Abstract
The localization of the type-2 angiotensin II receptor (AT2) in the adrenal glands of rats, guinea pigs, bovines, and humans was examined at the mRNA and protein levels. PCR products for AT2 were detected in the adrenal cortices and adrenal medullae of all the mammals examined with an RT-PCR technique. Three different anti-AT2 antibodies (Abs), whose specificity was confirmed in our hands, recognized a 50-kDa protein in the adrenal glands of the four mammals, and this recognition was abolished by the preabsorption of an Ab with an antigen. Immunoblotting and immunohistochemistry revealed that the 50-kDa protein was expressed consistently and variably in the adrenal cortices and medullae of various mammals, respectively. We conclude that the 50-kDa AT2 is consistently expressed in the adrenal cortex in a wide variety of mammals. (J Histochem Cytochem 58:585–593, 2010)
Keywords: AT2, adrenal cortex, adrenal medulla, immunoblot, immunohistochemistry, RT-PCR
The renin-angiotensin system plays an essential role in the homeostasis of fluid and electrolytes in the body. There are two kinds of angiotensin receptors, AT1 and AT2, which belong to the receptor superfamily with seven transmembrane domains (De Gasparo et al. 2000). The physiological actions of angiotensin II (AGII), such as aldosterone secretion and smooth-muscle contraction in the artery, are mediated by the AT1 receptor, which is coupled with phospholipase C through the Gq/11 type G protein (De Gasparo et al. 2000). Compared with the well-established functions of AT1, the function of AT2 receptors has been a matter of controversy (Landon and Inagami 2005; Mogi et al. 2007). AGII was reported to facilitate the secretion of ouabain from bovine adrenal cortical (AC) cells in culture (Laredo et al. 1997). This action of AGII was blocked by PD123319, an AT2 antagonist, but not by DuP753, an AT1 antagonist, whereas the opposite was true for AGII-induced secretion of aldosterone and cortisol. These results suggest that AT2 receptors are expressed widely in bovine adrenal cortex and are selectively involved in ouabain secretion. However, it is not yet known whether AT2 receptors are expressed in AC cells at the protein level. An in situ hybridization study revealed that mRNA for AT2 was present in the rat adrenal medulla and zona glomerulosa, but not in the zona fasciculata, the majority of the adrenal cortex (Peters et al. 2001). On the other hand, AT2-like immunoreactivity was detected only in the rat adrenal medulla, but not in any part of the adrenal cortex (Frei et al. 2001). The antibody (Ab) used in the immunohistochemistry recognized a 73-kDa band in rat adrenal homogenates, a value that differs from the molecular mass (40 to 50 kDa) of immunologically identified AT2 in rat skeletal muscle (Nora et al. 1998) and heart (Wang et al. 1998; Carneiro-Ramos et al. 2007). Such differences in the molecular mass of putative AT2 receptors may be ascribed to different levels of N-glycosylation (Servant et al. 1996), and/or the nonspecificity of Abs used. Belloni et al. (1998) reported that [125I]AGII binding was strong in the rat adrenal medulla and zona glomerulosa and that binding in the former and latter was markedly suppressed by CGP42112, an AT2 agonist, and DuP753, respectively. Their studies also showed that CGP42112 enhanced catecholamine secretion from adrenal medulla fragments in a dose-dependent manner and that the AGII-evoked catecholamine secretion was suppressed conspicuously by PD123319, an AT2 antagonist, and marginally by DuP753. On the basis of these autoradiographic findings and pharmacological analysis of catecholamine secretion, it was concluded that AT2 receptors are expressed in the rat adrenal medulla, and not in the adrenal cortex, and are involved in catecholamine secretion in response to AGII. Electrophysiological studies and Ca2+ measurements, however, clearly indicated that AGII induced Ca2+ mobilization from Ca2+ store sites and catecholamine secretion through an AT1–phospholipase C pathway in cultured bovine adrenal medullary (AM) cells (Cheek et al. 1993; Teschemacher and Seward 2000). This induction of Ca2+ mobilization was also noted in guinea pig AM cells (Warashina et al. 1994).
We have previously reported that exposure to a low concentration of ouabain in guinea pig AM cells results in an increase in the stored Ca2+ concentration, with the consequent facilitation of Ca2+ mobilization (Lin et al. 2005). Ouabain or ouabain-like substances have been found in AC cells of various mammals (Bagrov et al. 2009), such as bovines (Laredo et al. 1997), rats (Ludens et al. 1992), and humans (El-Masri et al. 2002). Therefore, if ouabain or a ouabain-like substance is released from AC cells in response to AT2 activation in such mammals (Schoner and Scheiner-Bobis 2007; Manunta et al. 2009; Nicholls et al. 2009), AT2 could be assumed to be physiologically important for catecholamine secretion from AM cells. The aim of the present experiment was to elucidate whether AT2 is expressed in AC cells in a wide range of mammals at the mRNA and protein levels.
Materials and Methods
All experimental procedures involving humans and animals were approved by the Institutional Ethics Committee and Animal Care and Use Committee of the University of Occupational and Environmental Health, respectively.
Immunoblot
The rats (n=5) and guinea pigs (n=5) were killed by cervical dislocation, and the adrenal glands were excised and immediately put into ice-cold Ca2+-deficient balanced salt solution, which contained 137 mM NaCl, 5.4 mM KCl, 0.5 mM MgCl2, 0.53 mM NaHPO4, 5 mM d-glucose, 5 mM Hepes, and 4 mM NaOH (pH 7.4). The bovine adrenal glands (n=2) were obtained from a local slaughterhouse, and human adrenal glands were excised from a 48-year-old male and a 57-year-old male during surgery for kidney disease following written agreement (approval no. 07-14). The adrenal cortex was removed from the adrenal gland using microscissors and forceps under stereoscopic observation. The preparations were minced and homogenized with a Potter-Elvehjem homogenizer in 10 volumes of a solution containing 10 mM Tris-HCl (pH 7.4), 150 mM NaCl, and a protease inhibitor cocktail (set 1, Calbiochem; San Diego, CA). Homogenates were centrifuged at 500 × g for 10 min at 4C to remove the nuclei, then the postnucleus supernatants were mixed with equal volumes of an SDS buffer containing 25 mM Tris-HCl (pH 6.8), 4% SDS, and 20% glycerol. Protein concentrations in samples were determined using a BCA protein assay kit (Pierce; Rockford, IL). After the addition of 5% (v/v) 2-mercaptoethanol and 1% (v/v) bromophenol blue to the sample, the same amount of proteins (8 μg) for each column was separated by 10% (w/v) SDS-PAGE, and then transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with 5% (w/v) fat-free powdered milk dissolved in PBS-T solution, which contained 2 mM NaH2PO4, 8 mM Na2HPO4, 145 mM NaCl, and 0.1% Tween 20. The PVDF membrane was incubated with a rabbit anti-AT2 Ab made by Alpha Diagnostic (AD) (AT21-s, San Antonio, TX; antigen, 18 amino acids in the C terminal of human AT2), by Santa Cruz Biotechnology (SC) (sc-9049, Santa Cruz, CA; antigen aa 221–363 of human AT2), or by Alomone (AL) (AAR-012, Jerusalem, Israel; antigen aa 21–35 of rat AT2 that is about 50% identical with human AT2). The immunoreaction was detected by incubating the membrane with a respective secondary Ab linked to horseradish peroxidase (Amersham; Buckinghamshire, UK), and then with ECL-Plus (Amersham). Immunoblotting was also performed with an anti-actin Ab (MAB1501R; Chemicon, Temecula, CA) to confirm that the same amount of proteins was loaded. The neutralization of an Ab with its antigen was performed according to the manufacturer's instructions.
Immunohistochemistry
The rats (n=2) and guinea pigs (n=2) were anesthetized with sodium pentobarbital (50 mg/kg−1 intraperitoneally) and perfused through the ascending aorta with 30 ml of saline, then 250 ml of Zamboni's fixative. The adrenal glands were fixed in the fixative overnight at 4C. After fixation, they were rinsed in PBS, and then the medium was successively exchanged with 10%, 20%, and 30% sucrose-containing PBS. Thin sections (10 μm thick) were obtained with a cryostat (OTF5000 CRYOSTAT; Bright, Huntingdon, UK) and mounted on glass slides (MAS-coated Superfrost; Matsunami, Kishiwada, Japan). After treatment with 0.2% casein for 60 min to reduce the nonspecific binding, the sections were incubated overnight with the SC-made anti-AT2 Ab diluted 1:50, followed by treatment with anti-rabbit IgG Abs conjugated with Alexa 488 or Alexa 546 (Molecular Probes; Eugene, OR). The fluorescence was observed with a confocal laser scanning microscope (LSM5 Pascal; Carl Zeiss, Oberkochen, Germany). For Alexa 488, a 488-nm laser was used, and emission of 510–560 nm was observed (FITC fluorescence); for Alexa 546, a 543-nm laser was used, and emission >570 nm was observed (rhodamine fluorescence).
The adrenal glands of bovines (n=3) were fixed in the fixative overnight at 4C. After fixation and rinsing in PBS, they were dehydrated through a graded ethanol series and embedded in paraffin (Histosec; Merck, Darmstadt, Germany). Thin sections (5 μm thick) were obtained with a microtome, mounted on glass slides, dried overnight, and deparaffinized. Next, the sections were rinsed for 10 min in PBS. Endogenous peroxidase activity was inhibited by pretreatment with 0.1% hydrogen peroxide in methanol for 20 min. After treatment with 0.2% casein for 60 min to reduce the nonspecific binding, the sections were incubated overnight with the AD-made anti-AT2 Ab. After rinsing in PBS, the immunoreaction was examined using the indirect immunoperoxidase method (Histofine Simple Stain Max-PO; Nichirei, Tokyo, Japan). The peroxidase complex was visualized by treatment with a freshly prepared solution of diaminobenzidine tetrahydrochloride (DAB) (DAB Substrate Kit; Nichirei), and the diaminobenzidine reaction was enhanced by the addition of nickel ammonium sulfate.
Immunocytochemistry
AM cells were dissociated from adrenal medullae of guinea pigs (n=2) with collagenase treatment, as described previously (Inoue et al. 2008). For indirect immunofluorescence studies, cells were treated overnight with the SC anti-AT2 Ab diluted 1:50 to 1:200. After incubation, the cells were washed three times in PBS and then treated with an anti-rabbit IgG Ab conjugated with Alexa 488 (Molecular Probes). Fluorescence was observed with an LSM5 Pascal microscope.
Cell Culture and Transfection
Human embryonic kidney (HEK) 293T cells were cultured in DMEM (Invitrogen; Carlsbad, CA) supplemented with 10% fetal bovine serum (Nichirei). Lipofectamine 2000 Reagent (Invitrogen) was used to transfect HEK293T cells with the expression vector for hemagglutinin (HA)-tagged human AT2 (Missouri S and T cDNA Resource Center; Rolla, MO) according to the manufacturer's instructions. The transfected cells were placed onto glass coverslips coated with collagen type I (BD Biosciences; San Jose, CA), and then cultured for 24 hr. The cells were fixed with 4% paraformaldehyde in PBS for 30 min at room temperature. After washing three times with PBS, the cells were incubated in PBS containing 0.1% Triton X-100 for 30 min and then with PBS containing 1% FBS for 1 hr at room temperature. The cells were treated with primary Abs and then with secondary Abs. The coverslips were mounted in 50% glycerol containing 1 mg/ml 1,4-diaminobenzene, and immunostaining was observed with an LSM5 Pascal microscope.
RT-PCR
The Micro-fast Track Kit (Invitrogen) was used according to the manufacturer's instructions to isolate poly(A)+ RNA from the brain, adrenal medulla, and/or adrenal cortex of rats (n=5), bovines (n=1), and humans (n=2). To digest the genomic DNA, mRNA prepared from rat and human tissues was treated with DNase I (Invitrogen). Oligo dT primer was utilized for the reverse transcriptase (RT) reaction to obtain cDNAs. PCR reactions were carried out with 1.25 μl of DNA template, 4 pmol of primer, 2 mM of dNTPs, 0.5 U KOD FX DNA polymerase (Toyobo; Osaka, Japan), and PCR buffers supplied with the kit, in a final volume of 25 μl. Table 1 lists the primers for the PCR. The PCR protocol used started with an initial 3-min denaturation step at 94C, followed by 30–40 cycles of the profile consisting of 30 sec of denaturation at 94C, 30 sec of annealing at 54C to 60C, and 30 sec of extension at 72C. To obtain the maximum fidelity, a hot-start procedure was used. In each PCR reaction, a 198-bp PCR product of β-actin mRNA was amplified simultaneously or separately and used as an internal standard. The PCR products were separated by 1.5% agarose gel electrophoresis, and stained with ethidium bromide.
Table 1.
Primer sequences used for PCR of AT2 receptor and β-actin
| Target gene | Forward primer | Reverse primer | Product size (bp) |
|---|---|---|---|
| Rat or bovine AGTR2 | 5′-gataagcatttagatgcaattcctgttc-3′ | 5′-cttctgaatttcataagccttc-3′ | 275 |
| Human AGTR2 | 5′-gtcaaggaaaagggtatactccaagg-3′ | 5′-gtgtgctcaggctcaccattg-3′ | 554 |
| β-actin | 5′-cctgggtatggaatcctgtggcat-3′ | 5′-ggagcaatgatcttgatcttc-3′ | 198 |
AT2, type-2 angiotensin II.
Results
RT-PCR
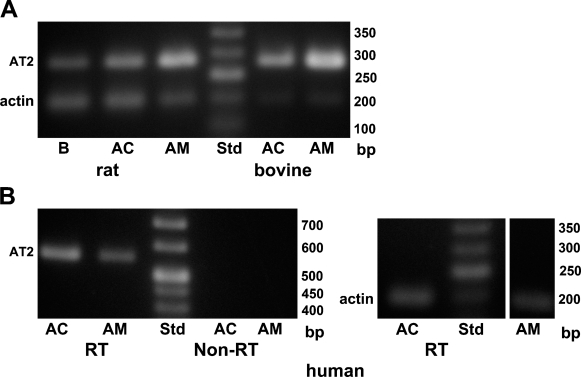
The expression of AT2 at the mRNA level was examined with the RT-PCR technique. Primer sequences for PCR analysis were selected so that an intron was present between the forward and backward primers. Therefore, PCR products originating from the genome were clearly differentiated from PCR products from cDNAs (Figure 1A). PCR products of 285 bp, the size estimated for AT2 mRNA, were detected in cDNA samples of rat brain, adrenal cortex, and adrenal medulla. PCR products of the same size were also recognized in samples of both bovine adrenal cortex and adrenal medulla (Figure 1A). Next, the same set of primers was used to examine whether mRNA for AT2 was expressed in human adrenal cortex and adrenal medulla. PCR products of 285 bp were detected in cDNA samples of human adrenal medulla, but not adrenal cortex, whereas β-actin amplicons were found in both samples (not shown). This failure of detection in the adrenal cortex was possibly due to deterioration of the human adrenal cortex, which was excised during surgery. To reduce the effect of deterioration, backward and forward primer sequences were selected to be near the beginning of the coding region in mRNA for AT2 without insertion of an intron. Therefore, non-RT samples were simultaneously subjected to PCR analysis to exclude the possible contamination of genomic DNA. Figure 1B shows that AT2 amplicons were detected in both adrenal cortex and adrenal medulla cDNAs, but not in non-RT samples, indicating that PCR products for AT2 originate from cDNA, but not genomic DNA. The results indicate that mRNAs for AT2 are produced in both the adrenal cortex and the adrenal medulla of rats, bovines, and humans.
Figure 1.
PCR analysis for expression of the type-2 angiotensin II receptor (AT2). (A) Electrophoresis of PCR products obtained with rat and bovine cDNA samples. The upper and lower bands represent PCR products of 275 bp and 198 bp for AT2 and β-actin, respectively. (B) Electrophoresis of PCR products obtained with human cDNA samples. RT and Non-RT indicate nucleotides obtained from RNAs treated with (RT) and without (Non-RT) reverse transcriptase, respectively (see Materials and Methods). The upper and lower bands represent PCR products of 554 bp and 198 bp for AT2 and β-actin, respectively. AC, adrenal cortex; AM, adrenal medulla; B, brain.
Immunoblotting
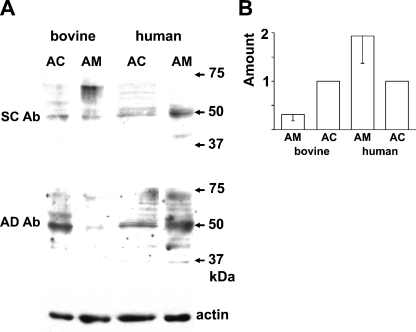
The molecular mass of AT2 identified immunologically or autoradiographically varied among the organs and the species (Ouali et al. 1993; Servant et al. 1996; Nora et al. 1998; Wang et al. 1998; Frei et al. 2001; Carneiro-Ramos et al. 2007). Thus, three anti-AT2 Abs that were raised against different antigens were used. Figure 2A shows immunoblotting with an AL-made anti-AT2 Ab. The Ab clearly recognized a 50-kDa band in the rat adrenal cortex but not in the adrenal medulla, and this detection was markedly suppressed by preabsorption of the Ab with the antigen, suggesting that the Ab is specific for AT2. The AL Ab also detected the 50-kDa band in guinea pig adrenal medulla as well as adrenal cortex (Figure 2B). Figure 2C summarizes the expression levels of AT2 in rat and guinea pig adrenal glands. The amount of protein expressed in the guinea pig adrenal medulla was about half the amount in the adrenal cortex, whereas the amount of AT2 in the rat adrenal medulla was one-tenth that in the adrenal cortex. Immunoblotting of rat and guinea pig adrenal glands was also performed with an SC-made anti-AT2 Ab with similar results (not shown). To explore the expression of AT2 in bovine and human adrenal glands, SC- and AD-made anti-AT2 Abs were used. As shown in Figure 3A, both Abs recognized 50-kDa bands in bovine adrenal cortex and medulla. Similarly, the 50-kDa band was recognized in human adrenal glands. However, the expression levels of the protein in humans differed from those in the other three mammals; the amount of 50-kDa protein in the human adrenal medulla was about twice that in the adrenal cortex (Figure 3B).
Figure 2.
Immunoblot of rat and guinea pig adrenal glands for AT2. (A) Immunoblots of rat AC and AM homogenates with AL-made anti-AT2 antibody (Ab) and anti-actin Ab. The middle panel represents an immunoblot in which the Ab was preabsorbed with an antigen (see Materials and Methods). (B) Immunoblots of guinea pig AC and AM homogenates with the AL-made Ab and anti-actin Ab. (C) Summary of expression levels of AT2 in AM and AC homogenates of rats and guinea pigs. The data represent means ± SEM (rat, n=3; guinea pig, n=4). The expression levels of AT2 in AM were expressed as fractions of those of AT2 in AC.
Figure 3.
Immunoblot of bovine and human adrenal glands for AT2. (A) Immunoblots of AC and AM homogenates of bovines and humans with SC- and AD-made anti-AT2 Abs and anti-actin Ab. (B) Summary of expression levels of AT2 in AM and AC homogenates of bovines and humans examined with the SC-made anti-AT2 Ab. The data represent means ± SEM (bovine, n=3; human, n=3).
Immunohistochemistry
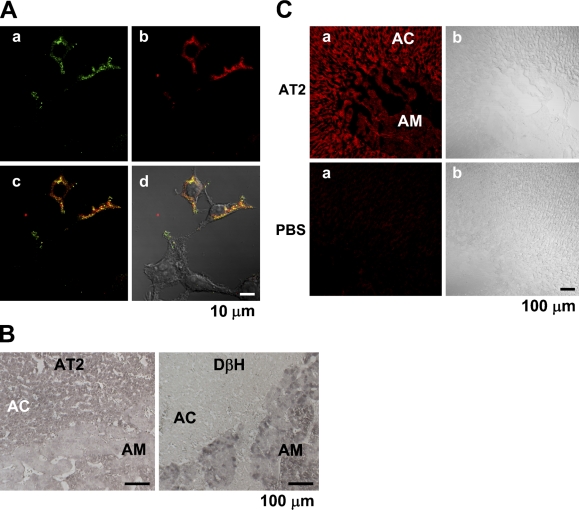
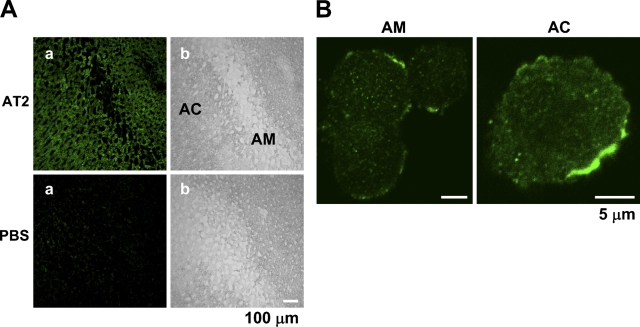
We first examined the specificity of the AD- and SC-made Abs in immunohistochemistry. Figure 4A shows that the AD-made Ab selectively stained the HEK294T cells transfected with an AT2 vector encoding the human AT2 and HA tag, indicating that the Ab is specific for AT2 in staining. Similar results were obtained with the SC-made Ab (not shown). The AD-made Ab produced significant staining in the bovine adrenal cortex (n=6), compared with that in the adrenal medulla, whereas an anti-dopamine-β-hydroxylase Ab stained the adrenal medulla and not the adrenal cortex (Figure 4B). Next, the rat adrenal gland was immunohistochemically examined with the SC anti-AT2 Ab. Consistent with the expression levels obtained with immunoblotting, the intensity of immunofluorescence in the adrenal cortex was significantly higher than that in the adrenal medulla (n=5). On the other hand, fluorescence was not observed in any part of the section not treated with the Ab (Figure 4C), suggesting that fluorescence was due to immunoreaction with the anti-AT2 Ab. The section of guinea pig adrenal gland was also examined with the SC-made AT2 Ab. As shown in Figure 5A, the adrenal medulla and cortex were rather homogeneously stained (n=6), whereas the section treated without the Ab did not show noticeable fluorescence. Finally, the intracellular localization of AT2 was examined in isolated guinea pig AM and AC cells. In both cells (AM, n=11; AC, n=8), AT2-like immunoreactivity was mainly located in the plasma membrane or in its vicinity (Figure 5B).
Figure 4.
Immunohistochemistry of bovine and rat adrenal glands for AT2. (A) Double staining for AT2 and hemagglutinin (HA) in HEK293T cells transfected with HA-AT2 vector. HEK293T cells were treated with rabbit AD-made anti-AT2 Ab (dilution, 1:50) and mouse anti-HA mAb (1:100) (A00186; GenScript, Piscataway, NJ). The reactions with the former and the latter were visualized with anti-rabbit IgG conjugated with Alexa 488 and anti-mouse IgG with Alexa 546, respectively. Panels a and b represent FITC-like and rhodamine-like fluorescence. Panel c is an overlay image of a and b with convergence in yellow, and d is an overlay image of c and differential interference contrast (DIC) image. (B) Immunostaining of bovine adrenal section with AD-made anti-AT2 Ab (1:100) and anti-DβH Ab (1:250) (AB1538; Chemicon). Each immunoreaction was visualized with the indirect immunoperoxidase method (see Materials and Methods). (C) Immunostaining of a rat adrenal section for AT2. The sections of rat adrenal gland were treated with or without (PBS) SC-made anti-AT2 Ab, and then with anti-rabbit IgG conjugated with Alexa 546.
Figure 5.
Immunostaining of guinea pig adrenal gland for AT2. (A) Immunohistochemical staining of guinea pig adrenal sections for AT2. The sections were treated with or without (PBS) SC-made anti-AT2 Ab and then with anti-rabbit IgG Ab conjugated with Alexa 488. a and b are FITC fluorescence and DIC, respectively. (B) Immunostaining of guinea pig adrenal medullary (AM) and adrenal cortical (AC) cells with SC-made anti-AT2 Ab and with anti-rabbit IgG conjugated with Alexa 546. Immunoreaction was visualized as FITC fluorescence. AM and AC cells were isolated with collagenase treatment (see Materials and Methods).
Discussion
Whether ouabain or a ouabain-like substance is secreted from AC cells remains controversial (Murrell et al. 2005; Schoner and Scheiner-Bobis 2007; Manunta et al. 2009; Nicholls et al. 2009). Ouabain or a ouabain-like substance, however, has consistently been found in the adrenal cortex of rats (Ludens et al. 1992), bovines (Laredo et al. 1997), and humans (El-Masri et al. 2002). If endogenous ouabain is released from AC cells following stimulation of AT2, as has been suggested in bovine AC cells (Laredo et al. 1997), the secreted ouabain may be expected to enhance catecholamine secretion in AM cells. In fact, application of a low concentration of ouabain resulted in an increase in Ca2+ mobilization– induced secretion in guinea pig AM cells (Lin et al. 2005). If this hypothesis is correct, ouabain would be expected to ameliorate, through its direct and catecholamine-mediated effects on the heart, a decrease in renal blood flow, which may be a cause for an increase in blood AGII in the classical renin-angiotensin system. From this point of view, it would be important to elucidate whether AT2 is consistently expressed in mammalian AC cells or not. In the present experiment, this issue was examined at the mRNA and protein levels. The RT-PCR analysis revealed that the AT2 receptor was expressed at the mRNA level in adrenal cortices and medullae of humans, bovines, and rats. On the other hand, PCR products were not detected in any cDNA samples of guinea pig brain, adrenal cortex, or adrenal medulla with a set of primers that was successful for PCR of rat samples. This failure was probably due to a slight difference in gene structure. Expression at the protein level was explored with three distinct anti-AT2 Abs, which had been raised against different epitopes. All three Abs clearly recognized a 50-kDa protein in the adrenal cortex in rats, guinea pigs, bovines, and/or humans, and recognition of the protein by the AL-made Ab was suppressed by preabsorption of the Ab with the antigen. On the other hand, the AT2 in the adrenal medulla was detected at low levels in bovines and guinea pigs and prominently in humans, but it was scarcely recognized in rats. These differences in expression of the 50-kDa protein between the adrenal cortices and medullae of bovines, rats, and guinea pigs were also observed in immunohistochemical studies with the SC- and AD-made Abs. On the basis of the immunoblotting and immunohistochemical findings, we conclude that the 50-kDa protein represents AT2 in the adrenal gland and is expressed consistently and variably in the adrenal cortex and medulla, respectively. The finding that AT2 was expressed predominantly in the rat adrenal cortex and scarcely in the adrenal medulla conflicts with previous results showing that AT2-like immunoreactive substances were exclusively present in the rat adrenal medulla and that mRNA for AT2 was detected in the rat adrenal medulla and zona glomerulosa, and not in the zona reticularis or zona fasciculata, with the in situ hybridization technique. The anti-AT2 Ab used in the previous immunohistochemical analyses recognized a protein of ∼70 kDa, which differed from the 50-kDa protein in the present experiment. Therefore, the immunoreactivity obtained with the previous Ab might have reflected a protein unrelated to AT2. Furthermore, the expression of mRNA for AT2 may not necessarily indicate that of AT2 at the protein level. In our previous experiments, TASK3 channels in the rat adrenal medulla were detected at the mRNA level but not at the protein level (Inoue et al. 2008). A similar discrepancy of expression between the mRNA and protein levels has been noted with membrane proteins, such as other K+ channels (Barry et al. 1995).
Belloni et al. (1998) noted that [125I]AGII binding in the rat adrenal gland occurred in the medulla and zona glomerulosa. The binding in the former and latter were markedly suppressed by CGP42112, an AT2 agonist, and DuP753, an AT1 antagonist, respectively, and AGII-induced catecholamine secretion was conspicuously inhibited by PD123319, an AT2 antagonist, but not by DuP753. These results seem to suggest that AT2 is selectively expressed in the rat adrenal medulla and is involved in catecholamine secretion in response to AGII. This notion, however, is not compatible with the electrophysiological findings that in bovine AM cells, AGII receptors involved in catecholamine secretion showed rapid desensitization (Teschemacher and Seward 2000), which is characteristic of AT1 and not of AT2 (De Gasparo et al. 2000; Landon and Inagami 2005; Mogi et al. 2007), that AGII produced a Ca2+ mobilization with the consequent release of catecholamine through a phospholipase C pathway (Stauderman and Pruss 1990; Cheek et al. 1993; Teschemacher and Seward 2000), and most importantly that AGII-induced secretion was completely suppressed by DuP753, but not by PD123319 (Teschemacher and Seward 2000). This difference would warrant a more detailed study to identify the subtype of AGII receptor expressed in the rat adrenal medulla at the protein level.
In summary, three different anti-AT2 Abs, whose specificity was confirmed in our hands, recognized a 50-kDa protein in adrenal glands of humans, rats, guinea pigs, and bovines, and the recognition of the protein was abolished by the preabsorption of an Ab with an antigen. Immunoblotting and immunohistochemistry revealed that the 50-kDa protein was expressed consistently and variably in adrenal cortices and medullae of various mammals, respectively. We conclude that AT2 receptors of 50 kDa are consistently expressed in the adrenal cortex in a wide variety of mammals. This consistent expression of AT2 in the adrenal cortex may suggest that AT2 receptors play an essential role in the functions of the adrenal cortex. One such function might be the regulation of the secretion of ouabain or a ouabain-like substance.
Acknowledgments
This work was supported in part by Grant-in-Aid for Scientific Research on Priority Areas (21026029 to MI) and Grant-in-Aid for Scientific Research (C) (21500360 to MI).
We are grateful to Y. Doi for guidance on the histochemical studies and T. Hatama for technical assistance.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Bagrov AY, Shapiro JI, Fedorova OV (2009) Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol Rev 61:9–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DM, Trimmer JS, Merlie JP, Nerbonne JM (1995) Differential expression of voltage-gated K+ channel subunits in adult rat heart. Relation to functional K+ channels? Circ Res 77:361–369 [DOI] [PubMed] [Google Scholar]
- Belloni AS, Andreis PG, Macchi V, Gottardo G, Malendowicz LK, Nussdorfer GG (1998) Distribution and functional significance of angiotensin-II AT1- and AT2-receptor subtypes in the rat adrenal gland. Endocr Res 24:1–15 [DOI] [PubMed] [Google Scholar]
- Carneiro-Ramos MS, Diniz GP, Almeida J, Vieira RL, Pinheiro SV, Santos RA, Barreto-Chaves ML (2007) Cardiac angiotensin II type I and type II receptors are increased in rats submitted to experimental hypothyroidism. J Physiol 583:213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheek TR, Morgan A, O'Sullivan AJ, Moreton RB, Berridge MJ, Burgoyne RD (1993) Spatial localization of agonist-induced Ca2+ entry in bovine adrenal chromaffin cells. Different patterns induced by histamine and angiotensin II, and relationship to catecholamine release. J Cell Sci 105:913–921 [DOI] [PubMed] [Google Scholar]
- De Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T (2000) International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 52:415–472 [PubMed] [Google Scholar]
- El-Masri MA, Clark BJ, Qazzaz HM, Valdes R Jr (2002) Human adrenal cells in culture produce both ouabain-like and dihydroouabain-like factors. Clin Chem 48:1720–1730 [PubMed] [Google Scholar]
- Frei N, Weissenberger J, Beck-Sickinger AG, Höfliger M, Weis J, Imboden H (2001) Immunocytochemical localization of angiotensin II receptor subtypes and angiotensin II with monoclonal antibodies in the rat adrenal gland. Regul Pept 101:149–155 [DOI] [PubMed] [Google Scholar]
- Inoue M, Harada K, Matsuoka H, Sata T, Warashina A (2008) Inhibition of TASK1-like channels by muscarinic receptor stimulation in rat adrenal medullary cells. J Neurochem 106:1804–1814 [DOI] [PubMed] [Google Scholar]
- Landon EJ, Inagami T (2005) Beyond the G protein: the saga of the type 2 angiotensin II receptor. Arterioscler Thromb Vasc Biol 25:15–16 [DOI] [PubMed] [Google Scholar]
- Laredo J, Shah JR, Lu ZR, Hamilton BP, Hamlyn JM (1997) Angiotensin II stimulates secretion of endogenous ouabain from bovine adrenocortical cells via angiotensin type 2 receptors. Hypertension 29:401–407 [DOI] [PubMed] [Google Scholar]
- Lin H, Ozaki S, Fujishiro N, Takeda K, Imanaga I, Prestwich GD, Inoue M (2005) Subunit composition and role of Na+,K+-ATPases in adrenal chromaffin cells. J Physiol 564:161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludens JH, Clark MA, Robinson FG, DuCharme DW (1992) Rat adrenal cortex is a source of a circulating ouabainlike compound. Hypertension 19:721–724 [DOI] [PubMed] [Google Scholar]
- Manunta P, Ferrandi M, Bianchi G, Hamlyn JM (2009) Endogenous ouabain in cardiovascular function and disease. J Hypertens 27:9–18 [DOI] [PubMed] [Google Scholar]
- Mogi M, Iwai M, Horiuchi M (2007) Emerging concepts of regulation of angiotensin II receptors: new players and targets for traditional receptors. Arterioscler Thromb Vasc Biol 27:2532–2539 [DOI] [PubMed] [Google Scholar]
- Murrell JR, Randall JD, Rosoff J, Zhao JL, Jensen RV, Gullans SR, Haupert GT Jr (2005) Endogenous ouabain: upregulation of steroidogenic genes in hypertensive hypothalamus but not adrenal. Circulation 30:1301–1308 [DOI] [PubMed] [Google Scholar]
- Nicholls MG, Lewis LK, Yandle TG, Lord G, McKinnon W, Hilton PJ (2009) Ouabain, a circulating hormone secreted by the adrenals, is pivotal in cardiovascular disease. Fact or fantasy? J Hypertens 27:3–8 [DOI] [PubMed] [Google Scholar]
- Nora EH, Munzenmaier DH, Hansen-Smith FM, Lombard JH, Greene AS (1998) Localization of the ANG II type 2 receptor in the microcirculation of skeletal muscle. Am J Physiol 275:H1395–1403 [DOI] [PubMed] [Google Scholar]
- Ouali R, LeBrethon MC, Saez JM (1993) Identification and characterization of angiotensin-II receptor subtypes in cultured bovine and human adrenal fasciculata cells and PC12W cells. Endocrinology 133:2766–2772 [DOI] [PubMed] [Google Scholar]
- Peters B, Clausmeyer S, Teubner P, Obermüller N, Kränzlin B, Gretz N, Inagami T, et al. (2001) Changes of AT2 receptor levels in the rat adrenal cortex and medulla induced by bilateral nephrectomy and its modulation by circulating ANG II. J Histochem Cytochem 49:649–656 [DOI] [PubMed] [Google Scholar]
- Schoner W, Scheiner-Bobis G (2007) Endogenous and exogenous cardiac glycosides: their roles in hypertension, salt metabolism, and cell growth. Am J Physiol Cell Physiol 293:C509–536 [DOI] [PubMed] [Google Scholar]
- Servant G, Dudley DT, Escher E, Guillemette G (1996) Analysis of the role of N-glycosylation in cell-surface expression and binding properties of angiotensin II type-2 receptor of rat pheochromocytoma cells. Biochem J 313:297–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauderman KA, Pruss RM (1990) Different patterns of agonist-stimulated increases of 3H-inositol phosphate isomers and cytosolic Ca2+ in bovine adrenal chromaffin cells: comparison of the effects of histamine and angiotensin II. J Neurochem 54:946–953 [DOI] [PubMed] [Google Scholar]
- Teschemacher AG, Seward EP (2000) Bidirectional modulation of exocytosis by angiotensin II involves multiple G-protein-regulated transduction pathways in chromaffin cells. J Neurosci 20:4776–4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZQ, Moore AF, Ozono R, Siragy HM, Carey RM (1998) Immunolocalization of subtype 2 angiotensin II (AT2) receptor protein in rat heart. Hypertension 32:78–83 [DOI] [PubMed] [Google Scholar]
- Warashina A, Habara Y, Yamaguchi K, Kanno T (1994) [Ca2+]i changes arising in individual guinea-pig chromaffin cells in response to various receptor agonist and their relation to catecholamine secretion in the perfused adrenal gland. Biomed Res 15:271–280 [Google Scholar]