Abstract
The humoral immune response of cats that were naturally infected with the feline leukemia virus (FeLV) was examined after antigenic stimulation with the synthetic antigen poly(L-Tyr, L-Glu)-poly(DL-Ala)-poly(L-Lys). The primary humoral antibody response in FeLV-infected cats was both delayed and greatly reduced, compared with that seen in uninfected control cats. A similar discordance was observed after secondary stimulation with the antigen, in the FeLV-infected cats had both a delayed response and a reduced response, compared with uninfected cats. The levels of total immunoglobulins of the immunoglobulin G and immunoglobulin M classes in the sera of FeLV-infected cats were significantly higher (two- and threefold, respectively) than were those of the uninfected control animals. The presence of an impaired humoral immune response to newly presented antigens in the presence of elevated immunoglobulin levels has been thoroughly documented in the case of people with the acquired immunodeficiency syndrome. This further emphasizes the potential value of FeLV-infected cats as a model for human acquired immunodeficiency syndrome.
Full text
PDF
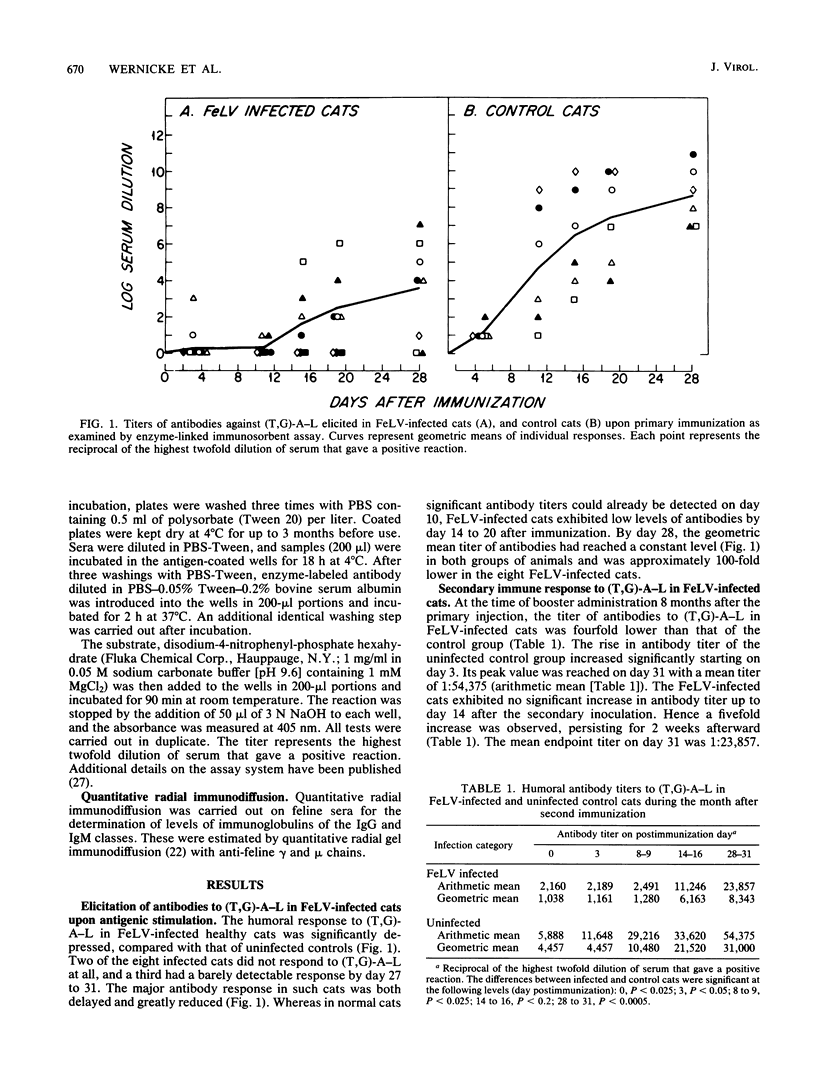
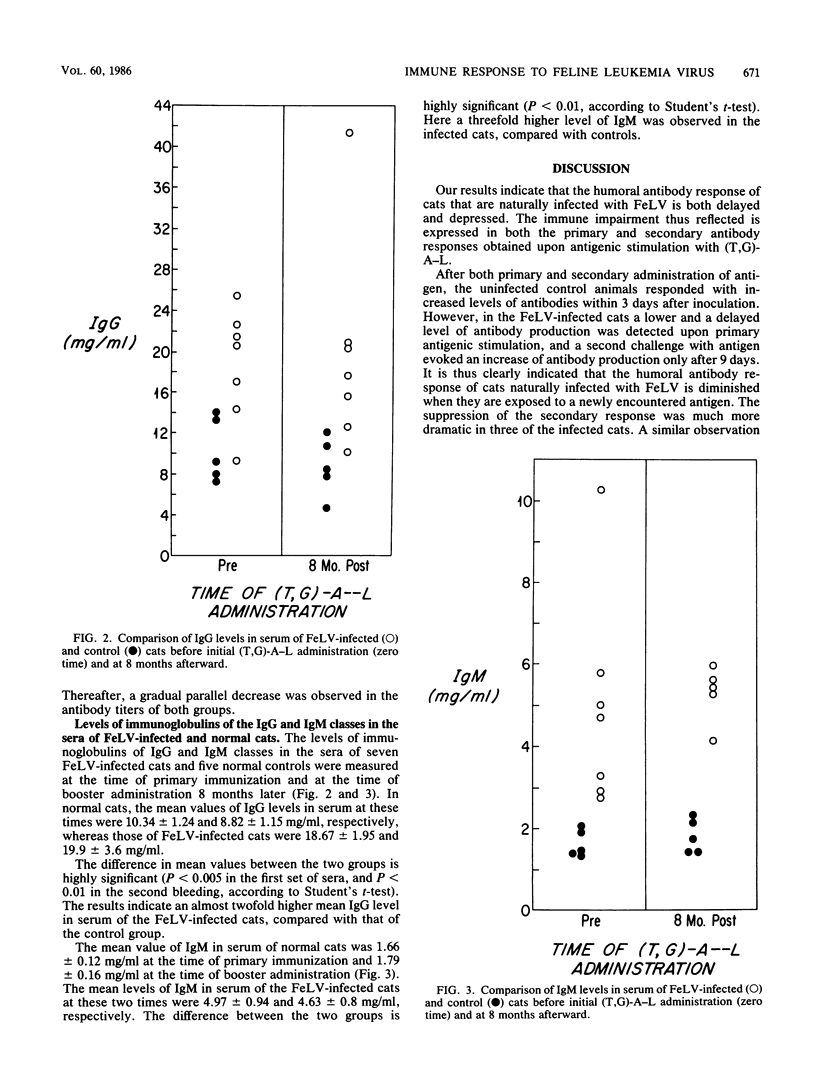


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. J., Jarrett W. F., Jarrett O., Laird H. M. Feline leukemia-virus infection of kittens: mortality associated with atrophy of the thymus and lymphoid depletion. J Natl Cancer Inst. 1971 Oct;47(4):807–817. [PubMed] [Google Scholar]
- Cianciolo G. J., Copeland T. D., Oroszlan S., Snyderman R. Inhibition of lymphocyte proliferation by a synthetic peptide homologous to retroviral envelope proteins. Science. 1985 Oct 25;230(4724):453–455. doi: 10.1126/science.2996136. [DOI] [PubMed] [Google Scholar]
- Cockerell G. L., Krakowka S., Hoover E. A., Olsen R. G., Yohn D. S. Characterization of feline T-and B-lymphocytes and identification of an experimentally induced T-cell neoplasm in the cat. J Natl Cancer Inst. 1976 Oct;57(4):907–913. doi: 10.1093/jnci/57.4.907. [DOI] [PubMed] [Google Scholar]
- Cotter S. M., Hardy W. D., Jr, Essex M. Association of feline leukemia virus with lymphosarcoma and other disorders in the cat. J Am Vet Med Assoc. 1975 Mar 1;166(5):449–454. [PubMed] [Google Scholar]
- Dent P. B. Immunodepression by oncogenic viruses. Prog Med Virol. 1972;14:1–35. [PubMed] [Google Scholar]
- Dunlap J. E., Nichols W. S., Hebebrand L. C., Mathes L. E., Olsen R. G. Mobility of lymphocyte surface membrane concanavalin A receptors of normal and feline leukemia virus-infected viremic felines. Cancer Res. 1979 Mar;39(3):956–958. [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Essex M., Hardy W. D., Jr, Cotter S. M., Jakowski R. M., Sliski A. Naturally occurring persistent feline oncornavirus infections in the absence of disease. Infect Immun. 1975 Mar;11(3):470–475. doi: 10.1128/iai.11.3.470-475.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex M. Horizontally and vertically transmitted oncornaviruses of cats. Adv Cancer Res. 1975;21:175–248. doi: 10.1016/s0065-230x(08)60973-2. [DOI] [PubMed] [Google Scholar]
- Francis D. P., Essex M., Jakowski R. M., Cotter S. M., Lerer T. J., Hardy W. D., Jr Increased risk for lymphoma and glomerulonephritis in a closed population of cats exposed to feline leukemia virus. Am J Epidemiol. 1980 Mar;111(3):337–346. doi: 10.1093/oxfordjournals.aje.a112905. [DOI] [PubMed] [Google Scholar]
- Hardy W. D., Jr Naturally occurring retroviruses (RNA tumor viruses). II. Cancer Invest. 1983;1(2):163–174. doi: 10.3109/07357908309042418. [DOI] [PubMed] [Google Scholar]
- Hardy W. D., Jr, Old L. J., Hess P. W., Essex M., Cotter S. Horizontal transmission of feline leukaemia virus. Nature. 1973 Aug 3;244(5414):266–269. doi: 10.1038/244266a0. [DOI] [PubMed] [Google Scholar]
- Hardy W. D., Jr, Zuckerman E. E., MacEwen E. G., Hayes A. A., Essex M. A feline leukaemia virus- and sarcoma virus-induced tumour-specific antigen. Nature. 1977 Nov 17;270(5634):249–251. doi: 10.1038/270249a0. [DOI] [PubMed] [Google Scholar]
- Hoover E. A., Kociba G. J., Hardy W. D., Jr, Yohn D. S. Erythroid hypoplasia in cats inoculated with feline leukemia virus. J Natl Cancer Inst. 1974 Nov;53(5):1271–1276. doi: 10.1093/jnci/53.5.1271. [DOI] [PubMed] [Google Scholar]
- Hoover E. A., Perryman L. E., Kociba G. J. Early lesions in cats inoculated with feline leukemia virus. Cancer Res. 1973 Jan;33(1):145–152. [PubMed] [Google Scholar]
- Lane H. C., Masur H., Edgar L. C., Whalen G., Rook A. H., Fauci A. S. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983 Aug 25;309(8):453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mathes L. E., Olsen R. G., Hebebrand L. C., Hoover E. A., Schaller J. P., Adams P. W., Nichols W. S. Immunosuppressive properties of a virion polypeptide, a 15,000-dalton protein, from feline leukemia virus. Cancer Res. 1979 Mar;39(3):950–955. [PubMed] [Google Scholar]
- Mozes E., Haimovich J. Antigen specific T-cell helper factor cross reacts idiotypically with antibodies of the same specificity. Nature. 1979 Mar 1;278(5699):56–57. doi: 10.1038/278056a0. [DOI] [PubMed] [Google Scholar]
- Perryman L. E., Hoover E. A., Yohn D. S. Immunologic reactivity of the cat: immunosuppression in experimental feline leukemia. J Natl Cancer Inst. 1972 Nov;49(5):1357–1365. [PubMed] [Google Scholar]
- SELA M., FUCHS S., ARNON R. Studies on the chemical basis of the antigenicity of proteins. 5. Synthesis, characterization and immunogenicity of some multichain and linear polypeptides containing tyrosine. Biochem J. 1962 Oct;85:223–235. doi: 10.1042/bj0850223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainin Z., Wernicke D., Ungar-Waron H., Essex M. Suppression of the humoral antibody response in natural retrovirus infections. Science. 1983 May 20;220(4599):858–859. doi: 10.1126/science.6302837. [DOI] [PubMed] [Google Scholar]
- Weijer K., Calafat J., Daams J. H., Hageman P. C., Misdorp W. Feline malignant mammary tumors. II. Immunologic and electron microscopic investigations into a possible viral etiology. J Natl Cancer Inst. 1974 Mar;52(3):673–679. doi: 10.1093/jnci/52.3.673. [DOI] [PubMed] [Google Scholar]



