128/jvi.47.3.478-486.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Green M. R., Roeder R. G. Transcripts of the adeno-associated virus genome: mapping of the major RNAs. J Virol. 1980 Oct;36(1):79–92. doi: 10.1128/jvi.36.1.79-92.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert D. N., Cutt J. R., Shenk T. Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host cell shutoff. J Virol. 1985 Oct;56(1):250–257. doi: 10.1128/jvi.56.1.250-257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa H., Kingston R. E., Sharp P. A. Inhibition of adenovirus early region IV transcription in vitro by a purified viral DNA binding protein. Nature. 1983 Apr 7;302(5908):545–547. doi: 10.1038/302545a0. [DOI] [PubMed] [Google Scholar]
- Hermonat P. L., Labow M. A., Wright R., Berns K. I., Muzyczka N. Genetics of adeno-associated virus: isolation and preliminary characterization of adeno-associated virus type 2 mutants. J Virol. 1984 Aug;51(2):329–339. doi: 10.1128/jvi.51.2.329-339.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Janik J. E., Huston M. M., Rose J. A. Locations of adenovirus genes required for the replication of adenovirus-associated virus. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1925–1929. doi: 10.1073/pnas.78.3.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay F. T., Laughlin C. A., Carter B. J. Eukaryotic translational control: adeno-associated virus protein synthesis is affected by a mutation in the adenovirus DNA-binding protein. Proc Natl Acad Sci U S A. 1981 May;78(5):2927–2931. doi: 10.1073/pnas.78.5.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J. Identification of the components necessary for adenovirus translational control and their utilization in cDNA expression vectors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):689–693. doi: 10.1073/pnas.82.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchingman G. R. Sequence of the DNA-binding protein of a human subgroup E adenovirus (type 4): comparisons with subgroup A (type 12), subgroup B (type 7), and subgroup C (type 5). Virology. 1985 Oct 15;146(1):90–101. doi: 10.1016/0042-6822(85)90055-8. [DOI] [PubMed] [Google Scholar]
- Klein H., Maltzman W., Levine A. J. Structure-function relationships of the adenovirus DNA-binding protein. J Biol Chem. 1979 Nov 10;254(21):11051–11060. [PubMed] [Google Scholar]
- Klessig D. F., Chow L. T. Incomplete splicing and deficient accumulation of the fiber messenger RNA in monkey cells infected by human adenovirus type 2. J Mol Biol. 1980 May 15;139(2):221–242. doi: 10.1016/0022-2836(80)90306-x. [DOI] [PubMed] [Google Scholar]
- Klessig D. F., Grodzicker T. Mutations that allow human Ad2 and Ad5 to express late genes in monkey cells map in the viral gene encoding the 72K DNA binding protein. Cell. 1979 Aug;17(4):957–966. doi: 10.1016/0092-8674(79)90335-0. [DOI] [PubMed] [Google Scholar]
- Klessig D. F., Quinlan M. P. Genetic evidence for separate functional domains on the human adenovirus specified, 72 kd, DNA binding protein. J Mol Appl Genet. 1982;1(4):263–272. [PubMed] [Google Scholar]
- Kruijer W., van Schaik F. M., Speijer J. G., Sussenbach J. S. Structure and function of adenovirus DNA binding protein: comparison of the amino acid sequences of the Ad5 and Ad12 proteins derived from the nucleotide sequence of the corresponding genes. Virology. 1983 Jul 15;128(1):140–153. doi: 10.1016/0042-6822(83)90325-2. [DOI] [PubMed] [Google Scholar]
- Kruijer W., van Schaik F. M., Sussenbach J. S. Structure and organization of the gene coding for the DNA binding protein of adenovirus type 5. Nucleic Acids Res. 1981 Sep 25;9(18):4439–4457. doi: 10.1093/nar/9.18.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Fatt R. B., Mak S. Mapping of an adenovirus function involved in the inhibition of DNA degradation. J Virol. 1982 Jun;42(3):969–977. doi: 10.1128/jvi.42.3.969-977.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin C. A., Jones N., Carter B. J. Effect of deletions in adenovirus early region 1 genes upon replication of adeno-associated virus. J Virol. 1982 Mar;41(3):868–876. doi: 10.1128/jvi.41.3.868-876.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin C. A., Tratschin J. D., Coon H., Carter B. J. Cloning of infectious adeno-associated virus genomes in bacterial plasmids. Gene. 1983 Jul;23(1):65–73. doi: 10.1016/0378-1119(83)90217-2. [DOI] [PubMed] [Google Scholar]
- Lemaire G., Gold L., Yarus M. Autogenous translational repression of bacteriophage T4 gene 32 expression in vitro. J Mol Biol. 1978 Nov 25;126(1):73–90. doi: 10.1016/0022-2836(78)90280-2. [DOI] [PubMed] [Google Scholar]
- Linné T., Philipson L. Further characterization of the phosphate moiety of the adenovirus type 2 DNA-binding protein. Eur J Biochem. 1980 Jan;103(2):259–270. doi: 10.1111/j.1432-1033.1980.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McPherson R. A., Ginsberg H. S., Rose J. A. Adeno-associated virus helper activity of adenovirus DNA binding protein. J Virol. 1982 Nov;44(2):666–673. doi: 10.1128/jvi.44.2.666-673.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M. W., Laughlin C. A., Jay F. T., Carter B. J. Adenovirus helper function for growth of adeno-associated virus: effect of temperature-sensitive mutations in adenovirus early gene region 2. J Virol. 1980 Jul;35(1):65–75. doi: 10.1128/jvi.35.1.65-75.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Enomoto T., Lichy J. H., Hurwitz J. Adenovirus DNA replication in vitro: identification of a host factor that stimulates synthesis of the preterminal protein-dCMP complex. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6438–6442. doi: 10.1073/pnas.79.21.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R., Winkler J. J. Regulation of early adenovirus transcription: a protein product of early region 2 specifically represses region 4 transcription. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1893–1897. doi: 10.1073/pnas.77.4.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas J. C., Ingrand D., Sarnow P., Levine A. J. A mutation in the adenovirus type 5 DNA binding protein that fails to autoregulate the production of the DNA binding protein. Virology. 1982 Oct 30;122(2):481–485. doi: 10.1016/0042-6822(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Osborne T. F., Schell R. E., Burch-Jaffe E., Berget S. J., Berk A. J. Mapping a eukaryotic promoter: a DNA sequence required for in vivo expression of adenovirus pre-early functions. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1381–1385. doi: 10.1073/pnas.78.3.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelich G., Stillman B. W. Functional characterization of thermolabile DNA-binding proteins that affect adenovirus DNA replication. J Virol. 1986 Mar;57(3):883–892. doi: 10.1128/jvi.57.3.883-892.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn C. O., Kitchingman G. R. Sequence of the DNA-binding protein gene of a human subgroup B adenovirus (type 7). Comparisons with subgroup C (type 5) and subgroup A (type 12). J Biol Chem. 1984 Apr 25;259(8):5003–5009. [PubMed] [Google Scholar]
- Richardson W. D., Westphal H. A cascade of adenovirus early functions is required for expression of adeno-associated virus. Cell. 1981 Nov;27(1 Pt 2):133–141. doi: 10.1016/0092-8674(81)90367-6. [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Westphal H. Requirement for either early region 1a or early region 1b adenovirus gene products in the helper effect for adeno-associated virus. J Virol. 1984 Aug;51(2):404–410. doi: 10.1128/jvi.51.2.404-410.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Suggs S. V., Wallace R. B., Hirose T., Kawashima E. H., Itakura K. Use of synthetic oligonucleotides as hybridization probes: isolation of cloned cDNA sequences for human beta 2-microglobulin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6613–6617. doi: 10.1073/pnas.78.11.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson C., Akusjärvi G. Adenovirus VA RNAI: a positive regulator of mRNA translation. Mol Cell Biol. 1984 Apr;4(4):736–742. doi: 10.1128/mcb.4.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmappaya B., Weinberger C., Schneider R. J., Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982 Dec;31(3 Pt 2):543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- Tigges M. A., Raskas H. J. Splice junctions in adenovirus 2 early region 4 mRNAs: multiple splice sites produce 18 to 24 RNAs. J Virol. 1984 Apr;50(1):106–117. doi: 10.1128/jvi.50.1.106-117.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tratschin J. D., Miller I. L., Carter B. J. Genetic analysis of adeno-associated virus: properties of deletion mutants constructed in vitro and evidence for an adeno-associated virus replication function. J Virol. 1984 Sep;51(3):611–619. doi: 10.1128/jvi.51.3.611-619.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Schold M., Johnson M. J., Dembek P., Itakura K. Oligonucleotide directed mutagenesis of the human beta-globin gene: a general method for producing specific point mutations in cloned DNA. Nucleic Acids Res. 1981 Aug 11;9(15):3647–3656. doi: 10.1093/nar/9.15.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg D. H., Ketner G. Adenoviral early region 4 is required for efficient viral DNA replication and for late gene expression. J Virol. 1986 Mar;57(3):833–838. doi: 10.1128/jvi.57.3.833-838.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. C., Nevins J. R., Blanchard J. M., Ginsberg H. S., Darnell J. E., Jr Metabolism of mRNA from the transforming region of adenovirus 2. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):447–455. doi: 10.1101/sqb.1980.044.01.048. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Levine A. J. DNA-binding proteins specific for cells infected by adenovirus. Nat New Biol. 1973 Dec 12;246(154):170–174. doi: 10.1038/newbio246170a0. [DOI] [PubMed] [Google Scholar]