Abstract
We test the hypothesis that cat jaw satellite cells belong to a distinct lineage preprogrammed to express masticatory-specific isoforms of myosin heavy-chain (m-MyHC), myosin-binding protein-C (m-MBP-C), and tropomyosin (m-Tm) during myogenesis in vitro. A monoclonal antibody (MAb) against m-MyHC and MAbs raised here against cat m-MBP-C and m-Tm were used to stain cryostat sections of cat masseter muscle and cultured myotubes derived from satellite cells of cat temporalis and limb muscles, using peroxidase immunohistochemistry. MAbs against m-MBP-C bound purified m-MBP-C in Western blots. MAbs against m-Tm failed to react with m-Tm in Western blots, but reacted with native m-Tm in gel electrophoresis–derived ELISA. In cat masseter sections, MAbs against m-MyHC, m-MBP-C, and m-Tm stained all masticatory fibers, but not the jaw-slow fibers. Cat jaw and limb muscle cultures mature significantly more slowly relative to rodent cultures. However, at 3 weeks, all three MAbs extensively stained temporalis myotubes, whereas they apparently stained isolated myotubes weakly in cat limb and rat jaw cultures. We conclude that satellite cells of masticatory fibers are preprogrammed to express these isoforms during myogenesis in vitro. These results consolidate the notion that masticatory and limb muscle allotypes are distinct. (J Histochem Cytochem 58:623–634, 2010)
Keywords: masticatory muscle, myogenesis, muscle allotype, satellite cell, tissue culture, cat, myosin heavy chain, myosin-binding protein-C, tropomyosin
Jaw-closing muscles of mammals are very different from muscles of the limb and trunk in fiber-type composition and function (Hoh 2002). There are up to four fiber types in mammalian limb muscles, each expressing a different myosin isoform that finely tunes the mechanics and energetics of these fiber types for posture and locomotion. Functional demands on jaw-closing muscles are more complex and varied, and their fiber types show considerable variation from a phylogenetic perspective. These muscles have a repertoire for myosin expression that includes isoforms found in limb, developmental, and cardiac muscles, including a jaw-specific isoform, masticatory myosin.
Masticatory or “superfast” myosin is a phylogenetically ancient motor protein with myosin heavy chains (MyHCs) (Rowlerson et al. 1981; Qin et al. 2002) and light chains (Rowlerson et al. 1981; Qin et al. 1994; Hoh et al. 2007b) distinct from those in limb muscles. Masticatory MyHC (m-MyHC) and masticatory light-chain-2 are regarded as distinct subclasses of MyHC and myosin light-chain-2, respectively, on the basis of their low homologies with other sarcomeric myosin subunits (Qin et al. 1994,2002). Masticatory myosin is found in carnivorous lower vertebrates (e.g., shark, crocodile) and in members of several orders of eutherian and marsupial mammals, including carnivores, chiropterans, primates, rodents, dasyurids, and diprotodonts (Rowlerson et al. 1983b; Hoh 2002; Reiser et al. 2009). Fibers expressing m-MyHC are associated with high force of contraction (Saeki et al. 1987; Toniolo et al. 2008), a property useful to carnivores for predation and to animals having to deal with tough vegetable matter and nuts. Masticatory fibers are also associated with high Ca sensitivity of tension development (Kato et al. 1985) and moderate cross-bridge cycling rate and speed of contraction (Hoh et al. 2007b; Toniolo et al. 2008).
Cat limb muscles have two types of fast fiber (2x, 2a) and one slow fiber type, which, respectively, express 2X, 2A, and slow MyHC (Lucas et al. 2000). Cat jaw muscles have two types of fibers: masticatory fibers that express m-MyHC (Rowlerson et al. 1981; Hoh et al. 1988a; Qin et al. 2002), and jaw-slow fibers that express slow MyHC associated with masticatory light chains (Hoh and Hughes 1989; Sciote et al. 1995; Hoh et al. 2007b). When cat masticatory muscle is transplanted into a limb fast muscle bed and allowed to regenerate and to be reinnervated by the limb fast muscle nerve, the regenerated muscle expresses m-MyHC (Hoh and Hughes 1988). When masticatory muscle regenerates in a slow muscle bed and is reinnervated by the slow muscle nerve, m-MyHC is initially expressed, but in the long term, most muscle fibers express slow MyHC (Hoh and Hughes 1988). The capacity of cat jaw muscle regenerates to express masticatory MyHC is independent of innervation (Hoh and Hughes 1991b) and the basal lamina (Hoh and Hughes 1991a) of the transplanted jaw muscle. These studies suggest that cat jaw muscle belongs to a different muscle allotype from that of limb muscles, implying that myogenic cells of jaw muscle are preprogrammed to express m-MyHC during myogenesis. To test this hypothesis more rigorously, it is now important to rule out possible roles of humoral and cellular factors present in vivo by demonstrating that jaw muscle satellite cells in culture have the capacity to express m-MyHC, and to ask if these cultured cells would also express other masticatory muscle-specific myofibrillar proteins as well.
Masticatory fibers in cat jaw-closing muscles also express a jaw-specific isoform of tropomyosin (Tm), referred to here as masticatory Tm (m-Tm) (Rowlerson et al. 1983a; Hoh et al. 1989). In contrast, limb fast and slow fibers each express two isoforms of Tm; fast fibers express αf-Tm and a β-Tm, whereas slow fibers express αs-Tm and β-Tm, in common with fast fibers (Hoh 1992). Recent work on dog masticatory fibers has shown the existence of a jaw-specific isoform of myosin-binding protein-C (MBP-C) or m-MBP-C (Wu et al. 2007). MBP-C is a thick filament protein that modulates cross-bridge function (Hofmann et al. 1991a), and exists as distinct isoforms in limb fast, limb slow, and cardiac muscles (Dhoot et al. 1985). The presence of m-MBP-C in cat masticatory fibers has not been demonstrated. Knowing the complement of myofibrillar proteins in cat masticatory ffibers would greatly help in understanding their special mechanical properties.
Earlier work using a polyclonal antibody raised against cat masticatory MyHC failed to demonstrate the expression of m-MyHC in cat jaw muscle cultures, but revealed that they express fetal (embryonic and neonatal) and slow MyHCs (Hughes and Hoh 1985). In the present work, we raised specific monoclonal antibodies (MAbs) against cat m-MBP-C and m-Tm, and used them, together with an MAb against m-MyHC (Kang et al. 1994; Hoh et al. 2006), to study the expression of these myofibrillar proteins in adult cat masticatory muscle, and in primary cultures of cat jaw and limb fast and slow muscle satellite cells. We show that these jaw-specific myofibrillar proteins are coexpressed in masticatory fibers, but not jaw-slow fibers, of adult jaw muscle, and that satellite cells of cat masticatory muscle are preprogrammed to express these jaw-specific myofibrillar protein isoforms during their myogenic progression in vitro. This work strongly supports the notion that cat jaw and limb muscles belong to different allotypes. Brief reports on this topic have appeared previously (Kang et al. 1991,1992; Hoh et al. 1993).
Materials and Methods
Development of Monoclonal Antibodies Against Cat m-MBP-C and m-Tm
Clone 3F10 against cat m-MBP-C was isolated from hybridomas resulting from immunizing Balb/c mice with crude m-MyHC as described previously (Kang et al. 1994). Clone 1H2 against m-Tm was isolated following in vitro immunization of spleen cells of Balb/c mice (Pardue et al. 1983) with native m-Tm prepared from temporalis muscle by the method of Smillie (1982), except that the final column chromatographic step was omitted. Hybridoma supernatants were initially screened using fluorescence immunohistochemistry on cryostat sections of a cat muscle tissue block comprising the following muscles: masseter, extensor digitorum longus (EDL, limb fast), and soleus (limb slow) muscles. A fluorescein-labeled rabbit anti-mouse immunoglobulin (Dako Corp.; Carpinteria, CA) was used as a secondary antibody, and the results were viewed with a Zeiss Axioplan microscope (Oberkochen, West Germany) equipped with epifluoresence optics. Supernatants of clones that selectively stained fibers in masseter but not limb muscles were further characterized by Western blotting or gel electrophoresis–derived ELISA (GEDELISA). The anti–m-MBP-C activity of 3F10 was identified by Western blotting using purified m-MBP-C prepared from masseter myofibrils by the method of Hartzell and Glass (1984). The use of Balb/c mice for hybridoma production and of cat and rodent tissues for tissue culture experiments was approved by the Animal Ethics Review Committee of the University of Sydney.
Satellite Cell Isolation and Cultures
Jaw and limb muscles were removed from adult cats, rats, and mice killed by anesthetic overdose. Cat tissues were obtained terminally from animals used in unrelated physiological experiments. Cat jaw and limb muscles were cut into pieces of ∼100 mg, placed in HBSS containing 20% fetal calf serum (FCS) and 8% dimethyl sulphoxide, frozen and cryogenically stored in liquid nitrogen, and were used within 4 weeks. All procedures were carried out under aseptic conditions.
For cat cell cultures, aliquots of frozen muscles were rapidly thawed at 37C and washed in HBSS containing antibiotics (500 IU/ml penicillin, 500 μg/ml streptomycin) three times. After removal of connective tissue, the muscles were minced and treated with pronase (0.01%) at 37C for 1–1.5 hr. Satellite cells were isolated by serial washes and trituration steps and then seeded into 12-well flat-bottom culture plates (Linbro Plastics; McLean, VA) precoated with fibronectin (2 μg/cm2; Sigma-Aldrich, St. Louis, MO) and maintained at 37C in a 5% CO2 incubator. The growth medium contained 80% (v/v) of a 4:1 mixture of DMEM and Medium 199, 20% FCS, and 2 mM glutamine, 200 μg/ml gentamycin, 20 μg/ml insulin, 6–8 ng/ml fibroblast growth factor, and 10−7 M dexamethasone. Once cells were confluent, FCS concentration was reduced to 10% (v/v) to favor myoblast fusion. Fusion occurred 10–14 days after seeding. Myotubes were fixed for immunohistochemistry 1–2 weeks after fusion.
For jaw and limb muscle cultures of rats and mice, satellite cells were isolated from fresh tissues. All tissue culture procedures were the same as those described above. Fusion occurred ∼5–7 days after seeding, and the cells were fixed 5–7 days after fusion.
Immunohistochemistry
Immunohistochemistry was used to analyze the expression of m-MyHC, m-MBP-C, and m-Tm in masseter muscle tissue sections and in tissue cultures. General procedures for horseradish peroxidase (HRP) immunohistochemistry of tissue sections were carried out as previously described (Hoh et al. 1988b). Immunoperoxidase staining of cat jaw and limb cultures was performed when myotubes appeared to be mature, usually 2 to 3 weeks after seeding. Cells were fixed with 0.2% acroleine in PBS for 20 min and washed in 1% nonidet P-40 for 20 min at room temperature. All other procedures were the same as those for tissue sections, but were viewed and photomicrographed as wet mounts. Primary antibodies used were anti–m-Tm MAb 1H2 and anti–m-MBPC MAb 3F10 developed above, anti–m-MyHC MAb 2F4 raised against cat masticatory MyHC (Kang et al. 1994) (available now from Developmental Studies Hybridoma Bank, University of Iowa), as well as the following, described previously (Hoh et al. 1988b): anti–slow MyHC MAb NOQ-7-5-4D, anti–fast MyHC MAb NOQ-7-5-2B, sheep polyclonal anti–fetal myosin (STE). HRP-labeled rabbit anti-mouse or anti-sheep immunoglobulin antibody was used as the secondary antibody.
SDS-PAGE, Western Blotting, and GEDELISA
SDS-PAGE of myofibrillar proteins was done in 15% gels as described previously for myosin light chains (Hoh et al. 2007a), whereas SDS-PAGE and Western blots for m-MBP-C were done as for MyHCs in 8% gels (Hoh et al. 2007a). The primary and secondary antibodies used were 3F10 and HRP-labeled rabbit anti-mouse immunoglobulin antibody, respectively. GEDELISA for m-Tm was performed as described for native myosin (Hoh et al. 1988b), after electrophoresis of native m-Tm in 4% pyrophosphate gels (Hoh et al. 1978) for 1.75 hr at 80V.
Results
Characterization of MAbs Against Jaw-specific Myofibrillar Proteins
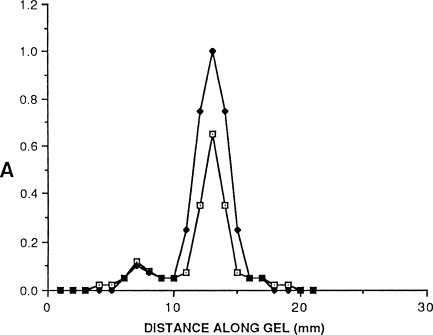
A hybridoma clone (1H2) was isolated from spleen cells immunized in vitro against purified m-Tm prepared from the superficial temporalis muscle, which contains only masticatory fibers. 1H2 reacted with masticatory fibers but not with limb fast or slow fibers. It did not react with denatured m-Tm in Western blots. However, 1H2 did react with purified native m-Tm in GEDELISA after gel electrophoresis under non-denaturing conditions. Figure 1 shows that the peak of the GEDELISA assay coincides with peak absorbance of a protein-stained companion gel loaded with the purified antigen, thus positively identifying the MAb as anti–m-Tm.
Figure 1.
Gel electrophoresis–derived ELISA (GEDELISA) of native masticatory-specific tropomyosin (m-Tm) using monoclonal antibody (MAb) 1H2. Ordinate: absorbance (A); abscissa: distance along gel. Filled squares mark the absorbance profile, scanned at 590 nm, of a protein-stained gel with purified native m-Tm after pyrophosphate gel electrophoresis in 4% gels at 80V for 1.75 hr. Open squares show GEDELISA absorbance readings at 650 nm for individual gel slices of a duplicate gel sliced at 1-mm intervals.
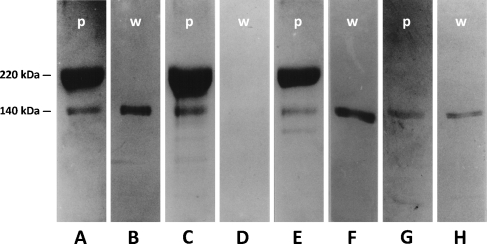
The screening of hybridomas from mice immunized with m-MyHC resulted in numerous jaw-specific clones, one of which, 3F10, reacted in Western blots, not with m-MyHC, but with a protein migrating just ahead of it in SDS-PAGE. Figure 2A presents SDS-PAGE of temporalis myosin, showing m-MyHC as a major band of 220 kDa, with a weaker band migrating ahead of it. Western blot of a companion gel using MAb 3F10 (Figure 2B) shows that this MAb binds the weaker band. Figure 2C shows the protein-stained gel with limb fast-muscle myosin, which also shows a weaker band ahead of the MyHC band, but MAb 3F10 does not react with it in a Western blot (Figure 2D). Figure 2E shows that masseter MyHC has two bands migrating just ahead of it, one of which has the same electrophoretic mobility as that in temporalis; the other is weaker, and has a faster mobility. Only the stronger band binds 3F10 in a Western blot (Figure 2F). Because MBP-C is the only known abundant myofibrillar protein of this molecular mass range (∼140 kDa), we suspect that the band that binds 3F10 (Figure 2B) is m-MBP-C. This is confirmed by the preparation of purified MBP-C from temporalis muscle. This protein in SDS-PAGE (Figure 2G) shows a mobility matching that of the presumptive m-MBP-C (Figures 2A and 2E), and binds MAb 3F10 in a Western blot (Figure 2H).
Figure 2.
SDS-PAGE of myosin-binding protein-C (MBP-C) from jaw and limb muscles in 8% gels and their Western blots using MAb 3F10 (p, protein-stained gels; w, Western blots). (A) Temporalis myofibrillar proteins, showing masticatory-specific isoforms of myosin heavy-chain (m-MyHC; 220 kDa) as the main band and the presumptive m-MBP-C band (140 kDa) just ahead of it. (B) Western blot of temporalis myofibrillar proteins. (C) Extensor digitorum longus (EDL) myofibrillar proteins. (D) Western blot of EDL myofibrillar proteins. (E) Masseter myofibrillar proteins. (F) Western blot of masseter myofibrillar proteins. (G) Purified m-MBP-C from temporalis muscle. (H) Western blot of purified m-MBP-C.
Expression of Myofibrillar Proteins in Cat Masseter Muscle Fibers
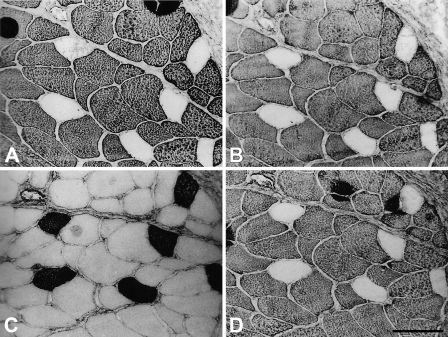
Figure 3 shows serial sections of the cat masseter stained with anti–m-MyHC (Figure 3A), anti–m-MBP-C (Figure 3B), anti–slow MyHC (Figure 3C), and anti–m-Tm (Figure 3D). It shows clearly that m-MyHC, m-MBP-C, and m-Tm are coexpressed only in masticatory fibers, whereas none of the three masticatory myofibrillar proteins are expressed in jaw-slow fibers. These MAbs also did not stain limb fast- and slow-muscle fibers (data not shown).
Figure 3.
Immunoperoxidase staining of serial sections of cat masseter muscle with anti–m-MyHC MAb 2F4 (A), anti–m-MBP-C MAb 3F10 (B), anti–slow MyHC MAb NOQ-7-5-4D (C), and anti–m-Tm MAb 1H2 (D). Bar = 50 μm.
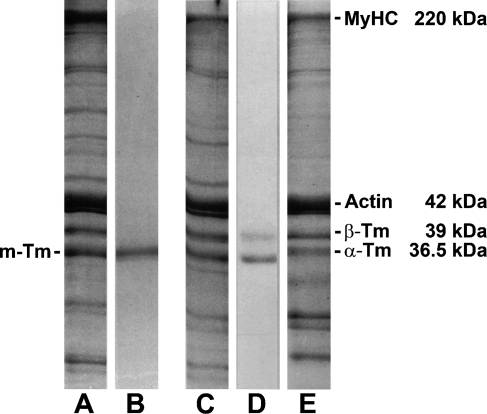
Because jaw-slow fibers in the masseter do not express m-Tm, the question arises as to what Tm isoform they express. A comparison of SDS-PAGE of the jaw and limb myofibrillar proteins is useful in this connection. Myofibrillar proteins from masseter muscle with masticatory and jaw-slow fibers (Figure 4A) show two components in the molecular size range for Tm isoforms (which migrate just ahead of actin), whereas purified m-Tm (Figure 4B) from the homogeneous temporalis muscle shows only the faster migrating of these components. Fast EDL myofibrillar proteins (Figure 4C), purified Tm prepared from the EDL (Figure 4D), and myofibrillar proteins from the slow soleus muscle (Figure 4E) all showed two Tm components, α-Tm and β-Tm. Comparing Figures 4D and 4E reveals that the α-Tm isoform in fast muscle has a slightly higher mobility compared with that in slow muscle. These isoforms are known to be structurally different, and are designated αf-Tm and αs-Tm, respectively (Hoh 1992). Purified temporalis m-Tm has a mobility that is close to that of limb muscle α-Tm isoforms, whereas the slower component of masseter Tm corresponds to β-Tm. Thus, jaw-slow fibers in the masseter express limb β-Tm or a Tm isoform of a molecular size similar to that of limb β-Tm.
Figure 4.
SDS-PAGE in 15% gels of (A) crude myofibrillar proteins of cat masseter, (B) purified m-Tm from cat temporalis, (C) crude myofibrillar proteins of cat EDL muscle, (D) purified Tm preparation from cat EDL, and (E) crude myofibrillar proteins of cat soleus muscle. Shown are bands corresponding to MyHC, actin, m-Tm, α-Tm, and β-Tm; molecular mass at right.
Expression of Jaw-specific Myofibrillar Proteins in Jaw and Limb Myotubes
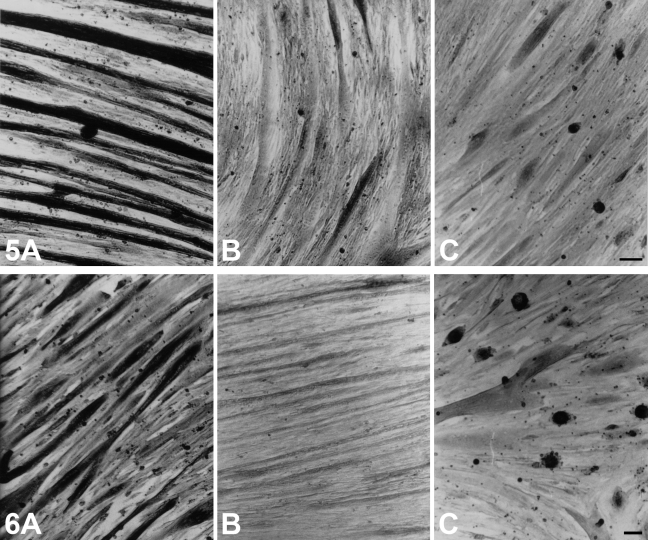
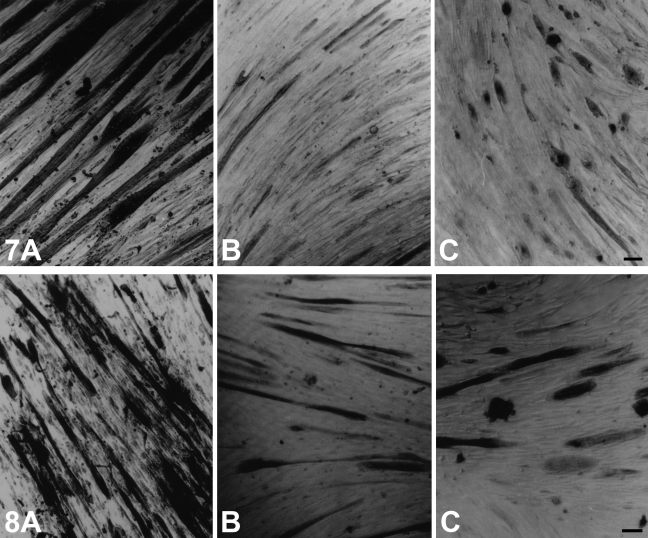
The expression of m-MyHC, m-MBP-C, and m-Tm was studied in myotubes cultured from satellite cells of cat and rat jaw and limb muscles. Preliminary studies without growth factor supplementation showed that cat jaw and limb cultures were much slower in growth and maturation compared with rat cultures in the same medium. Rat cultures fused 5–7 days after seeding, and cat cultures took 10–14 days. The routine use of growth factors did not appear to improve the situation. At ∼3 weeks, myotubes in virtually all cat cultures appeared to be quite mature, after which they began to deteriorate and lift from the culture plate.
Figure 5 shows immunohistochemical analysis using anti–m-MyHC MAb of 3-week-old cultures of cat temporalis muscle (Figure 5A), cat EDL muscle (Figure 5B), and cat soleus muscle (Figure 5C). Figure 6 shows the staining of these three types of cultures using anti-m–MBP-C MAb, whereas Figure 7 shows cultures stained with anti–m-Tm MAb. Although each of these MAbs strongly stained virtually all myotubes of cat temporalis cultures, the vast majority of cat EDL and soleus myotubes were not stained. However, a few fibers in these culture plates appeared to be stained. The limb myotubes seen in Figures 5–7 were views selected to show regions of the culture plate with the highest density of stained fibers. The intensity of staining of the few limb myotubes was much weaker, and staining was often confined to the perinuclear region.
Figure 5.
Immunoperoxidase staining of cultured myotubes derived from cat temporalis (A), cat EDL (B), and cat soleus (C) muscle using anti–m-MyHC MAb 2F4. Bar = 100 μm.
Figure 6.
Immunoperoxidase staining with anti–m-MBP-C MAb 3F10 of cultured myotubes derived from cat temporalis (A), cat EDL (B), and cat soleus (C) muscle. Bar = 100 μm.
Figure 7.
Immunoperoxidase staining with anti–m-Tm MAb 1H2 of cultured myotubes derived from cat temporalis (A), cat EDL (B), and cat soleus (C) muscle. Bar = 100 μm.
Immunohistochemical analysis with a polyclonal anti–fetal myosin antibody showed uniform staining of myotubes in jaw and limb muscle cultures. However, MAb NOQ-7-5-2B against limb fast MyHC stained myotubes derived from limb fast and slow muscles, but not those from jaw muscle (data not shown).
Figure 8 shows anti–slow MyHC MAb staining of matured myotubes in the three types of cat muscle cultures. Nearly all myotubes in jaw and limb fast cultures and some myotubes in the slow-muscle culture were strongly stained, in contrast to the selective pattern of staining using MAbs against m-MyHC, m-MBP-C, and m-Tm.
Figure 8.
Immunoperoxidase staining with anti–slow MyHC MAb NOQ-7-5-4D of cultured myotubes derived from cat temporalis (A), cat EDL (B), and cat soleus (C) muscle. Bar = 100 μm.
In summary, these results show that virtually all matured myotubes in cat jaw cultures coexpress fetal, slow, and masticatory MyHCs, m-MBP-C, and m-Tm, whereas the vast majority of myotubes derived from limb muscles coexpress fetal, slow, and fast MyHCs, as shown previously (LaFramboise et al. 2003), but do not express m-MyHC, m-MBP-C, or m-Tm.
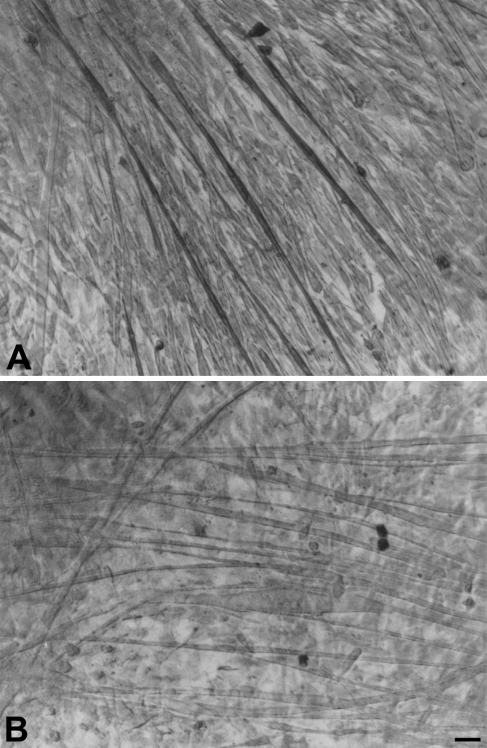
To compare with cat cultures, we investigated whether m-MBP-C is expressed in cultures of jaw and limb muscle of the rat, a species that does not have masticatory fibers. Figure 9 shows rat jaw and limb fast-muscle cultures stained with anti–m-MBP-C MAb. Surprisingly, a few rat jaw myotubes are weakly stained by this MAb, whereas limb myotubes are not stained.
Figure 9.
Immunoperoxidase staining with anti–m-MBP-C MAb 3F10 of cultured myotubes derived from rat masseter (A) and rat gastrocnemius (B) muscle. Bar = 50 μm.
Discussion
Specificities of the MAbs Against Masticatory Myofibrillar Proteins
Clone 1H2 was raised against purified native m-Tm, and its supernatant reacted immunohistochemically with masticatory but not with jaw-slow fibers, nor with limb fast or slow fibers. In Western blots, it failed to react with m-Tm. However, it reacted with native m-Tm after electrophoresis under non-denaturating conditions, as shown by GEDELISA. It also reacted with m-Tm in unfixed cryostat sections, as well as m-Tm in acroleine-fixed masticatory myotubes. The epitope to which 1H2 binds is presumably an aspect of the 100% α-helical coiled-coil structure of native m-Tm used as antigen, which apparently survives tissue fixation, but would have been destroyed by uncoiling during SDS-PAGE.
Although clone 3F10 was isolated following immunization with cat m-MyHC, and its supernatant reacted specifically with masticatory fibers immunohistochemically, it did not react with m-MyHC in Western blots, but bound a band in SDS-PAGE that migrated just ahead of m-MyHC. This band has the same mobility as MBP-C in limb fast muscle (Figure 2), which has a molecular mass of ∼140 kDa, suggesting that it could be m-MBP-C. Clone 3F10 presumably arose in response to m-MBP-C present in the antigen as a contaminant. This suspicion was confirmed by the preparation of MBP-C from cat temporalis muscle using a standard protocol for MBP-C extraction (Hartzell and Glass 1984). The purified protein showed the same mobility as presumptive m-MBP-C in the temporalis myosin preparation, and bound 3F10 in Western blots. 3F10 did not bind MBP-C from limb fast muscle, or another protein of slightly lower molecular mass in masseter myofibrillar proteins, which is probably the slow isoform of MBP-C from the slow fibers in the masseter. Our results demonstrate the existence of m-MBP-C in the cat masticatory muscle. An m-MBP-C has recently been reported in dog masticatory fibers, and the gene for this protein has also been shown to exist in the genome of humans, who have no masticatory fibers (Wu et al. 2007).
Functional Significance of the Coexpression of Jaw-specific Myofibrillar Proteins in Masticatory Fibers
Our immunohistochemical analysis of cat masseter muscle using the mMAbs developed here shows that m-Tm and m-MBP-C are coexpressed with m-MyHC in adult cat masticatory fibers, but not in jaw-slow fibers of the same muscle. This provides another instance in which a specific isoform of MyHC is associated with specific isoforms of MBP-C and Tm; the other examples are limb fast and slow fibers (Hoh 1992; Yu et al. 2002). The coordinated expression of these myofibrillar proteins is likely to be critical for producing the functional characteristics of these respective fiber types. In view of this coordinated expression of specific myofibrillar protein isoforms, it appears unlikely that other craniofacial muscles, such as extraocular and laryngeal muscles, would not express m-MBP-C and m-Tm, inasmuch as these muscles do not express m-MyHC (Hoh 2010).
The functional characteristics of masticatory fibers include high Ca sensitivity (Kato et al. 1985), high tension cost (Saeki et al. 1987), high force-per-unit cross-sectional area (Saeki et al. 1987; Toniolo et al. 2008), moderate cross-bridge cycling rate as indicated by dynamic stiffness analysis (Hoh et al. 2007b), and moderate speed of shortening (Toniolo et al. 2008). The high-tension cost is probably related to the high ATPase activity of the masticatory myosin (Rowlerson et al. 1981), whose MyHCs and light chains are structurally distinct from those of limb muscle (Rowlerson et al. 1981; Qin et al. 1994,2002). It is very likely that the high force and moderate speed features can also be attributed to this myosin (Hoh et al. 2007b; Toniolo et al. 2008). X-ray diffraction studies on dog masticatory fibers have recently shown that their thick filaments are disordered and that myosin heads on the thick filaments protrude out toward thin filaments (Yamaguchi et al. 2010), and are therefore closer to their binding sites on the thin filaments than in limb muscle. These features may promote high force by accelerating cross-bridge attachment rate. However, m-MBP-C may also be involved in bringing this about.
MBP-C is a thick filament protein that can modulate cross-bridge function. In skeletal muscle, MBP-C appears to tether cross-bridges close to the thick filament backbone, tending to reduce fiber force at submaximal Ca activation (Hofmann et al. 1991b) and limiting its shortening speed (Hofmann et al. 1991a). The role of MBP-C has been more extensively studied in cardiac muscle, where cardiac MBP-C is phosphorylated during β-adrenergic stimulation. This reversibly causes cross-bridges to extend toward thin filaments (Weisberg and Winegrad 1996; Levine et al. 2001), thereby increasing the cross-bridge attachment rate and promoting force development. This is functionally important during β-adrenergic stimulation, because it compensates for the reduced force due to the accelerated cross-bridge detachment rate needed to bring about rapid relaxation during adrenergically mediated tachycardia. The rapid relaxation is partly due to the β-adrenergically stimulated cardiac troponin-I phosphorylation, which would otherwise reduce isometric force by reducing Ca sensitivity (Turnbull et al. 2002).
It is possible that m-MBP-C causes masticatory myosin cross-bridges to protrude toward actin filaments constitutively, rather than reversibly by phosphorylation as in cardiac muscle, thereby contributing to the high force feature. At present, it is not clear whether the protrusion of masticatory myosin cross-bridges toward thin filaments is due to the peculiar structure of masticatory myosin itself, or to the interaction of masticatory myosin with m-MBP-C, or to both these factors. Expressing each of these genes in transgenic animals will greatly help in elucidating this problem.
Tm, in collaboration with troponin and Ca, regulates muscle activation in striated muscles (Gordon et al. 2001). In adult limb muscles, the isoforms of Tm expressed are fiber type–specific: αf-Tm and β-Tm in fast fibers, and αs-Tm and β-Tm in slow fibers (Pette and Staron 1990). Our results (Figure 4) suggest that αf-Tm migrates slightly faster than αs-Tm. These differences in Tm isoforms are associated with fast and slow isoforms of troponin subunits, and result in higher Ca sensitivities in fast fibers (Hoh 1992). It is likely that the expression of m-Tm in masticatory fibers, possibly in conjunction with unique isoforms of masticatory troponin subunits, underlies the higher Ca sensitivity of masticatory fibers compared with limb fast fibers (Saeki et al. 1987).
Our immunohistochemical results suggest that the jaw-slow fibers, which express the same slow MyHC as limb slow fibers (Hoh et al. 2007b), express isoforms of Tm and MBP-C different from those in masticatory fibers. SDS-PAGE of myofibrillar proteins of the cat masseter included a band identical in mobility to limb β-Tm (Figure 4D) and another band with a slightly higher mobility than m-MBP-C (Figure 2E). Because the masseter contains jaw-slow fibers in addition to masticatory fibers, the simplest explanation for these findings would be that limb β-Tm and a slow isoform, MBP-C, are expressed in jaw-slow fibers. Whether jaw-slow fibers also express αs-Tm as in limb slow fibers remains to be resolved.
The suites of myofibrillar proteins in masticatory and jaw-slow fibers among mammals that express m-MyHC vary across species. For example, jaw-slow fibers of the cat express masticatory light chains (Hoh et al. 2007b), whereas jaw-slow fibers in the ringtail possum do not (Kang L and Hoh JFY, unpublished data). Jaw-slow fibers in marsupial mammals abundantly express α-cardiac MyHC in addition to slow MyHC in mammalian jaw-slow fibers (Hoh et al. 2006). These variations probably represent evolutionary adaptations to varied functional demands on jaw-slow fibers in animals procuring and eating different types of food.
Cat Primary Muscle Cultures
In preliminary studies, we observed that cat jaw and limb muscle satellite cells are slow to grow and mature, relative to rodent cultures in the same standard medium. Whereas rodent cultures formed mature myotubes within 1 week, cat cultures take up to 2 weeks to begin fusion, and start to deteriorate within 3 weeks. This difference was not due to the use of frozen cat tissues, because similar results were obtained under the same culture conditions with fresh kitten muscle tissues (Hughes and Hoh 1985). In an attempt to enhance satellite cell proliferation and differentiation in cat cultures, for the bulk of this study, we included various growth factors in the culture media, including fibroblast growth factor (Florini and Magri 1989; Grounds 1991), dexamethasone (Grigoriadis et al. 1988; Grounds 1990), and insulin (Florini et al. 1991) to promote satellite cell proliferation. Furthermore, fibronectin was used to coat the culture plates for better cell adhesion (Grounds 1991), which is deemed important for myotube maturation. These attempts did not appear to significantly improve the situation.
One study recently reported no apparent difference in the rate of myoblast maturation between cat and rodent cultures (Radojevic et al. 2002). The culture media used by these authors differed from those used here; in particular, their growth medium contained 5% horse serum in addition to 10% FCS, compared with our 20% FCS, whereas their differentiation medium contained 10% horse serum without FCS, compared with our 10% FCS. Because there is a developmental surge of thyroid hormones in mammals (Hulbert 2000), our culture condition is probably hypothyroid relative to that of Radojevic and colleagues (2002). The postnatal surge of thyroid hormones in mammals is essential for normal muscle development, promoting the withdrawal of embryonic and neonatal MyHC expression concurrent with the induction of adult MyHC isoforms (Adams et al. 1999). In vitro studies also show that thyroid hormone promotes myotube maturation (Carnac et al. 1992; Cassar-Malek et al. 1999). We hypothesize that the presumed higher thyroid hormone level in the study by Radojevic and colleagues (2002) contributed to the accelerated maturation rate of their cat cultures compared with ours.
Expression of Jaw-specific Myofibrillar Proteins in Cultures of Cat and Rodent Jaw and Limb Satellite Cells
Our results showed that virtually all matured myotubes derived from satellite cells of cat jaw muscle coexpressed fetal, slow, and masticatory MyHCs, m-MBP-Cs, and m-Tms, but not the limb-specific fast MyHCs, whereas the vast majority of myotubes derived from limb muscles coexpressed fetal, slow, and fast MyHCs, but not m-MyHC, m-MBP-C, and m-Tm. These findings consolidate the notion of jaw muscle allotype by demonstrating the capacity of cat jaw satellite cells to express the three masticatory myofibrillar proteins independent of cellular and humoral factors found in vivo. These myogenic cells, therefore, belong to a distinct lineage that is preprogrammed to express, during myogenesis, a spectrum of masticatory-specific myofibrillar proteins normally expressed in adult cat masticatory fibers.
Although the satellite cells used in our study were derived from homogeneously masticatory fibers, both m-MyHC and slow MyHC isoforms found in adult cat jaw-closing muscle fibers were coexpressed in all myotubes. This is analogous to satellite cell cultures derived from limb fast, slow, and other somitic muscles uniformly coexpressing fast 2A MyHC and slow MyHC isoforms (LaFramboise et al. 2003). Satellite cells from masticatory fibers can serve as stem cells for both masticatory and jaw-slow fibers. Most myotubes in early regenerates of masticatory muscles in vivo also coexpress m-MyHC and slow MyHC (Hoh and Hughes 1991b). However, slow MyHC expression is selectively suppressed upon innervation by a nerve to a fast muscle to produce masticatory fibers, whereas m-MyHC is selectively suppressed by a nerve to a slow muscle to generate jaw-slow fibers (Hoh and Hughes 1988).
The notion of jaw muscle allotype suggests that satellite cells of jaw-closing muscles of other species that do not express m-MyHCs, m-MBP-C, and m-Tm would also be preprogrammed to express those myofibrillar protein isoforms found in their jaw muscles during myogenesis. Thus, as jaw muscles of kangaroos express α-cardiac MyHC and slow myosin light chains (Hoh et al. 2000), their satellite cells would be expected to express these myosin subunits.
What is the cellular basis for the allotypic differences between myogenic cells of masticatory and limb muscles? It is known that craniofacial and limb muscles use the same MyoD family of transcription factors to control their development (Hacker and Guthrie 1998; Noden et al. 1999). However, upstream regulatory cascades that commit mesodermal cells to myogenesis differ in muscles of different regions of the body (Tajbakhsh et al. 1997; Lu et al. 2002; Mootoosamy and Dietrich 2002). In limb and trunk muscles, pax-3 and myf-5 cooperate to induce myogenesis at these sites, so that in mutants in which these genes are deleted, limb and trunk muscles are absent, whereas head muscles are intact (Tajbakhsh et al. 1997). When regulatory genes myoR and capsulin are deleted, jaw-closing muscles are absent, whereas development of trunk and limb muscles is intact (Lu et al. 2002). Although Wnt family members expressed in the dorsal neural tube cooperate with Sonic Hedgehog expressed in the notochord to activate myogenesis in the epaxial component of the myotome, recent work has shown that inhibitors of Wnt and BMP signaling (Noggin, Gremlin, and Frzh) secreted by cranial neural crest cells promote the formation of vertebrate head muscle (Tzahor et al. 2003). How differences in upstream cascades involved in myoblast specification lead to different patterns of myofibrillar protein gene expression during the subsequent myogenic progression is now becoming an exciting question for future research.
Although the vast majority of cat limb and rat limb myotubes express limb-specific MyHC, a few myotubes per culture plate in these cultures appeared to stain with antibodies against masticatory myofibrillar proteins. We have not ruled out the possibility that such aberrant staining may be due to some artifact. It will be important for future work to confirm by mRNA analysis using PCR whether this represents weak expression of masticatory myofibrillar proteins in rat and cat limb cultures. The perinuclear staining of limb myotubes would be consistent with weak expression of these masticatory genes, because their mRNA would be more abundant there. Expression of masticatory myofibrillar proteins in cat limb muscle cultures in a very small fraction of myotubes may be regarded as noise at the level of gene regulation, and may have little functional significance. Expression of m-MyHC was not observed in innervated regenerates of limb muscles (Hoh and Hughes 1988). Interestingly, weak expression of fast MyHC has been observed in chronically denervated masticatory muscle regenerating in a fast muscle bed (Hoh and Hughes 1991b). This may be an example of aberrant MyHC expression in myogenic cells of the jaw allotype, but the possibility that this may be due to migration of limb satellite cells from neighboring fast muscle has not been excluded. Expression of jaw-specific myofibrillar proteins in rat muscle cultures would imply that these genes are present in the rat genome. Masticatory fibers are not found in rats, mice, or guinea pigs, but are present in some members of the rodent family Sciuridae (Reiser et al. 2009).
In summary, we have demonstrated the presence of m-MBP-C in cat jaw muscle and have developed monoclonal antibodies against m-MBP-C and m-Tm. We have shown that myotubes derived from satellite cells of cat masticatory muscle specifically express in vitro a suite of myofibrillar proteins normally found in cat masticatory fibers. These results consolidate the notion that myogenic cells in masticatory muscle are allotypically different from those in limb muscle.
Acknowledgments
This work was supported by grants from the Muscular Dystrophy Association, USA, the National Health and Medical Research Council of Australia, and the Australian Research Council.
We thank Professor W. Burke for providing cat muscle tissues.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Adams GR, McCue SA, Zeng M, Baldwin KM (1999) Time course of myosin heavy chain transitions in neonatal rats: importance of innervation and thyroid state. Am J Physiol 276:R954–961 [DOI] [PubMed] [Google Scholar]
- Carnac G, Albaglicuriel O, Vandromme M, Pinset C, Montarras D, Laudet V, Bonnieu A (1992) 3,5,3′-Triiodothyronine positively regulates both MyoD1 gene transcription and terminal differentiation in C2 myoblasts. Mol Endocrinol 6:1185–1194 [DOI] [PubMed] [Google Scholar]
- Cassar-Malek I, Langlois N, Picard B, Geay Y (1999) Regulation of bovine satellite cell proliferation and differentiation by insulin and triiodothyronine. Domest Anim Endocrinol 17:373–388 [DOI] [PubMed] [Google Scholar]
- Dhoot GK, Hales MC, Grail BM, Perry SV (1985) The isoforms of C protein and their distribution in mammalian skeletal muscle. J Muscle Res Cell Motil 6:487–505 [DOI] [PubMed] [Google Scholar]
- Florini JR, Ewton DZ, Magri KA (1991) Hormones, growth factors, and myogenic differentiation. Annu Rev Physiol 53:201–216 [DOI] [PubMed] [Google Scholar]
- Florini JR, Magri KA (1989) Effects of growth factors on myogenic differentiation. Am J Physiol 256:C701–711 [DOI] [PubMed] [Google Scholar]
- Gordon AM, Regnier M, Homsher E (2001) Skeletal and cardiac muscle contractile activation: tropomyosin “rocks and rolls.” News Physiol Sci 16:49–55 [PubMed] [Google Scholar]
- Grigoriadis AE, Heersche JNM, Aubin JE (1988) Differentiation of muscle, fat, cartilage, and bone from progenitor cells present in a bone-derived clonal cell population: effect of dexamethasone. J Cell Biol 106:2139–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grounds MD (1990) Factors controlling skeletal muscle regeneration in vivo. In Kakulas BA, Mastaglia FL, eds. Pathogenesis and Therapy of Duchenne and Becker Muscular Dystrophy. New York, Raven Press, 171–185
- Grounds MD (1991) Towards understanding skeletal muscle regeneration. Pathol Res Pract 187:1–22 [DOI] [PubMed] [Google Scholar]
- Hacker A, Guthrie S (1998) A distinct developmental programme for the cranial paraxial mesoderm in the chick embryo. Development 125:3461–3472 [DOI] [PubMed] [Google Scholar]
- Hartzell HC, Glass DB (1984) Phosphorylation of purified cardiac muscle C-protein by purified cAMP-dependent and endogenous Ca2+-calmodulin-dependent protein kinases. J Biol Chem 259:15587–15596 [PubMed] [Google Scholar]
- Hofmann PA, Greaser ML, Moss RL (1991a) C-protein limits shortening velocity of rabbit skeletal muscle fibres at low levels of Ca2+ activation. J Physiol 439:701–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann PA, Hartzell HC, Moss RL (1991b) Alterations in Ca2+ sensitive tension due to partial extraction of C-protein from rat skinned cardiac myocytes and rabbit skeletal muscle fibers. J Gen Physiol 97:1141–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoh JF (1992) Muscle fiber types and function. Curr Opin Rheumatol 4:801–808 [PubMed] [Google Scholar]
- Hoh JF (2002) ‘Superfast’ or masticatory myosin and the evolution of jaw-closing muscles of vertebrates. J Exp Biol 205:2203–2210 [DOI] [PubMed] [Google Scholar]
- Hoh JF (2010) Laryngeal muscles as highly specialized organs in airway protection, respiration and phonation. In Brudzynski SM, ed. Handbook of Mammalian Vocalization. Oxford, Academic Press, 13–22
- Hoh JF, Hughes S (1988) Myogenic and neurogenic regulation of myosin gene expression in cat jaw-closing muscles regenerating in fast and slow limb muscle beds. J Muscle Res Cell Motil 9:59–72 [DOI] [PubMed] [Google Scholar]
- Hoh JF, Hughes S (1989) Immunocytochemical analysis of the perinatal development of cat masseter muscle using anti-myosin antibodies. J Muscle Res Cell Motil 10:312–325 [DOI] [PubMed] [Google Scholar]
- Hoh JF, Hughes S (1991a) Basal lamina and superfast myosin expression in regenerating cat jaw muscle. Muscle Nerve 14:398–406 [DOI] [PubMed] [Google Scholar]
- Hoh JF, Hughes S (1991b) Expression of superfast myosin in aneural regenerates of cat jaw muscle. Muscle Nerve 14:316–325 [DOI] [PubMed] [Google Scholar]
- Hoh JF, Hughes S, Chow C, Hale PT, Fitzsimons RB (1988a) Immunocytochemical and electrophoretic analyses of changes in myosin gene expression in cat posterior temporalis muscle during postnatal development. J Muscle Res Cell Motil 9:48–58 [DOI] [PubMed] [Google Scholar]
- Hoh JF, Hughes S, Hale PT, Fitzsimons RB (1988b) Immunocytochemical and electrophoretic analyses of changes in myosin gene expression in cat limb fast and slow muscles during postnatal development. J Muscle Res Cell Motil 9:30–47 [DOI] [PubMed] [Google Scholar]
- Hoh JF, Kang LH, Sieber LG, Lim JH, Zhong WW (2006) Myosin isoforms and fibre types in jaw-closing muscles of Australian marsupials. J Comp Physiol [B] 176:685–695 [DOI] [PubMed] [Google Scholar]
- Hoh JF, Kim Y, Lim JH, Sieber LG, Lucas CA, Zhong WW (2007a) Marsupial cardiac myosins are similar to those of eutherians in subunit composition and in the correlation of their expression with body size. J Comp Physiol [B] 177:153–163 [DOI] [PubMed] [Google Scholar]
- Hoh JF, Kim Y, Sieber LG, Zhong WW, Lucas CA (2000) Jaw-closing muscles of kangaroos express alpha-cardiac myosin heavy chain. J Muscle Res Cell Motil 21:673–680 [DOI] [PubMed] [Google Scholar]
- Hoh JF, Li ZB, Qin H, Hsu MK, Rossmanith GH (2007b) Cross-bridge kinetics of fast and slow fibres of cat jaw and limb muscles: correlations with myosin subunit composition. J Muscle Res Cell Motil 28:329–341 [DOI] [PubMed] [Google Scholar]
- Hoh JF, McGrath PA, Hale PT (1978) Electrophoretic analysis of multiple forms of rat cardiac myosin: effects of hypophysectomy and thyroxine replacement. J Mol Cell Cardiol 10:1053–1076 [DOI] [PubMed] [Google Scholar]
- Hoh JF, Walker ML, Lin JJC (1989) A unique isoform of skeletal tropomyosin in cat jaw-closing muscle and its developmental expression. Proc Aust Physiol Pharmacol Soc 20:192P [Google Scholar]
- Hoh JFY, Hughes S, Kang LHD, Rughani A, Qin H (1993) The biology of cat jaw-closing muscle cells. J Comput Assist Microsc 5:65–70 [Google Scholar]
- Hughes S, Hoh JF (1985) Myotubes grown in tissue culture from juvenile cat jaw and limb muscles express a slow myosin epitope. Proc Aust Physiol Pharmacol Soc 16:260P [Google Scholar]
- Hulbert AJ (2000) Thyroid hormones and their effects: a new perspective. Biol Rev Camb Philos Soc 75:519–631 [DOI] [PubMed] [Google Scholar]
- Kang LHD, Bestak R, Rughani A, Hoh JF (1991) Expression of superfast myosin heavy chain and jaw-specific tropomyosin in cultures of cat jaw muscle cells. Proc Aust Physiol Pharmacol Soc 22:14P [Google Scholar]
- Kang LHD, Hughes S, Pettigrew JD, Hoh JF (1994) Jaw-specific myosin heavy chain gene expression in sheep, dog, monkey, flying fox and microbat jaw-closing muscles. Basic Appl Myol 4:381–392 [Google Scholar]
- Kang LHD, Rughani A, Bestak R, Hoh JF (1992) Expression of a unique isoform of C-protein in cat jaw-closing muscle cells during development and regeneration. Proc 11th Meeting Aust Soc Cell Biol 81P–82P
- Kato C, Saeki Y, Yanagisawa K (1985) Ca2+ sensitivities and transient tension responses to step-length stretches in feline mechanically-stripped single-fibre jaw-muscle preparations. Arch Oral Biol 30:429–432 [DOI] [PubMed] [Google Scholar]
- LaFramboise WA, Guthrie RD, Scalise D, Elborne V, Bombach KL, Armanious CS, Magovern JA (2003) Effect of muscle origin and phenotype on satellite cell muscle-specific gene expression. J Mol Cell Cardiol 35:1307–1318 [DOI] [PubMed] [Google Scholar]
- Levine R, Weisberg A, Kulikovskaya I, McClellan G, Winegrad S (2001) Multiple structures of thick filaments in resting cardiac muscle and their influence on cross-bridge interactions. Biophys J 81:1070–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JR, Bassel-Duby R, Hawkins A, Chang P, Valdez R, Wu H, Gan L, et al. (2002) Control of facial muscle development by MyoR and capsulin. Science 298:2378–2381 [DOI] [PubMed] [Google Scholar]
- Lucas CA, Kang LH, Hoh JF (2000) Monospecific antibodies against the three mammalian fast limb myosin heavy chains. Biochem Biophys Res Commun 272:303–308 [DOI] [PubMed] [Google Scholar]
- Mootoosamy RC, Dietrich S (2002) Distinct regulatory cascades for head and trunk myogenesis. Development 129:573–583 [DOI] [PubMed] [Google Scholar]
- Noden DM, Marcucio R, Borycki AG, Emerson CPJ (1999) Differentiation of avian craniofacial muscles: I. Patterns of early regulatory gene expression and myosin heavy chain synthesis. Dev Dyn 216:96–112 [DOI] [PubMed] [Google Scholar]
- Pardue RL, Brady RC, Perry GW, Dedman JR (1983) Production of monoclonal antibodies against calmodulin by in vitro immunization of spleen cells. J Cell Biol 96:1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pette D, Staron RS (1990) Cellular and molecular diversities of mammalian skeletal muscle fibers. Rev Physiol Biochem Pharmacol 116:2–76 [DOI] [PubMed] [Google Scholar]
- Qin H, Hsu MK, Morris BJ, Hoh JF (2002) A distinct subclass of mammalian striated myosins: structure and molecular evolution of ‘superfast’ or masticatory myosin heavy chain. J Mol Evol 55:544–552 [DOI] [PubMed] [Google Scholar]
- Qin H, Morris BJ, Hoh JF (1994) Isolation and structure of cat superfast myosin light chain-2 cDNA and evidence for the identity of its human homologue. Biochem Biophys Res Commun 200:1277–1282 [DOI] [PubMed] [Google Scholar]
- Radojevic V, Oppliger C, Gaschen F, Burgunder JM (2002) Restoration of dystrophin expression in cultured hybrid myotubes. Neuropathol Appl Neurobiol 28:397–409 [DOI] [PubMed] [Google Scholar]
- Reiser PJ, Bicer S, Chen Q, Zhu L, Quan N (2009) Masticatory (‘superfast’) myosin heavy chain and embryonic/atrial myosin light chain 1 in rodent jaw-closing muscles. J Exp Biol 212:2511–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlerson A, Heizmann CW, Jenny E (1983a) Type-specific proteins of single IIM fibres from cat muscle. Biochem Biophys Res Commun 113:519–525 [DOI] [PubMed] [Google Scholar]
- Rowlerson A, Mascarello F, Veggetti A, Carpene E (1983b) The fibre-type composition of the first branchial arch muscles in carnivora and primates. J Muscle Res Cell Motil 4:443–472 [DOI] [PubMed] [Google Scholar]
- Rowlerson A, Pope B, Murray J, Whalen RG, Weeds AG (1981) A novel myosin present in cat jaw-closing muscles. J Muscle Res Cell Motil 2:415–438 [Google Scholar]
- Saeki Y, Kato C, Satomi M, Yanagisawa K (1987) ATPase activity and tension development in mechanically-skinned feline jaw muscle. Arch Oral Biol 32:207–210 [DOI] [PubMed] [Google Scholar]
- Sciote JJ, Rowlerson AM, Carlson DS (1995) Myosin expression in the jaw-closing muscles of the domestic cat and American opossum. Arch Oral Biol 40:405–413 [DOI] [PubMed] [Google Scholar]
- Smillie LB (1982) Preparation and identification of α- and β-tropomyosins. Methods Enzymol 85:234–241 [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, Cossu G (1997) Establishing myogenic identity during somitogenesis. Curr Opin Genet Dev 7:634–641 [DOI] [PubMed] [Google Scholar]
- Toniolo L, Cancellara P, Maccatrozzo L, Patruno M, Mascarello F, Reggiani C (2008) Masticatory myosin unveiled: first determination of contractile parameters of muscle fibers from carnivore jaw muscles. Am J Physiol Cell Physiol 295:C1535–1542 [DOI] [PubMed] [Google Scholar]
- Turnbull L, Hoh JF, Ludowyke RI, Rossmanith GH (2002) Troponin I phosphorylation enhances crossbridge kinetics during beta-adrenergic stimulation in rat cardiac tissue. J Physiol 542:911–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzahor E, Kempf H, Mootoosamy RC, Poon AC, Abzhanov A, Tabin CJ, Dietrich S, et al. (2003) Antagonists of Wnt and BMP signaling promote the formation of vertebrate head muscle. Genes Dev 17:3087–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg A, Winegrad S (1996) Alteration of myosin cross bridges by phosphorylation of myosin-binding protein C in cardiac muscle. Proc Natl Acad Sci USA 93:8999–9003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Li ZF, Brooks R, Komives EA, Torpey JW, Engvall E, Gonias SL, et al. (2007) Autoantibodies in canine masticatory muscle myositis recognize a novel myosin binding protein-C family member. J Immunol 179:4939–4944 [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Takemori S, Kimura M, Tanishima Y, Nakayoshi T, Kimura S, Ohno T, et al. (2010) Protruding masticatory (superfast) myosin heads from staggered thick filaments of dog jaw muscle revealed by X-ray diffraction. J Biochem 147:53–61 [DOI] [PubMed] [Google Scholar]
- Yu F, Stal P, Thornell LE, Larsson L (2002) Human single masseter muscle fibers contain unique combinations of myosin and myosin binding protein C isoforms. J Muscle Res Cell Motil 23:317–326 [DOI] [PubMed] [Google Scholar]