Abstract
Background
Left ventricular hypertrophy (LVH) has been known as an important predictor of prognosis of cardiovascular disease. Carboxy-terminal propeptide of procollagen type I (PIP) is related with myocardial fibrosis. We sought to analyze the differences in the characteristics of LVH, myocardial fibrosis, and LV functions among hypertension (HBP), diabetes mellitus (DM) and chronic renal failure (CRF).
Methods
We enrolled consecutive patients with LVH. Patients were grouped as HBP (n=50), DM (n=41), CRF (n=31). Age and sex-matched normal control was also enrolled (n=32). Echocardiography and blood sampling for serum PIP level measuring was performedin all participants.
Results
There were no differences in baseline characteristics except systolic blood pressure among four groups. In three patients groups, their LV mass indices were significantly increased than control. Serum PIP level in CRF was much higher than others (CRF 1505.5 vs. HBP 868.7 vs. DM 687.5 vs. control 826.4, p<0.0001). LV diastolic and systolic function evaluated by E', E/E, S' and midwall fractional shortening was significantly decreased in three patients groups. However, LAVi was significantly elevated and LV ejection fraction was significantly decreased in CRF compared to others. In correlation analysis, indices of diastolic function were weakly, but statistically correlated with PIP (E': r=0.234, p=0.006; LAVi: r=0.231, p=0.006).
Conclusion
In CRF, LV function was more deteriorated and serum PIP was more elevated when compared to HBP or DM. Therefore, myocardial fibrosis may play an important role to LV dysfunction as well as LV hypertrophy in CRF in some degree.
Keywords: Left ventricular hypertrophy, Carboxy-terminal propeptide of procollagen type I, Fibrosis
Introduction
Essential hypertension (HBP), type 2 diabetes mellitus (DM) and chronic renal failure (CRF) induce left ventricular hypertrophy (LVH), and patients with LVH is significantly associated with higher occurrence of cardiovascular death compared with normal population.1-3) However, the common echocardiographic findings of LVH without combined severe cardiovascular complications are merely expressed diastolic dysfunction, grade I (impaired relaxation abnormality) and normal systolic function in patients with above three diseases in general.4) Also in clinical situation, the stage of only LVH in three diseases is not regarded as abnormal cardiac function, so active diagnostic process and treatment has not been applied. Nevertheless many clinical researches have shown that LVH itself is an important prognostic predictor for occurrence of cardiovascular diseases and cardiovascular mortality.5),6)
In HBP, it has been explained that the mechanism of LVH is due to hypertrophy of cardiomyocyte caused by the adaptation of increased afterload, but recent studies give an account that abnormal fibrosis in myocardial interstitium plays an important role in LVH.1),7),8) Furthermore, in DM and CRF it has been reported that the increased fibrosis in myocardial interstitium affect LVH.3),4) Fibrosis in myocardial interstitium induces the diastolic dysfunction due to decreased the elasticity of myocardium and results in systolic dysfunction. The histopathological proof by direct biopsy of myocardium is the best method in the existence of myocardial fibrosis, but in real world it is impossible to perform myocardial biopsy in all patients. On the other hand, it has been known that the plasma level of carboxy-terminal propeptide of procollagen type I (PIP) reflecting the synthesis of type I collagen is well correlated with fibrosis in myocardial interstitium.9-11) So the degree of myocardial fibrosis can be estimated by the measurement of the serum PIP level.
As above, abnormally increased myocardial fibrosis plays an important role in the mechanism of LVH, hinder the elasticity of myocardium and systolic function and then decrease blood supply resulting in inducing cardiac dysfunction in all three diseases.
The aims of present study were 1) to analyze the differences in the characteristics of LV hypertrophy, myocardial fibrosis, and LV systolic and diastolic function between HBP, DM and CRF, 2) to search the relation between myocardial fibrosis and LV function in patients with LVH.
Materials and Methods
Study populations
We enrolled consecutive patients with LVH, who underwent transthoracic echocardiography from June 2006 to October 2008 at Kangnam St. Mary's Hospital. LVH was defined as left ventricular mass index >125 g/m2 regardless of gender measured by 2-dimension guided M-mode echocardiography. LV mass was calculated using the formula of Devereux and indexed to body surface area. These patients were evaluated by chart review and history taking, and finally 50 patients with essential hypertension (HBP group), 41 patients with type 2 diabetes mellitus (DM group), and 31 patients with chronic renal failure (CRF group) were included and classified for the study. Age and sex-matched 32 normal persons were also enrolled as control. Inclusion criteria were as below: 1) HBP: patients who had systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg at consecutive 2 or more visits, or who took anti-hypertensive medications already without history of DM or CRF, and their fasting blood sugar and creatinine were within normal limit; 2) DM: patients who had fasting blood glucose ≥126 mg/dL two times or more or who took hypoglycemic drugs already without history of HBP or CRF, and their creatinine was within normal limit; 3) CRF: patients who have creatinine clearance (CCr) <15 mL/min/1.73 m2 by Cockcroft-Gault equation during recent 3 months or longer, or already treated by dialysis. Exclusion criteria were as below: 1) LV ejection fraction <45%; 2) myocardial infarction, ischemic cardiomyopathy or dilated cardiomyopathy; 3) pulmonary hypertension or right heart failure; 4) moderate or severe pericardial effusion; 5) atrial arrhythmia including atrial fibrillation; 6) moderate or severe valvular heart disease; 7) history of valve replacement, open chest surgery or permanent pacemaker insertion; 8) rheumatologic disease; 9) hematologic or oncologic disease; 10) hypertropic cardiomyopathy; 11) infiltrative cardiomyopathy such as amyloidosis; 12) current use of steroid. The institutional medical ethics committee approved the study protocol. All participants gave informed consent to undergo the study.
Echocardiogarphy
Transthoracic echocardiography was performed by using commercially available ultrasound equipments (Acuson Sequoia 512, Siemens Medical, Mountain View, CA, USA; Vivid-7, GE Medical Systems, Milwaukee, WI, USA) for the obtaining 2-dimensional, M-mode, color Doppler imaging and tissue Doppler imaging according to standardized method by American Society of Echocardiography.12-14) All echo indices were calculated by the mean of two measurements. In present study, we measured cardiac echo indices as below: 1) interventricular septum and posterior wall thickness at end-diastole; 2) LV dimension at end-diastole and end-systole; 3) left atrial (LA) dimension at end-systole; 4) LV volume at end-diastole and end-systole; 5) LA volume at end-systole; 6) left ventricular mass index (LVMi); 7) relative wall thickness; 8) transmitral inflow Doppler: early diastolic wave (E), late diastolic wave (A), duration of A (A dur) and decelerating time of E (DT); 9) tissue velocity on septal mitral annulus: systolic (S'), early diastolic (E'), late diastolic (A') velocity; 10) pulmonary venous flow Doppler: duration of reverse A wave (Ar dur); 11) indices of diastolic function: E', E/E', DT, Ar dur-A dur, left atrial volume index (LAVi); 12) indices of systolic function: LV ejection fraction (LVEF), S', midwall fractional shortening (FS midwall).
Measurement of serum PIP
Venous blood was sampled with minimal stimulus in all participants for the measuring of serum PIP level in all participants. And then serum was extracted by centrifuge (2,000 G, 10 minutes) and kept at -70℃ refrigerator until quantitative analysis. Level of PIP in serum was quantified by precoated type I step sandwich enzyme immunoassay (measurement range: 10-640 ng/mL, detection sensitivity: 10 ng/mL; Takara bio, Shiga, Japan) using monoclonal anti-human procollagen type I. 9-11),15)
Statistical analysis
Continuous data are expressed as mean±SD. Data of 4 groups were compared by ANOVA and post-hoc analysis (Turkey-b). The correlation of plasma level of PICP and diastolic or systolic functional echo indices was analyzed by Pearson correlation. For all analyses, a two-sided p<0.05 was considered statistically significant. Statistical analysis was performed with SPSS (version 11.5 for window, SPSS, Inc., Chicago, IL, USA).
Results
Clinical baseline characteristics of study population
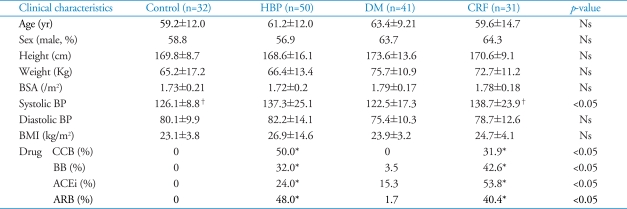
Table 1 shows clinical baseline characteristics of four groups. There was no significant difference in age, ratio of male gender, body surface area and body mass index among four groups. In HBP and CRF groups, systolic blood pressure (SBP) was higher than in DM and normal groups (HBP 137.3 mmHg vs. DM 122.5 mmHg vs. CRF 138.7 mmHg vs. normal 126.1 mmHg, p<0.05), but in case of diastolic blood pressure (DBP) did not show significant differences among four groups. Antihypertensive agents were prescribed optimally in all patients with HBP and CRF. In DM group, blood sugar level was well controlled by oral hyperglycemic agent.
Table 1.
Baseline characteristics of study population
*p<0.05 versus control and diabetes. Ns: non-specific, HBP: hypertension, DM: diabetes, CRF: chronic renal failure, BSA: body surface area, BP: blood pressure, BMI: body mass index, CCB: calcium channel blocker, BB: beta blocker, ACEi: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blocker
Cardiac indices by echocardiography
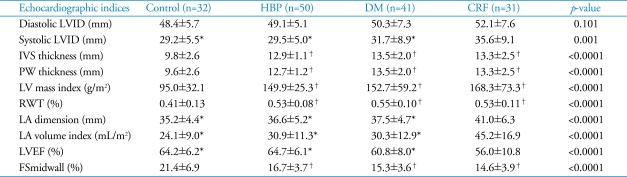
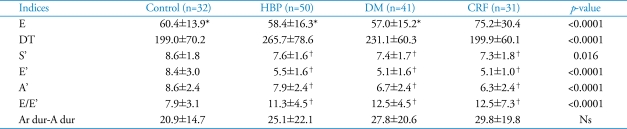
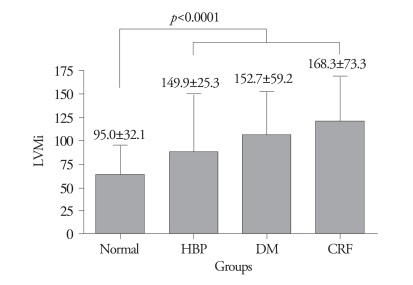
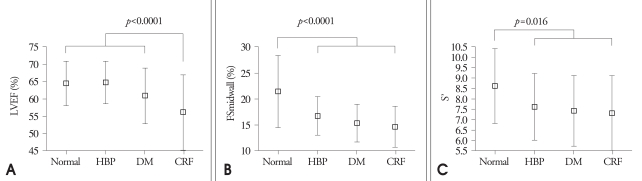
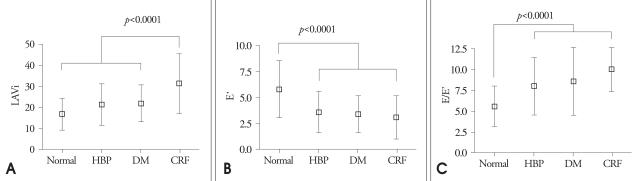
The results of 2-dimensional and M-mode echocardiography were manifested in Table 2 and Table 3. In CRF group, end-systolic LV internal dimension, LA dimension, LAVi were larger than other three groups (p=0.001, p<0.0001, p<0.0001, respectively) (Fig. 1). Interventricular septum, LV posterior wall, relative wall thickness and LV mass index of 3 disease groups (HBP, DM, CRF) were significantly larger than control (p<0.0001, all). LV systolic function evaluated by systolic mitral annular tissue velocity (S') and midwall fractional shortening (FSmidwall) were significantly decreased in 3 disease groups than control. However, there were no differences in S' and FSmidwall between 3 disease groups. LV ejection fraction, a load dependent marker of LV contraction, was significantly decreased in CRF compared with HBP, DM and control (Fig. 2). LV diastolic function evaluated by E' and E/E' was significantly decreased in 3 disease groups than control. However, there were no differences in E' and E/E between 3 disease groups. LAVi, an index of chronic diastolic load, was significantly elevated in CRF compared to others (Fig. 3). Ar dur-A dur, an index of LV diastolic compliance, was not different among 4 groups.
Table 2.
M-mode and 2-dimentional echocardiographic indices
*p≤0.001 versus chronic renal failure, †p<0.0001 versus control. LVID: left ventricular internal dimension, IVS: interventricular septum, PW: posterior wall, LV: left ventricle, RWT: relative wall thickness, LA: left atrium, LVEF: left ventricular ejection fraction, FS: fractional shortening
Table 3.
Transmitral inflow Doppler and tissue Doppler indices by echocardiography
*p<0.0001 versus chronic renal failure, †p<0.05 versus control. Ns: non-specific, DT: decelerating time, dur: duration
Fig. 1.
Left ventricular mass index (LVMi) by M-mode among four groups. HBP: hypertension, DM: diabetes, CRF: chronic renal failure.
Fig. 2.
Indices of systolic function among four group. A: Left ventricular ejection fraction (LVEF) among four groups: in CRF groups, LVEF was statistically decreased. B: Fractional shortening midwall (FSmidwall) among four groups: in HBP, DM and CRF group, FSmidwall was significantly decreased. C: S' among four groups: in HBP, DM and CRF group, S' was significantly decreased. CRF: chronic renal failure, HBP: hypertension, DM: diabetes.
Fig. 3.
Indices of diastolic function among four group. A: Left atiral volume index (LAVi) among four groups: in CRF group, LAVi was significantly increased. B: E' among four groups: in HBP, DM and CRF group, E' was significantly decreased. C: E/E' among four groups: in HBP, DM and CRF group, E/E' was significantly increased. CRF: chronic renal failure, HBP: hypertension, DM: diabetes.
The serum level of PIP
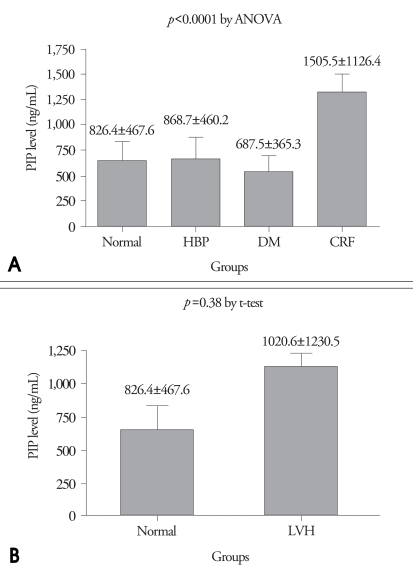
Fig. 4 shows the serum level of PIP among 4 groups. In CRF group, the serum PIP was significantly higher than other 3 groups (CRF 1505.5 ng/mL vs. HBP 868.7 ng/mL vs. DM 687.5 ng/mL vs. normal 826.4 ng/mL, p<0.0001). However, when compared all patients with LVH with control, there was no significant difference in serum PIP.
Fig. 4.
A: Serum level of Carboxy-terminal propeptide of procollagen type I (PIP) among four groups: in CRF group, serum PIP level was significantly higher than other three groups. B: Serum level of PIP between patients with LVH and normal group. HBP: hypertension, DM: diabetes, CRF: chronic renal failure.
Correlation between the serum PIP and systolic function or diastolic function
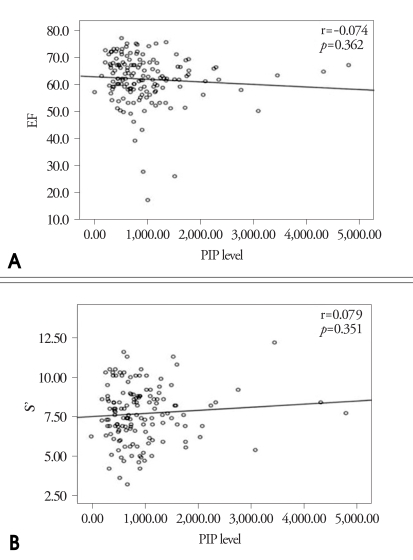
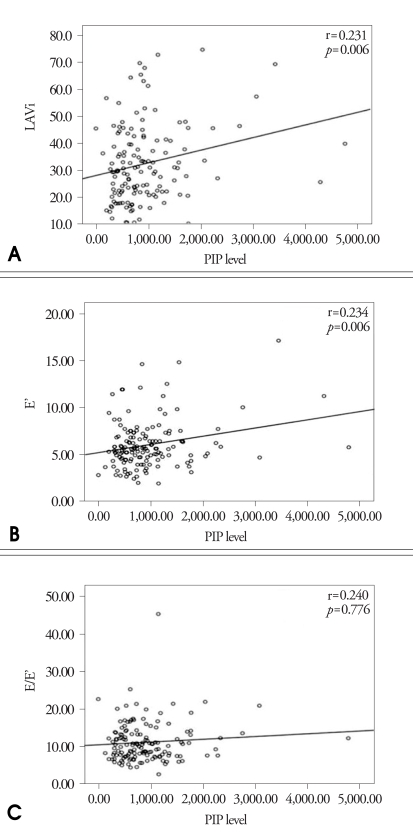
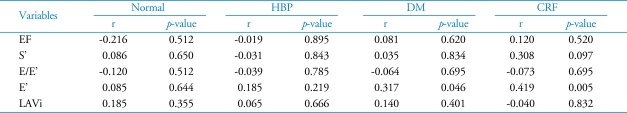
The correlation of serum PIP and the index of LV systolic function such as LVEF and S' are expressed in Fig. 5. There was no significant correlation between serum PIP and LV systolic function. Fig. 6 shows the correlation between serum PIP and the index of LV diastolic function such as LAVi, E' and E/E'. There were weakly positive correlations between serum PIP and E' or LAVi (r=0.234, p=0.006; r=0.231, p=0.006, respectively). But there was no significant correlation between serum PIP and E/E'. In the analysis of each group, only E' was statistically correlated with PIP level in patients with DM and CRF (Table 4).
Fig. 5.
Correlation between Serum Carboxy-terminal propeptide of procollagen type I (PIP) level and index of systolic function. A: Correlation between serum PIP level and LVEF. B: Correlation between serum PIP level and S'.
Fig. 6.
Correlation between serum Carboxy-terminal propeptide of procollagen type I (PIP) level and index of diastolic function. A: Correlation between serum PIP level and LAVi. B: Correlation between serum PIP level and E'. C: Correlation between serum PIP level and E/E'.
Table 4.
Correlation between Carboxy-terminal propeptide of procollagen type I (PIP) and variable echocardiographic indices according to groups
r: correlation coefficient. HBP: hypertension, DM: diabetes, CRF: chronic renal failure
Discussion
In present study, the shape of LVH in HBP, DM and CRF was all concentric, degree of LVH was not different each other. In the results of this study, decreased LV diastolic function was noted in all 3 disease groups. Especially, LA volume index, a result of chronic load of diastolic dysfunction was larger only in CRF. It means that CRF has worst diastolic function among 3 disease groups. LV systolic function represented as S' and FSmidwall was markedly decreased in 3 disease groups. In addition, LVEF was significantly decreased in CRF group than other 3 groups. It means that CRF have worst systolic function among 3 disease groups. The serum PIP which was known to reflect the state of myocardial fibrosis well9-11) was much higher in CRF group in spite of optimal anti-hypertensive agents compared with other three groups. It suggests that myocardial fibrosis may be related to LV dysfunction as well as the mechanism of LVH in CRF in some degree. In other words, myocardial fibrosis may be more excessive and may induce diastolic and systolic dysfunction in CRF although the degree of LVH in CRF is similar to HBP or DM.
About the development of LVH, several mechanisms are suggested. In HBP, arteriosclerosis of large arteries and increased resistance of muscular arterioles induce elevated afterload to heart and finally hypertrophy of cardiomyocytes develops for the maintaining the tension of ventricular wall.16) It has been so called Laplace's law. In case of DM, non-enzymatic glycosylation plays a key role for LVH. It provokes the changes of chemical composition of proteins in cardiomyocytes and then the structure and function of cells are changed. This process induces the obstacle of calcium delivery, the suppression of myocardial systolic proteins and the inhibition of the metabolism of fatty acid, so due to these phenomena abnormal cardiomyocytes hypertrophy may progresses.17),18) In another theory, advanced glycation end-product (AGE) links with myocardial collagen and extracellular proteins, and then the combination products deposit in tissue. These deposited combination product stimulate tissue growth factor and transforming growth factor-beta1 (TGF-β1). Finally, this process induce cardiomyoctye hypertrophy and fibroblast hypertrophy resulting in myocardial fibrosis.19),20) In CRF, it has been explained that the causes of LVH are pressure overload due to HBP and volume overload of retained intravascular fluid and anemia.3),21),22) As above, it has been known that the mechanism of LVH is different among three diseases, but recently it has been established that myocardial fibrosis is common mechanism.1),7),8),17-20),23) However, it has not been known in which disease the degree of myocardial fibrosis is more excessive, the present study suggests that myocardial fibrosis in CRF may be more severe than HBP or DM in view of increased serum PIP level. Also serum PIP is known to be excreted not by kidney, but by liver,11) and may reflect myocardial fibrosis in CRF regardless of creatinine level.
Myocardial fibrosis is more prominent due to pressure overload than volume overload. Also myocardial fibrosis is associated with aging, ischemia, catecholamine, angiotensin II, aldosterone, TGF and inflammatory cell and it has been suggested that inflammatory mediated substances such as interleukin-6, monoctye chemoattractant protein-1 induce inflammatory process and affect myocardial fibrosis.3),24),25) In view of above mechanisms, severe inflammatory reaction due to uremia may enhance severe myocardial fibrosis in some degree. But, in a few articles, elevated PIP in patients with CRF might be a biomarker of bone metabolism.26),27) On the other hand, it was published that elevated PIP was not correlated with classic bone turnover markers such as intact parathyroid hormone, osteocalcin and alkaline phosphatase,27) and bone mineral density.28) In other words, it should not be interpreted that elevated PIP level was due to only bone turnover or only myocardial fibrosis. So elevated PIP level in patients with CRF may be resulted from a few causes such as myocardial fibrosis or bone metabolism.
However, in Pearson correlation, serum PIP level was not directly correlated with the indices of systolic function such as LVEF and S'. The causes of these results may be the feature of selected patients with preserved left ventricular systolic function. In case of diastolic function, there was weakly positive correlation between serum PIP level and the indices of diastolic function such as LAVi or E' but not E/E'. Furthermore, in view of the analysis according to each group, only E' was statistically correlated with PIP level in patients with DM and CRF. These results may be caused by the examination time, so to speak most patients are not diagnosed initially, but already have been treated optimally according to disease. These phenomena are also observed in other study.29) Also in a few studies, LV mass, diastolic function and systolic function were improved after optimal antihypertensive treatment in patients with HBP,30),31) after kidney transplantation in patients with dialysis.32) In other words, if echocardiographic examination and PIP level measuring was performed at diagnosis as HBP, DM and CRF initially, there might be the potentiality that PIP level might be correlated with echocardiographic indices. In the present study, echocardiographic examination and blood sampling time was very various according to each patient, so it might reflect these results.
In summary, LV systolic and diastolic function was more deteriorated and serum PIP was more elevated in CRF. It may suggest that myocardial fibrosis may play an important role to LV dysfunction as well as LV hypertrophy in CRF in some degree.
There are a few limitations in the present study. First, the present study is not prospective random trial but cross-section study, so patients who were newly diagnosed as HBP, DM or CRF were not selected but have been treated optimally. Due to these selections, serum PIP level was not differently in patients with HBP, DM compared with normal population. Second, the follow up measurements of echocardiography and serum PIP level were not performed regularly, so the improvement of cardiac function and serum PIP level was not grasped. In future, the study that regular follow up measurement of echocardiography and serum PIP level is performed and the change of indices of variable echocardiographic findings and serum PIP level are analyzed will need.
Acknowledgements
The study was supported by the industry and academy collaboratory research fund 2006 of Korean Society of Echocardiography.
References
- 1.Díez J, González A, López B, Querejeta R. Mechanisms of disease: pathologic structural remodeling is more than adaptive hypertrophy in hypertensive heart disease. Nat Clin Pract Cardiovasc Med. 2005;2:209–216. doi: 10.1038/ncpcardio0158. [DOI] [PubMed] [Google Scholar]
- 2.Devereux RB, Roman MJ, Paranicas M, O'Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Howard BV. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000;101:2271–2276. doi: 10.1161/01.cir.101.19.2271. [DOI] [PubMed] [Google Scholar]
- 3.Guerin AP, Adda H, London GM, Marchais SJ. Cardiovascular disease in renal failure. Minerva Urol Nefrol. 2004;56:279–288. [PubMed] [Google Scholar]
- 4.Aurigemma GP, Silver KH, Priest MA, Gaasch WH. Geometric changes allow normal ejection fraction despite depressed myocardial shortening in hypertensive left ventricular hypertrophy. J Am Coll Cardiol. 1995;26:195–202. doi: 10.1016/0735-1097(95)00153-q. [DOI] [PubMed] [Google Scholar]
- 5.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 6.Casale PN, Devereux RB, Milner M, Zullo G, Harshfield GA, Pickering TG, Laragh JH. Value of echocardiographic measurement of left ventricular mass in predicting cardiovascular morbid events in hypertensive men. Ann Intern Med. 1986;105:173–178. doi: 10.7326/0003-4819-105-2-173. [DOI] [PubMed] [Google Scholar]
- 7.Rossi MA. Pathologic fibrosis and connective tissue matrix in left ventricular hypertrophy due to chronic arterial hypertension in humans. J Hypertens. 1998;16:1031–1041. doi: 10.1097/00004872-199816070-00018. [DOI] [PubMed] [Google Scholar]
- 8.Ciulla M, Paliotti R, Hess DB, Tjahja E, Campbell SE, Magrini F, Weber KT. Echocardiographic patterns of myocardial fibrosis in hypertensive patients: endomyocardial biopsy versus ultrasonic tissue characterizatien. J Am Soc Echocardiogr. 1997;10:657–664. doi: 10.1016/s0894-7317(97)70028-2. [DOI] [PubMed] [Google Scholar]
- 9.Querejeta R, Varo N, López B, Larman M, Artiñano E, Etayo JC, Martínez Ubago JL, Gutierrez-Stampa M, Emparanza JI, Gil MJ, Monreal I, Mindán JP, Díez J. Serum cardoxy-terminal propeptide of procollagen type I is a marker of myocardial fibrosis in hypertensive heart disease. Circulation. 2000;101:1729–1735. doi: 10.1161/01.cir.101.14.1729. [DOI] [PubMed] [Google Scholar]
- 10.Díez J, Panizo A, Gil MJ, Monreal I, Hernández M, Pardo Mindán J. Serum markers of collagen type I metabolism in spontaneously hypertensive rats: relation to myocardial fibrosis. Circulation. 1996;93:1026–1032. doi: 10.1161/01.cir.93.5.1026. [DOI] [PubMed] [Google Scholar]
- 11.López B, Querejeta R, Varo N, González A, Larman M, Martínez Ubago JL, Díez J. Usefulness of serum carboxy-terminal propeptide of procollagen type I in assessment of the cardioreparative ability of antihypertensive treatment in hypertensive patients. Circulation. 2001;104:286–291. doi: 10.1161/01.cir.104.3.286. [DOI] [PubMed] [Google Scholar]
- 12.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 13.Schiller NB, Shah PM, Crawford M, Demaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 14.Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence or absence of asynergy. Am J Cardiol. 1976;37:7–11. doi: 10.1016/0002-9149(76)90491-4. [DOI] [PubMed] [Google Scholar]
- 15.Ihm SH, Youn HJ, Kim SR, Park CS, Chang KY, Seung KB, Kim JH, Hong SJ, Choi KB. Relation between serum carboxy-terminal propeptide of type 1 procollagen (PIP), a marker of myocardial fibrosis, and left ventricular diastolic function in patients with early type 2 diabetes mellitus. Korean Circulation J. 2005;35:500–506. [Google Scholar]
- 16.Koren MJ, Devereux RB. Mechanism, effects, and reversal of left ventricular hypertrophy in hypertension. Curr Opin Nephrol Hypertens. 1993;2:87–95. doi: 10.1097/00041552-199301000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes: part I: general concepts. Circulation. 2002;105:1727–1733. doi: 10.1161/01.cir.0000012466.50373.e8. [DOI] [PubMed] [Google Scholar]
- 18.Young ME, McNulty P, Taegtmeyer H. Adaptation and maladaptation of the heart in diabetes: part II: potential mechanisms. Circulation. 2002;105:1861–1870. doi: 10.1161/01.cir.0000012467.61045.87. [DOI] [PubMed] [Google Scholar]
- 19.Candido R, Forbes JM, Thomas MC, Thallas V, Dean RG, Burns WC, Tikellis C, Ritchie RH, Twigg SM, Cooper ME, Burrell LM. A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes. Circ Res. 2003;92:785–792. doi: 10.1161/01.RES.0000065620.39919.20. [DOI] [PubMed] [Google Scholar]
- 20.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 21.Ha SK, Park HS, Kim SJ, Park CH, Kim DS, Kim HS. Prevalence and patterns of left ventricular hypertrophy in patients with predialysis chronic renal failure. J Korean Med Sci. 1998;13:488–494. doi: 10.3346/jkms.1998.13.5.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ulasi II, Arodiwe EB, Ijoma CK. Left ventricular hypertrophy in African Black patients with chronic renal failure at first evaluation. Ethn Dis. 2006;16:859–864. [PubMed] [Google Scholar]
- 23.Vlahakos DV, Hahalis G, Vassilakos P, Marathias KP, Geroulanos S. Relationship between left ventricular hypertrophy and plasma renin activity in chronic hemodialysis patients. J Am Soc Nephrol. 1997;8:1764–1770. doi: 10.1681/ASN.V8111764. [DOI] [PubMed] [Google Scholar]
- 24.Weber KT, Brilla CG, Campbell SE, Zhou G, Matsubara L, Guarda E. Pathologic hypertrophy with fibrosis: the structural basis for myocardial failure. Blood Press. 1992;1:75–85. doi: 10.3109/08037059209077497. [DOI] [PubMed] [Google Scholar]
- 25.Nicoletti A, Michel JB. Cardiac fibrosis and inflammation: interaction with hemodynamic and hormonal factors. Cardiovasc Res. 1999;41:532–543. doi: 10.1016/s0008-6363(98)00305-8. [DOI] [PubMed] [Google Scholar]
- 26.Rix M, Andreassen H, Eskildsen P, Langdahl B, Olgaard K. Bone mineral density and biochemical markers of bone turnover in patients with predialysis chronic renal failure. Kidney Int. 1999;56:1084–1093. doi: 10.1046/j.1523-1755.1999.00617.x. [DOI] [PubMed] [Google Scholar]
- 27.Coen G, Mazzaferro S, Ballanti P, Bonucci E, Bondatti F, Manni M, Pasquali M, Perruzza I, Sardella D, Spurio A. Procollagen type I C-terminal extension peptide in predialysis chronic renal failure. Am J Nephrol. 1992;12:246–251. doi: 10.1159/000168453. [DOI] [PubMed] [Google Scholar]
- 28.Ha SK, Park CH, Seo JK, Park SH, Kang SW, Choi KH, Lee HY, Han DS. Studies on bone markers and bone mineral density in patients with chronic renal failure. Yonsei Med J. 1996;37:350–356. doi: 10.3349/ymj.1996.37.5.350. [DOI] [PubMed] [Google Scholar]
- 29.Yoon SJ, Ha JW, Lee SH, Cho DK, Choi S, Seo HS, Hwang HJ, Choi EY, Chung N. Fibrillar collagen turnover index - The clinical importance in the assessment of ventricular hypertrophy and diastolic function in hypertensive patients. Korean Hypertension J. 2007;13:32–40. [Google Scholar]
- 30.Cheong ER, Chae SC, Jun JE, Park WH. LV mass and left ventricular systolic function after antihypertensive therapy. J Kor Soc Echo. 1994;2:187–191. [Google Scholar]
- 31.Son KH, Lee KN, Kang HS, Choue CW, Kim KS, Kim MS, Song JS, Bae JH. Assessment of left ventricular mass and diastolic function in patients with essential hypertension after one year antihypertensive therapy. J Kor Soc Echo. 1994;2:71–79. [Google Scholar]
- 32.Lee H, Lee S, Lee C, Park K, Choi Y, Shin G, Cho HK, Park S. Effects of renal transplantation on echocardiographic changes. J Kor Soc Echo. 2000;8:31–35. [Google Scholar]