Abstract
Primary cardiac lymphoma (PCL) is an extranodal non-Hodgkin's lymphoma exclusively located in the heart and/or pericardium. It is rare in immunocompetent patients and represents 1.3% of primary cardiac tumors and 0.5% of extranodal lymphomas. The clinical behavior is aggressive and the early symptoms are cardiac failure, syncope, arrhythmia, or pericardial effusion. Although echocardiography, computed tomography (CT) scan, magnetic resonance image (MRI) are the mainly used imaging techniques to detect cardiac tumors, pathologic examination is always required to confirm the diagnosis. Diagnosis of PCL is difficult due to non-specific clinical manifestations and requires invasive approach to get histopathologic evidence. While surgery with systemic chemotherapy or in combination with irradiation has been attempted, the only effective treatment is chemotherapy. However, the prognosis remains poor. We report on a 42-year-old woman who is diagnosed histopathologically as PCL by cardiac catheterization assisted percutaneous endomyocardial biopsy and treated successfully by anthracycline based chemotherapy.
Keywords: Non-Hodgkin's lymphoma, Cardiac tamponade, Pericardial effusion
Introduction
PCL is very rare and difficult to diagnose because of its non-specific clinical manifestations.1) The main diagnostic imaging techniques are echocardiography, CT and MRI.2),3) Besides histologic and immunohistochemical examination of the involved tissue is always required to confirm diagnosis.4) But, it is not easy to do open biopsy because its aggressive behavior.2) In addition, surgery for mass reduction is not usually recommended because it does not seem to have improved the prognosis.4) So, non-invasive and early diagnosis is fundamental to allow prompt initiation of chemotherapy.5) The following describes the case report of PCL diagnosed by percutaneous endomyocardial biopsy and successfully treated by chemotherapy with literature review.
Case
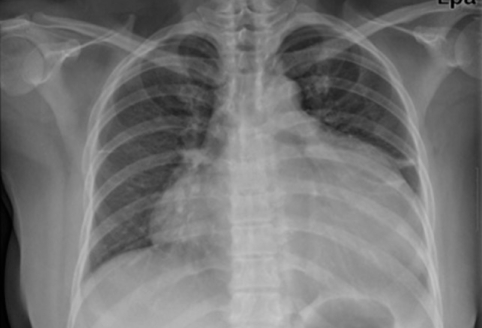
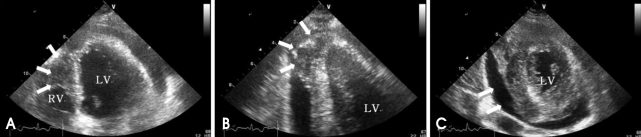
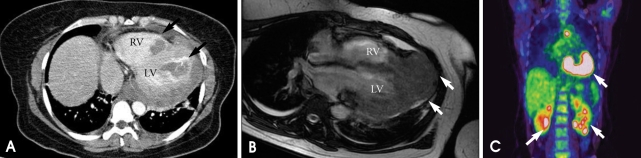
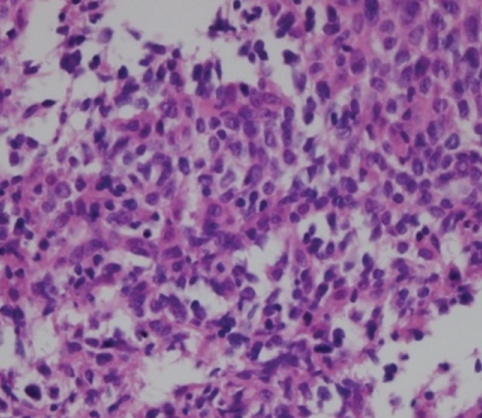
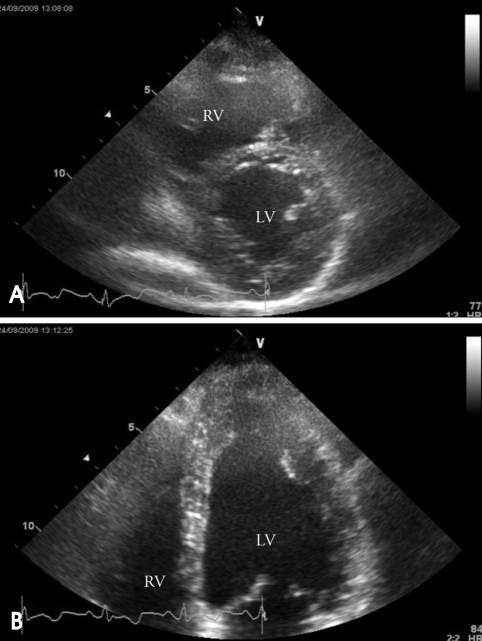
A 42-year-old female visited our outpatient clinic due to progressive exertional dyspnea and retrosternal pain for 2 weeks. She was not a smoker but drunk alcohol for 1 or 2 times a week. She did not have any disease nor operation history. Her brother had acute myocardial infarction history. The patient's blood pressure was 110/70 mmHg, body temperature was 37.0℃, respiratory rate was 20/min and heart rate was 90 bpm. And she had jugular venous distension and carotid pulses were regular without bruits. Cardiac auscultation revealed regular and muffled heart sound without murmur and the lung sound was clear. There was no hepatosplenomegaly but she had pretibial pitting edema. The initial electrocardiography (ECG) displayed regular sinus rhythm with low voltage of QRS and asymmetric T wave inversion at inferior limb lead and precordial lead V4-6. The chest radiography demonstrated cardiomegaly with globular shape of heart (Fig. 1). Complete blood cell count revealed normocytic normochromic anemia without leukocytosis. Biochemical parameters showed raised erythrocyte sedimentation rate, C-reactive protein, lactic dehydrogenase and elevated pro B-type natriuretic peptide. Echocardiography disclosed abnormally thickened myocardium of left ventricle (LV) and right ventricle (RV) with heterogenous echo-texture owing to the invasion of mass (Fig. 2A and B). Moderate amount of pericardial effusion was also seen (Fig. 2C). Pericardiocentesis was performed urgently, yielding 400 mL of serosanguinous pericardial effusion. Pericardial fluid analysis proved abundant red blood cells and lymphocyte dominant nucleated cells without malignant cells. Computed tomography (CT) scan showed lobulating mass on both ventricular myocardium protruding to base of heart with obscure boundary (Fig. 3A). Magnetic resonance imaging (MRI) demonstrated lobulating mass on LV base which extended to RV and pericardium with ovoid mass on interventricular septum (IVS), enhanced heterogeneously (Fig. 3B). Coronary angiography displayed normal coronary arteries without any visible feeding vessels. The left ventriculogram showed spade shaped LV due to protruding mass. Percutaneous endomyocardial biopsy disclosed that tumor cells were atypical lymphocytes regarded as lymphoma cells in hematoxylin and eosin stain (Fig. 4) and a diffuse large B-cell lymphoma in immunohistochemical stain (CD20+, CD3+, Ki67+). Positron emission tomography/computed tomography (PET/CT) revealed malignant tumor involving the LV, RV and pericardial region as well as intense fluorodeoxyglucose (FDG) uptake at left atrium (LA) and left lower paratracheal region and both kidneys (Fig. 3C). Abdomen CT demonstrated simple cysts in both kidneys and there was no evidence of metastasis in the abdominal cavity. Bone marrow biopsy proved normocellular bone marrow with lymphoma involvement with lymphoma cells that are about 2% of all nucleated elements. Cerebrospinal fluid examination was negative for malignancy. And thus, the patient had stage IV extranodal lymphoma with an international prognostic index (IPI) score of 3. As the patient's LV ejection fraction was normal, she was treated with Rituximab, Cyclophosphamide, Doxorubicin (chemical name; hydroxydaunorubicin), Vincristine (originally known as Oncovin) and Prednisolone (R-CHOP) and intrathecal chemotherapy with Methotrexate plus Ara-C. The PET/CT and echocardiogram were performed after 3rd cycle of chemotherapy. The PET/CT showed regressed FDG uptake with score of standardized uptake values (SUV) from 18.92 to 4.43 and there was no perceptible FDG uptake at LA, left lower paratracheal region and both kidneys. After 8th cycle R-CHOP and four courses of intrathecal chemotherapy, the patient was asymptomatic with complete remission. The cardiac infiltrative mass completely disappeared on follow-up echocardiogram (Fig. 5). At now, she is in very good condition with no relapse of the lymphoma for 4 months after completion of the treatment.
Fig. 1.
Chest radiography demonstrates marked cardiomegaly with globular shape of heart.
Fig. 2.
A: Apical 4 chamber view demonstrates infiltrative tumor mass in RV apical cavity (white arrow). B: On apical 2 chamber view, protruding mass (white arrow) is observed in LV apex with moderate amount of pericardial effusion. C: Parasternal short axis view at the mid-ventricular level shows moderate amount of pericardial effusion (white arrow) and mass in posteroinferior LV myocardium. LV: left ventricle, RV: right ventricle.
Fig. 3.
A: Computed tomography reveals intracardiac mass involving both ventricles (black arrow). B: Magnetic resonance imaging (MRI) demonstrates mass on LV base which extended from pericardium to RV and LV (white arrow). C: PET/CT revealing malignant tumor involving the LV, RV and pericardial region as well as intense FDG uptake at LA and left lower paratracheal region and both kidneys. LV: left ventricle, RV: right ventricle, PET/CT: positron emission tomography/computed tomography, FDG: fluorodeoxyglucose, LA: left atrium.
Fig. 4.
High power view of the left ventricular endomyocardial tumor. The tumor cells are atypical lymphocytes regarded as lymphoma cells. Hematoxylin and eosin stain (×100).
Fig. 5.
A: Parasternal short axis view at the mid-ventricular level shows no more observed tumor mass and pericardial effusion. B: On apical 4 chamber view, there is no infiltrating cardiac mass in LV and RV apex. LV: left ventricle, RV: right ventricle.
Discussion
PCL is an extranodal non-Hodgkin's lymphoma exclusively located in the heart and/or pericardium.1) PCL is rare in immunocompetent patients, and it represents 1.3% of primary cardiac tumors and 0.5% of extranodal lymphomas.2) However, it is being encountered with increasing frequency in patients with immunosupression by human immunodeficiency virus (HIV) infection or in transplant recipients.6)
Although, in our case, lymphoma is involved not only in the heart but also in other organs, we concluded our patient as PCL. Because PCL is a lymphoma primary to the heart if there are cardiac symptoms from lymphomatous cardiac infiltration at the time of initial diagnosis.7) Furthermore, in spite of high FDG uptake at kidneys and bone marrow invasion, there were only simple renal cysts in both kidneys at CT and lymphoma cells in BM were only about 2% of all nucleated elements.
According to literature by Anghel et al., PCLs occur more frequently in male and typically in the elderly, with lesions mainly localized in the heart's right chambers (classically in the RA) or pericardium4) in contrast with myxoma which involve mainly left atrium.1) There are no pathognomic symptoms or signs characteristic of a PCL, but some of more common presentations may depend on flow obstruction, infiltration of adjacent tissues, tumor embolization and symptoms are associated with heart failure, arrhythmias, atrioventricular disturbances, pericardial effusion, superior vena cava syndrome and stroke.5),8) Chest X-ray is not usually helpful, showing non-specific signs such as pleural effusion and/or cardiomegaly. Transthoracic echocardiography (TTE) is currently the most widely used diagnostic procedure for the detection of cardiac tumors. Transesophageal echocardiography (TEE) is reported to provide superior diagnostic imaging than TTE, also useful at surgical resection and transvenous biopsy.2) CT and MRI have better contrast resolution than echocardiography and can give a specific diagnosis for a number of tumors.3) Gallium-67 and Technesium-99m (Tc-99m) Sestamibi scan also has been reported to be valid myocardial perfusion tracers for detecting PCL and during the follow-up.1) CT scan and MRI are the mainly used imaging techniques to detect cardiac tumors. But histologic and immunohistochemical examination of the involved tissue, as well as cytologic examination with cytogenetic studies of pericardial effusion, is always required to confirm diagnosis.4) Although PCL was a near fatal disease in the past, recent advances in imaging diagnosis and chemotherapy have dramatically improved survival.3) So, non-invasive and early diagnosis is fundamental to allow prompt initiation of aggressive treatment; however, it is difficult to do so, because of its aggressive behavior.2),5)
Reviewed by Ceresoli et al.9) in 1997, cytology of pericardial effusion was diagnostic only in 67% of cases, while thoracotomy and biopsy yielded a positive result in all. Jurkovich et al. in 2000 reported PCL diagnosed by percutaneous intracardiac biopsy with combined fluoroscopic and TEE imaging. Recently Higo et al.10) reported cardiac lymphoma diagnosed by intracardiac echocardiography-guided biopsy in 2009. Histologically, most of the tumors were B-cell neoplasms and were of diffuse large cell type.4)
Although a gold standard therapy for cardiac lymphoma was still not established, surgery as well as systemic chemotherapy solely or in combination with irradiation is the main therapeutic approaches. According to reviews, prompt anthracycline-based chemotherapy results in 61% of complete remission (mean follow-up times 17 months; range 3-40 months).1),4) Meanwhile, surgery did not seem to have improved the prognosis. In Korea, there were some case reports of PCL, most of them diagnosed by invasive method, including thoracotomy.11-13) Furthermore, when they chose the primary therapeutic approach as a mass reduction by open heart surgery, patients showed poor prognostic features.14),15)
In our case, compared with previously reported cases, PCL was diagnosed by various modern imaging techniques and endomyocardial biopsy via percutaneous cardiac approach.
References
- 1.Piccaluga PP, Vigna E, Placci A, Agostinelli C, Laterza C, Papayannidis C, Leone O, Martinelli G, Zinzani PL, Baccarani M, Pileri SA. Primary cardiac non-Hodgkin lymphoma presenting with atrial flutter and pericardial effusion. Br J Haematol. 2006;134:356. doi: 10.1111/j.1365-2141.2006.06168.x. [DOI] [PubMed] [Google Scholar]
- 2.Chalabreysse L, Berger F, Loire R, Devouassoux G, Cordier JF, Thivolet-Bejui F. Primary cardiac lymphoma in immunocompetent patients: a report of three cases and review of the literature. Virchows Arch. 2002;441:456–461. doi: 10.1007/s00428-002-0711-0. [DOI] [PubMed] [Google Scholar]
- 3.Utsunomiya D, Awai K, Urata J, Hirayama T, Yamashita Y. Primary cardiac lymphoma: computed tomography and magnetic resonance imaging features. Jpn J Radiol. 2009;27:243–246. doi: 10.1007/s11604-009-0329-0. [DOI] [PubMed] [Google Scholar]
- 4.Anghel G, Zoli V, Petti N, Remotti D, Feccia M, Pino P, Majolino I. Primary cardiac lymphoma: report of two cases occurring in immunocompetent subjects. Leuk Lymphoma. 2004;45:781–788. doi: 10.1080/10428190310001617259. [DOI] [PubMed] [Google Scholar]
- 5.Motto A, Ballo P, Zito D, Cadenotti L, Moroni M, Dessanti P, Fedeli F. Primary cardiac lymphoma presenting as sick sinus syndrome. J Clin Oncol. 2008;26:6003–6005. doi: 10.1200/JCO.2008.19.4803. [DOI] [PubMed] [Google Scholar]
- 6.Nascimento AF, Winters GL, Pinkus GS. Primary cardiac lymphoma: clinical, histologic, immunophenotypic, and genotypic features of 5 cases of a rare disorder. Am J Surg Pathol. 2007;31:1344–1350. doi: 10.1097/PAS.0b013e3180317341. [DOI] [PubMed] [Google Scholar]
- 7.Zaharia L, Gill PS. Primary cardiac lymphoma. Am J Clin Oncol. 1991;14:142–145. doi: 10.1097/00000421-199104000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Ryu SJ, Choi BW, Choe KO. CT and MR findings of primary cardiac lymphoma: report upon 2 cases and review. Yonsei Med J. 2001;42:451–456. doi: 10.3349/ymj.2001.42.4.451. [DOI] [PubMed] [Google Scholar]
- 9.Ceresoli GL, Ferreri AJ, Bucci E, Ripa C, Ponzoni M, Villa E. Primary cardiac lymphoma in immunocompetent patients: diagnostic and therapeutic management. Cancer. 1997;80:1497–1506. doi: 10.1002/(sici)1097-0142(19971015)80:8<1497::aid-cncr18>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Higo T, Takemoto M, Ogawa K, Inoue S, Eshima K, Tada H, Sunagawa K. Intracardiac echocardiography-guided cardiac tumor biopsy. Circ J. 2009;73:381–383. doi: 10.1253/circj.cj-08-0043. [DOI] [PubMed] [Google Scholar]
- 11.Jang EH, Kim WS, Kim ST, Kang JH, Park GW, Jung CW, Park KC. Primary cardiac lymphoma presented as 3 degree AV block and right ventricular failure. Korean J Intern Med. 2006;70:341–346. [Google Scholar]
- 12.Park KS, Ahn WS, Lee S, Kwon OC, Ko MS, Jheon SH. Primary non-Hodgkin's lymphoma in right ventricle with right atrial invasion. Report of 1 case. Korean J Thorac Cardiovasc Surg. 2004;37:376–381. [Google Scholar]
- 13.Kim JY, Woo CM, Lee JY, Lee JB, Ryu JK, Choi JY, Chang SG, Shin JH, Lee WS, Seo JH, Kim YS. A case of primary cardiac non-Hodgkin's lymphoma. Korean Cir J. 2004;34:808–812. [Google Scholar]
- 14.Kang SB, Jin SW, Lee EK, Park YH, Choi YH, Kim YK, Park JC. A case of non-Hodgkin's lymphoma with massive involvement of the right atrium. Korean Cir J. 2000;30:492–496. [Google Scholar]
- 15.Choi WS, Han IY, Jun HJ, Lee YH, Hwang YH, Cho KH. Primary malignant cardiac lymphoma in right atrium. A case report. Korean J Thorac Cardiovasc Surg. 2008;41:369–372. [Google Scholar]