Abstract
In rodents, the Otx2 gene is expressed in the diencephalon, mesencephalon, and cerebellum and is crucial for the development of these brain regions. Together with Otx1, Otx2 is known to cooperate with other genes to develop the caudal forebrain and, further, Otx1 is also involved in differentiation of young neurons of the deeper cortical layers. We have studied the spatial and temporal expression of the two homeobox genes OTX2 and OTX1 in human fetal brains from 7 to 14 weeks postconception by in situ hybridization and immunohistochemistry. OTX2 was expressed in the diencephalon, mesencephalon, and choroid plexus, with a minor expression in the basal telencephalon. The expression of OTX2 in the hippocampal anlage was strong, with no expression in the adjacent neocortex. Contrarily, the OTX1 expression was predominantly located in the proliferative zones of the neocortex. At later stages, the OTX2 protein was found in the subcommissural organ, pineal gland, and cerebellum. The early expression of OTX2 and OTX1 in proliferative cell layers of the human fetal brain supports the concept that these homeobox genes are important in neuronal cell development and differentiation: OTX1 primarily in the neocortex, and OTX2 in the archicortex, diencephalon, rostral brain stem, and cerebellum. (J Histochem Cytochem 58:669–678, 2010)
Keywords: neocortex, fetus, homo sapiens, neuroanatomy
Transription factors are proteins that often function as markers of ontogenic units of the developing central nervous system and label and delineate specific brain areas and nuclei. Among the transcription factors important during development are homeobox genes, which are expressed during specific time windows of the ontogenesis.
The Otx2 and Otx1 homeobox genes are vertebrate orthologs to the Drosophila orthodenticle homeobox gene (Simeone et al. 1992). Otx2 is expressed as early as in the murine blastula (Simeone et al. 1993) in the entire epiblast, but becomes progressively restricted to the rostral part of the embryo during the gastrulation process (Simeone et al. 1993; Ang et al. 1994; Acampora et al. 1995; Mallamaci et al. 1996). Rodent studies have revealed that Otx2 is expressed in prosencephalon and mesencephalon, with a sharp caudal boundary at the mesencephalic–metencephalic border (Simeone et al. 1992,1993; Acampora et al. 1995; Broccoli et al. 1999; Millet et al. 1999). At later embryonic stages, Otx2 is also expressed in caudal areas of the forebrain, e.g., the ventricular zone of the ganglionic eminence, hippocampus, choroid plexus, and the diencephalon. Further, Otx2 is expressed in the posterior commissure of the mesencephalon and in the cerebellum (Frantz et al. 1994; Rath et al. 2006; Rakic et al. 2009).
Otx1 expression starts when the anterior neuroectoderm develops into prosencephalon and mesencephalon. At this stage of development, the expression of Otx1 is in the same areas as Otx2 but is more restricted. At later embryonic stages, Otxl is expressed in the cortical ventricular zone and the emerging cortical plate of the neocortex, in contrast to the Otx2 gene. An expression of Otx1 is also found in the choroid plexus of the lateral ventricles, the ventricular zone of the ganglionic eminence, the olfactory bulb, the hippocampus, and the cerebellum (Simeone et al. 1992,1993; Frantz et al. 1994).
Knockout mice have revealed important functions of the Otx genes in the brain. Thus, forebrain and midbrain regions are not developed in Otx2-homozygous knockout mice, owing to defective anterior neuroectoderm specification during gastrulation (Acampora et al. 1995; Ang et al. 1996; Rhinn et al. 1998).
Homozygous Otx1 knockout mice show spontaneous epileptic behavior and abnormalities affecting neocortical areas, the hippocampus, the mesencephalon, and the cerebellum (Acampora et al. 1996). Furthermore, a reduction in both the cortical thickness and the number of cells in the cortex has been reported (Pantò et al. 2004).
Although expression patterns of Otx2 and Otx1 have been extensively studied in non-human species, little is known about the expression of these genes during development of the human brain. In this study, we have examined the spatio-temporal expression of OTX2 and OTX1 in the human fetal brain in fetal weeks 7 to 14. OTX1 was predominantly expressed in the neocortex, whereas OTX2 was expressed in the archicortex, diencephalon, mesencephalon, and, in later fetal stages, also in the cerebellum.
Materials and Methods
Fetal Brains
Five human fetal brains were used for in situ hybridization and immunohistochemistry. Three of the brains were used for cryostat sectioning and two brains were embedded in paraffin and sectioned.
Materials for Cryostat Sectioning (In Situ Hybridization and Immunohistochemistry)
Brains from three fetuses, ages 7 and 9 fetal weeks (7 fetal weeks: n=1; 9 fetal weeks: n=2), were collected from legal abortions at Frederiksberg Hospital, Copenhagen, Denmark, in 2007. The study was approved by the local ethical committee for Copenhagen and Frederiksberg, and parental consent was obtained (see Lutterodt et al. 2009 for further information). Within 3–5 min after intrauterine removal, the fetuses were fixed by immersion in cold 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 24 hr. This was followed by cryosubstitution for 2–3 days in 30% sucrose in PBS. All samples were frozen in crushed carbon dioxide and stored at −20C until they were sectioned in a cryostat.
Materials for Paraffin Sectioning (Immunohistochemistry)
Out of a series of fetuses, obtained during surgery for extra uterine pregnancies, Caesarean sections, or prostaglandin-induced abortions in connection with legal abortions, two fetuses, ages 13 and 14 fetal weeks, were collected. Informed consent was obtained according to the guidelines of Helsinki Declaration II. The fetal age was based on information about the last menstrual period and measurements of crown–rump lengths of the fetuses (Stagaard Janas et al. 1991). The brains were immediately removed, and the specimens were dissected into appropriate blocks and fixed for 12–24 hr at 4C in 10% buffered formalin. The specimens were dehydrated in a graded ethanol series, cleared in toluene, and embedded in paraffin wax (Merck, Whitehouse Station, NJ; melting point, 52C).
The fetal age, in weeks postconception, was expressed in commenced fetal weeks, e.g., 13 fetal weeks ranged from 12 completed weeks plus 1 day to the termination of week 13. Both human embryos (from conception to day 56 postconception) and fetuses (from day 57 postconception) (O'Rahilly 1979) are referred to as “fetuses” in this study.
In Situ Hybridization Histochemistry
The brains were sectioned in 14-μm-thick frozen sections and mounted on Superfrost Plus slides (Menzel; Braunschweig, Germany). The sections were then washed in PBS and acetylated in 0.25% acetic anhydride for 10 min. This was followed by dehydration in a graded series of ethanols, delipidation in chloroform, and partial rehydration in 100% and 95% ethanol. For hybridization, 38-mer antisense DNA probes and sense control probes were used (Table 1).
Table 1.
Probes used for in situ hybridization
| mRNA detected | Probe sequence | Antisense/sense | Position | Database number |
|---|---|---|---|---|
| Human OTX2 | 5′-TACCAACAGTAAATTACAGATGAATTAGTGTCCTTTTG-3′ | Antisense | 1897–1860 | NM_172337 |
| Human OTX2 | 5′-GATTGGTTTGTCCATTTCATGTTGCTGGTTTGTAGGCC-3′ | Antisense | 1240–1203 | NM_172337 |
| Human OTX2 | 5′-TAACCTGAAGCCTGAGTATAGGTCATGGGATAGGACCT-3′ | Antisense | 725–688 | NM_172337 |
| Human OTX2 | 5′-CAAAAGGACACTAATTCATCTGTAATTTACTGTTGGTA-3′ | Sense | 1860–1897 | NM_172337 |
| Human OTX1 | 5′-TATGTGCTGTATATATCTTAAAGTTCTGTTTCCTGGGT-3′ | Antisense | 2132–2095 | NM_014562 |
| Human OTX1 | 5′-GGGGAAATAATACATCTCTCCTGTATAGAATGTGTAGG-3′ | Antisense | 1583–1546 | NM_014562 |
| Human OTX1 | 5′-GCTGCCGGGGCCGTTCACCATCTACCTGGATCTCTCAC-3′ | Antisense | 128–91 | NM_014562 |
| Human OTX1 | 5′-ACCCAGGAAACAGAACTTTAAGATATATACAGCACATA-3′ | Sense | 2095–2132 | NM_014562 |
The probes were diluted in diethyl pyrocarbonate–treated water to a concentration of 5 pmol/μl. Probes for detection of the same transcript were mixed before labeling. A total of 3 μl was then labeled with [35S]-dATP (Perkin Elmer; Boston, MA) using terminal transferase (Roche; Penzberg, Germany) to a specific activity of 1 × 1018 dpm/mol. Five μl of the labeled probe was diluted in 1 ml hybridization buffer consisting of 50% (v/v) formamide, 4 × SSC (SSC: 150 mM NaCl, 15 mM sodium citrate, pH 7.0), 1 × Denhardt solution (0.02% BSA, 0.02% polyvinylpyrrolidone, 0.02% ficoll), 10% (w/v) dextran sulfate, 10 mM dithiothreitol, 0.5 mg/ml salmon sperm DNA, and 0.5 mg/ml yeast tRNA. The sections were then hybridized in a humid chamber overnight at 37C. After hybridization, the slides were washed in 1 × SSC for 4 × 15 min at 55C and 2 × 30 min at room temperature, and rinsed in deionized water. The sections were dried and exposed to an X-ray film for 2–3 weeks. The X-ray film was subsequently developed in X-ray Developer (Kodak LX24; Foxdal, Odder, Denmark), diluted 1:3 for 4 min, and fixed in X-ray Fixer (Kodak AL 4) diluted 1:3 for 5 min, and finally, rinsed in tap water for 10 min before drying. The images of the sections on the X-ray film were transferred by a black-and-white video camera to a computer and quantified with ScionImage 1.42 software (National Institutes of Health; Bethesda, MD).
Immunohistochemistry for the OTX2 Protein
The brains were sectioned in 14-μm-thick frozen sections or 3–5-μm-thick paraffin-embedded sections and mounted on Superfrost Plus slides or silanized slides, respectively. The paraffin-embedded sections were then dewaxed and rehydrated through toluene and an alcohol series. Fourteen-μm-thick frozen sections were cut and mounted on Superfrost Plus. The sections were then washed in PBS for 3 × 5 min, followed by 10 min in 1% H2O2 to inhibit endogenous peroxidase, and preincubated in 5% normal swine serum diluted in PBS for 20 min. This was followed by incubation overnight in polyclonal goat anti-human OTX2 diluted to 10 μg/ml (R and D Systems; Abingdon, UK). The sections were then washed for 3 × 10 min in PBS with 0.25% bovine serum albumin and 0.1% Triton X-100, and then incubated for 1 hr in biotinylated secondary antibody donkey anti-goat (Santa Cruz Biotechnology; Santa Cruz, CA) diluted 1:400 in the same buffer. The sections were then washed 3 × 5 min in PBS with 0.25% bovine serum albumin and 0.1% Triton X-100 and incubated for 45 min in an ABC-Vectastain solution (Vector Laboratories; Burlingame, CA) diluted 1:100 in the same buffer. The sections were then washed for 5 min in PBS with 0.25% bovine serum albumin and 0.1% Triton X-100, followed by 5 × 3 min in PBS with 0.1% Triton X-100, 5 min in PBS, and 10 min in 0.05 M Tris (pH 7.6). They were subsequently incubated for peroxidase activity in 1.4 mM diaminobenzidine and 0.01% H2O2 in 0.05 M Tris/HCL buffer (pH 7.6) for 15 min. The reaction was stopped by washing the sections in excessive amounts of deionized water. Finally, the sections were dried and embedded in Pertex (Histolab; Gothenburg, Sweden). The specificity of the OTX2 antibody has previously been characterized (Rath et al. 2007).
Comparable Stages in Development
Throughout this article, comparisons of the developmental stages of the human brain to those of the rodent are based on the studies of Altman and Dittmer (1962), O'Rahilly (1979), Finlay and Darlington (1995), and Theiler (1972).
Results
OTX2 Expression
In Situ Hybridization
The expression of OTX2 mRNA was investigated by in situ hybridization on frozen brain sections from fetuses 7 and 9 fetal weeks old. In the young fetus, aged 7 fetal weeks, a strong signal was observed in the mesencephalon and the diencephalon (Figure 1B). A moderate signal was present in the basal telencephalon, the choroid plexus, and the hippocampal anlage, whereas no signal was seen in the area of the future neocortex (Figure 1B).
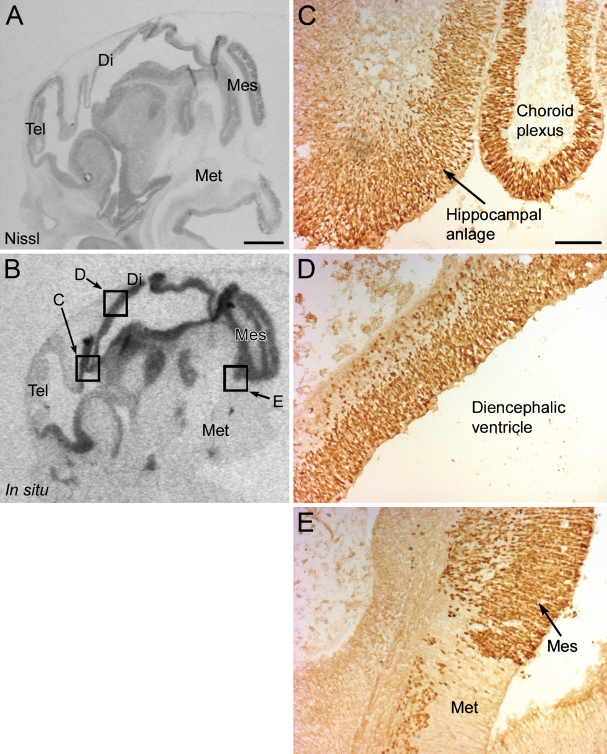
Figure 1.
Expression of OTX2 in the human fetal brain at 7 fetal weeks. (A) A Nissl-stained sagittal section of the brain. (B) X-ray image of the in situ signal of OTX2 in the same brain showing expression in the tectum, basal telencephalon, hippocampal anlage, choroid plexus, and walls of the diencephalic and mesencephalic superventricle. Squares marked in B highlight the areas of the positive immunohistochemical reactions of the OTX2 protein in C–E. (C–E) Photomicrographs of immunohistochemical reactions for the OTX2 protein in the hippocampal anlage and choroid plexus (C), diencephalic wall (D), and the caudal limit of OTX2 expression in the mesencephalon (E). Tel, telencephalon; Di, diencephalon; Mes, mesencephalon; Met, metencephalon. Bars: A,B = 1 mm; C–E = 100 μm.
At 9 fetal weeks, a strong OTX2 in situ signal was seen in the epithelial lining of the choroid plexus in the lateral ventricle and in the hippocampus, with a moderate signal in the ganglionic eminence on sections from the telencephalon (Figures 2A and 2E).
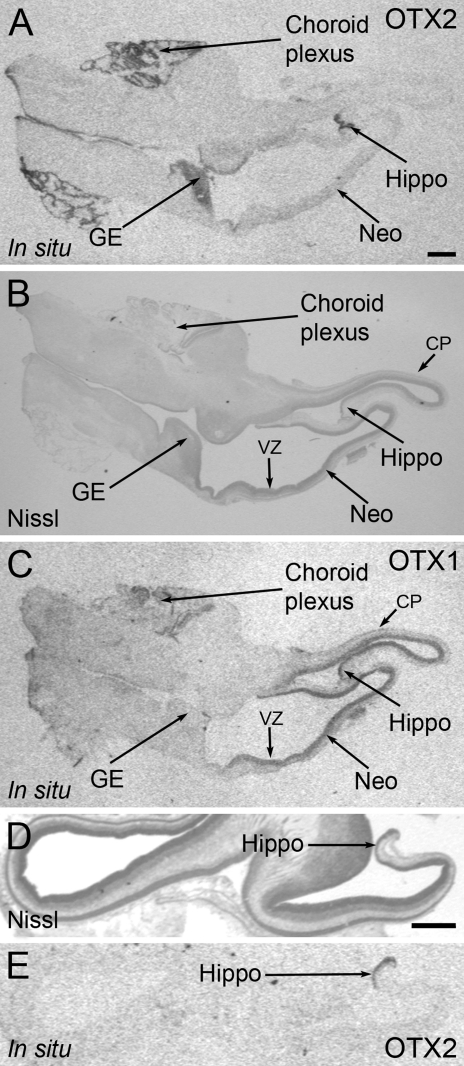
Figure 2.
Expression of OTX2 and OTX1 mRNA in the human fetal forebrain at the age of 9 fetal weeks. (A) X-ray image of the in situ signal of OTX2 in the human fetal forebrain showing strong expression in the choroid plexus, hippocampus, and ganglionic eminence. (B) Photomicrograph of a Nissl-stained parallel section of the same brain. (C) X-ray image of the in situ signal of OTX1 in the same brain. Expression of OTX1 is most notable in the proliferative zones of the neocortex. (D) Photomicrograph of a Nissl staining of the hippocampus in an additional fetus. (E) X-ray image of the hippocampal in situ signal of OTX2 in this fetus. GE, ganglionic eminence; Hippo, hippocampus; Neo, neocortex; CP, cortical plate; VZ, ventricular zone. Bar = 1 mm.
Immunohistochemistry
The expression of OTX2 protein was investigated by immunohistochemistry on frozen and paraffin-embedded brain sections from of fetuses 7 to 14 fetal weeks old.
At 7 fetal weeks, the localization of the protein corresponded with the localization of mRNA. Thus, strong immunoreactivity was observed in the diencephalon (Figure 1D), in the mesencephalon (Figure 1E), and in the epithelium of the choroid plexus (Figure 1C). A moderate signal was present in the basal telencephalon and in the hippocampal anlage (Figure 1C). Many highly immunoreactive neurons were observed in the mesencephalon, with no immunoreactivity in the metencephalon (Figure 1E). The immunoreactivity was confined to the cell nuclei, with no reactivity in the cytoplasm (Figures 1C–1E).
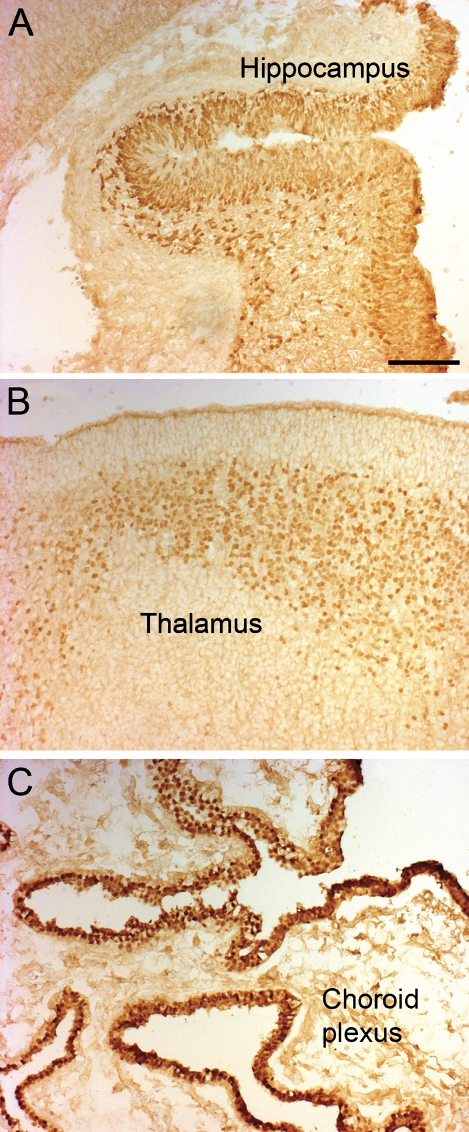
On sections from the telencephalon from fetuses aged 9 fetal weeks, a strong immunoreactivity was observed in the hippocampal anlage (Figure 3A), in the epithelium of the choroid plexus (Figure 3C), and in the thalamus in an area below the periventricular zone (Figure 3B). A weak immunoreactivity was present in the ventricular zone of the ganglionic eminence. There was no signal in the neocortex.
Figure 3.
Expression of the OTX2 protein in the human forebrain, age 9 weeks. (A) Photomicrograph of an immunohistochemical reaction for the OTX2 protein in the hippocampus. (B) Photomicrograph of the OTX2 protein in the thalamus. (C) Photomicrograph of the OTX2 protein in the choroid plexus. Bar = 100 μm.
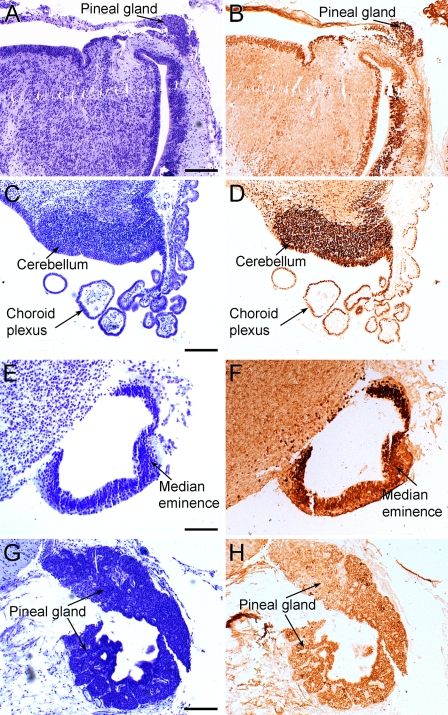
At 13 fetal weeks, strong immunoreactivity was present in the nuclei of the epithelial cells of the choroid plexus of the third ventricle (Figure 4D) and in the periventricular area of the same ventricle on sections from the diencephalon, mesencephalon, and metencephalon. The immunoreactivity became stronger toward the caudal end of the third ventricle, with very strong immunoreactivity in the periventricular cells of the pineal recess and in the pineal gland (Figure 4B). Many strong immunoreactive neurons were seen in the tectum and in the rhombic lip, from where the choroid plexus emerges as a dense collection of immunoreactive cells. Finally, a very strong immunoreactivity was observed in the internal layer of the median eminence (Figure 4F).
Figure 4.
Expression of the OTX2 protein in the human fetal brain at 13–14 fetal weeks. (A) Photomicrograph of a Nissl-stained paraffin-embedded section from a fetus, age 13 weeks. (B) Photomicrograph of a parallel section immunohistochemically reacted for the OTX2 protein. A very strong immunoreactivity is seen in the periventricular cells of the pineal recess and in the pineal gland. (C) Photomicrograph of a Nissl-stained paraffin-embedded section from a 13-week-old fetus. (D) Photomicrograph of the OTX2 protein in the cerebellar rhombic lip (cerebellum) and choroid plexus in a parallel section. (E) Photomicrograph of a Nissl-stained paraffin-embedded section from a fetus, age 13 weeks. (F) Photomicrograph of a parallel section with a very strong OTX2 immunoreactivity in the internal layer of the median eminence. (G) Photomicrograph of a Nissl-stained paraffin-embedded section from a 14-week-old fetus. (H) Photomicrograph of the OTX2 protein in the pineal gland. Bars: A,B,D = 200 μm; C = 100 μm.
In the 14-week-old fetus, a strong immunoreactivity was observed in the pineal gland on a section from this area (Figure 4H).
OTX1 Expression
In Situ Hybridization
The expression of OTX1 was investigated on frozen telencephalic sections from fetuses aged 9 fetal weeks. In both forebrains of the fetuses, OTX1 mRNA was located predominantly in the ventricular zone of the neocortex, and to a lesser extent, in the emerging cortical plate in the most lateral part of the telencephalon, and in the ventricular zone of the ganglionic eminence (Figure 2C). Only in situ hybridization results are reported for the expression of OTX1, because the immunohistochemical reactions with the available antibodies did not give satisfactory results.
Discussion
This study demonstrates that the two homeobox genes OTX2 and OTX1 are expressed during early human fetal brain development. The OTX2 gene was predominantly expressed in the diencephalon, mesencephalon, and archicortex, with no expression in the adjacent neocortex; therefore, it might be used as a hippocampal marker in the telencephalon. Contrarily, the OTX1 gene is a neocortical transcription factor expressed in the proliferative zones. At later stages, the OTX2 protein is also found in the cerebellum. These expression patterns are in accordance with the expressions observed in the developing rodent brain (Simeone et al. 1992,1993; Frantz et al. 1994; Rath et al. 2006).
Otx genes are involved in specification, regionalization, and terminal differentiation of the rostral part of the central nervous system (Boyl et al. 2001), and the function of Otx1 overlaps with that of Otx2 in the development of mouse forebrain and midbrain (Suda et al. 1996,1999).
In rodent fetuses, the Otx2 homeobox gene is expressed in the diencephalon and mesencephalon (Simeone et al. 1992; Rath et al. 2006), and knockout studies have shown that Otx2 is crucial for the development of these brain regions (Acampora et al. 1995; Matsuo et al. 1995; Ang et al. 1996). The caudal limit of Otx2 expression delineates the border between the midbrain and the hindbrain (Broccoli et al. 1999; Millet et al. 1999). At later developmental stages in the rat brain, the expression of Otx2 decreases, and in the adult rat, expression is only found in the choroid plexus and pineal gland (Rath et al. 2006). Otx2 also appears to play a role in the development and function of the retina, in which the gene is expressed at both prenatal and postnatal stages (Bovolenta et al. 1997; Martinez-Morales et al. 2001; Ragge et al. 2005; Rath et al. 2007). In the retina, Otx2 is necessary for the development and differentiation of the rods and cones via transactivation of another homeobox gene, the cone-rod gene (Crx) (Nishida et al. 2003). OTX2 was also recently detected in the human fetal retina in the pigment epithelium and the neural retina (Larsen et al. 2009).
In this study of the human fetus, both mRNA encoding the OTX2 protein, and the protein itself, were detected in the basal telencephalon, hippocampal anlage, choroid plexus, diencephalon, and mesencephalon of fetuses aged 7 fetal weeks. In the telencephalon, OTX2 continued to be expressed at a very high level in the choroid plexus and in the hippocampus at 9 fetal weeks. This spatio-temporal expression pattern in the telencephalon corresponds to that present in rodents (Simeone et al. 1993; Frantz et al. 1994; Rath et al. 2006) and supports the concept that Otx2 is involved in the generation of the caudal forebrain (Frantz et al. 1994; Kimura et al. 2005). Furthermore, we show the expression of the OTX2 protein in the pineal gland and cerebellum in the human brain at later fetal stages. The presence of Otx2 in the adult pineal gland has also been shown in the Sprague-Dawley rat (Rath et al. 2006; Bailey et al. 2009). Pineal Otx2 expression does not show circadian rhythmicity, and the expression is regulated neither by the sympathetic system nor by norepinephine (Rath et al. 2006). Therefore, the function of this transcription factor in the adult pineal gland remains enigmatic.
In zebrafish, Otx2 establishes the zona limitans intrathalamica between the ventral and dorsal thalamus (Scholpp et al. 2007). Furthermore, Otx2 controls the identity and fate of glutamatergic progenitors of the thalamus by repressing GABAergic differentiation. Therefore, any malfunction of Otx2 may cause neurological and psychiatric disorders, inasmuch as GABAergic and glutamatergic neurons modulate inhibitory and excitatory networks in the brain (Puelles et al. 2006). The pivotal role of Otx2 in thalamic development is in accordance with our observation of OTX2 expression in the human thalamus during brain development at all stages examined in this study.
During vertebrate development, an organizing signaling center, the isthmic organizer, is present at the boundary between the midbrain and the hindbrain (Martinez 2001). This organizer locally controls growth and patterning along the anteroposterior axis of the neural tube (Hidalgo-Sánchez et al. 2005). A strong expression of Otx2 is present in the isthmic organizer and is necessary for positioning the organizer and for the establishment of molecular interactions that induce other gene expressions. Thus, Otx2 is an essential regulator of the identity, extent, and fate of neuronal progenitor domains in the ventral midbrain (Puelles et al. 2003,2004) and controls proliferation and differentiation of dopaminergic progenitors (Omodei et al. 2008). The dopaminergic neurons of the mesencephalon are involved in voluntary movements, cognition, and reward responses, and their degeneration is associated with Parkinson's disease; therefore, Otx2 might be a potential target for cell replacement–based therapeutic approaches in Parkinson's disease (Omodei et al. 2008). In this study of the human fetus, we observed a strong OTX2 signal in the mesencephalon, with a sharp delineation toward the metencephalon corresponding to the isthmic organizer region in other vertebrates. Localization of the protein supports this finding, because our immunohistochemistry showed the OTX2 protein to be primarily located in the periventricular cells of the mesencephalon, also with a sharp delineation toward the metencephalon. Thus, our results show that the expression of OTX2 can also be used as a marker for the isthmic organizer in human fetuses and are in agreement with the involvement of OTX2 in neuronal phenotype determination in the mesencephalon in humans.
Both Otx1 and Otx2 take part in the development of the cerebellum (Frantz et al. 1994; Rath et al. 2006). However, neither gene is expressed in cells of the cerebellar ventricular zone, but both are expressed within the external germinal layer, a proliferative zone close to the pia, which is responsible for production of granule cells (Rakic 1971; Hallonet et al. 1990). The cells in the cerebellar external granular layer differentiate into the granule cells of the cerebellar cortex. In contrast, the other cerebellar cortical cells develop from the ventricular zone (Kuhar et al. 1993).
With regard to Otx1, this gene is also expressed in the rodent telencephalic hemispheres in both fetuses and adults. In the neocortex, Otx1 is expressed in layers 5 and 6 (Frantz et al. 1994) and play a role in the differentiation of the neurons of the deeper cortical layers (Weimann et al. 1999, Zhang et al. 2002). Further, Otx1 plays a synergistic role with Otx2 during development of the midbrain and part of the forebrain (Suda et al. 1996,1999). Otx1 is also expressed in sense organs (Acampora et al. 1996), especially in the anterior part of the retina (Larsen et al. 2009), where it is involved in formation of the ciliary body.
Some studies have investigated the impact of Otx1 on regionalization of the brain, indicating an involvement of Otx1 in the volume of the neocortex (Beatty and Laughlin 2006), especially in the proportions of the visual cortex, but not in the development of visual cortical identity (Ando et al. 2008). As mentioned above, in fetal rodents, Otx1 is expressed in the ventricular zone and in the emerging cortical plate of the telencephalic hemispheres and is confined to layers 5 and 6 of the neocortex postnatally (Frantz et al. 1994). In mice lacking functional Otx1 protein, layer 5 neurons in the visual cortex fail to prune their normally transient axon collaterals in the inferior colliculus and in the spinal cord (Weimann et al. 1999, Zhang et al. 2002). In a study by Zhang et al. (2002), it was shown that the Otx1 protein is confined to the cytoplasm of progenitor cells in the rat ventricular zone, and remains in the cytoplasm as neurons migrate and begin to differentiate. After arrival of the neurons at layer 5 in the cortex, the Otx1 protein is translocated to the nucleus and is involved in pruning of long-distance axonal projections. The importance of OTX1 for the development of the neocortex is also seen in human pathology, where the protein, together with the PAX6 proteins, is expressed in balloon cells in focal cortical dysplasia, a developmental malformation of the cerebral cortex highly associated with epilepsy. These balloon cells are derived from cortical radial glia and not from the ganglionic eminence (Lamparello et al. 2007). Both OTX1 and OTX2 are involved in the pathology of the medulloblastoma, which is the most common malignant brain tumor in children. Thus, several studies have reported that OTX2 is an important oncogenic driver in this tumor (Michiels et al. 1999; Boon et al. 2005; Di et al. 2005; Adamson et al. 2010); in a study by de Haas et al. (2006), it was revealed that the expression of both OTX1 and OTX2 correlated with the pathological classification of medulloblastomas. In this study, we demonstrate for the first time expression of OTX1 in the human fetal forebrain by locating mRNA encoding OTX1 protein in the proliferative zone of the human brain at 9 fetal weeks, as well as in the developing cortical plate, mostly in the lateral regions of the dorsal cortex, which are the first to mature. This finding indicates that in humans, OTX1 also plays a role in neocortical development and that functions of this gene differ from the functions of the OTX2 gene at later developmental stages.
In summary, our study shows expression of OTX2 and OTX1 in the brains of early human fetuses. OTX2 is mostly expressed in the diencephalon, in the mesencephalon, in the choroid plexus, and in the hippocampal anlage as a marker of this region in the telencephalon, whereas OTX1 is strongly expressed in the forebrain in the proliferative layers of the neocortex. At later stages, OTX2 is expressed in the cerebellum and pineal gland.
Acknowledgments
This study was supported by the Lundbeck Foundation, the Danish Medical Research Council (grant number 271-06-0412), the Novo Nordisk Foundation, the Carlsberg Foundation, the Simon Fougner Hartmann Family Foundation, and by a grant from the Danish Eye Health Society. K.B.L. was supported by a PhD grant from the Faculty of Health Sciences, University of Copenhagen.
We thank Ursula Rentzmann for expert technical assistance.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Acampora D, Mazan S, Avantaggiato V, Barone P, Tuorto F, Lallemand Y, Brûlet P, et al. (1996) Epilepsy and brain abnormalities in mice lacking the Otx1 gene. Nat Genet 14:218–222 [DOI] [PubMed] [Google Scholar]
- Acampora D, Mazan S, Lallemand Y, Avantaggiato V, Maury M, Simeone A, Brûlet P (1995) Forebrain and midbrain regions are deleted in Otx2−/− mutants due to a defective anterior neuroectoderm specification during gastrulation. Development 121:3279–3290 [DOI] [PubMed] [Google Scholar]
- Adamson DC, Shi Q, Wortham M, Northcott PA, Di C, Duncan CG, Li J, et al. (2010) OTX2 is critical for the maintenance and progression of Shh-independent medulloblastomas. Cancer Res 70:181–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman PL, Dittmer DS (1962) Growth, Including Reproduction and Morphological Development. Washington, Federation of American Societies for Experimental Biology
- Ando K, Yagi H, Suda Y, Aizawa S, Sakashita M, Nagano T, Terashima T, et al. (2008) Establishment of framework of the cortical area is influenced by Otx1. Neurosci Res 60:457–459 [DOI] [PubMed] [Google Scholar]
- Ang SL, Conlon RA, Jin O, Rossant J (1994) Positive and negative signals from mesoderm regulate the expression of mouse Otx2 in ectoderm explants. Development 120:2979–2989 [DOI] [PubMed] [Google Scholar]
- Ang SL, Jin O, Rhinn M, Daigle N, Stevenson L, Rossant J (1996) A targeted mouse Otx2 mutation leads to severe defects in gastrulation and formation of axial mesoderm and to deletion of rostral brain. Development 122:243–252 [DOI] [PubMed] [Google Scholar]
- Bailey MJ, Coon SL, Carter DA, Humphries A, Kim JS, Shi Q, Gaildrat P, et al. (2009) Night/day changes in pineal expression of >600 genes: central role of adrenergic/cAMP signaling. J Biol Chem 284:7606–7622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty J, Laughlin R (2006) Genomic regulation of natural variation in cortical and noncortical brain volume. BMC Neurosci 7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon K, Eberhart CG, Riggins GJ (2005) Genomic amplification of orthodenticle homologue 2 in medulloblastomas. Cancer Res 65:703–707 [PubMed] [Google Scholar]
- Bovolenta P, Mallamaci A, Briata P, Corte G, Boncinelli E (1997) Implication of OTX2 in pigment epithelium determination and neural retina differentiation. J Neurosci 17:4243–4252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyl PP, Signore M, Acampora D, Martinez-Barbera JP, Ilengo C, Annino A, Corte G, et al. (2001) Forebrain and midbrain development requires epiblast-restricted Otx2 translational control mediated by its 3′ UTR. Development 15:2989–3000 [DOI] [PubMed] [Google Scholar]
- Broccoli V, Boncinelli E, Wurst W (1999) The caudal limit of Otx2 expression positions the isthmic organizer. Nature 401:164–168 [DOI] [PubMed] [Google Scholar]
- de Haas T, Oussoren E, Grajkowska W, Perek-Polnik M, Popovic M, Zadravec-Zaletel L, Perera M, et al. (2006) OTX1 and OTX2 expression correlates with the clinicopathologic classification of medulloblastomas. J Neuropathol Exp Neurol 65:176–186 [DOI] [PubMed] [Google Scholar]
- Di C, Liao S, Adamson DC, Parrett TJ, Broderick DK, Shi Q, Lengauer C, et al. (2005) Identification of OTX2 as a medulloblastoma oncogene whose product can be targeted by all-trans retinoic acid. Cancer Res 65:919–924 [PubMed] [Google Scholar]
- Finlay BL, Darlington RB (1995) Linked regularities in the development and evolution of mammalian brains. Science 268:1578–1584 [DOI] [PubMed] [Google Scholar]
- Frantz GD, Weimann JM, Levin ME, McConnell SK (1994) Otx1 and Otx2 define layers and regions in developing cerebral cortex and cerebellum. J Neurosci 14:5725–5740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallonet MER, Teillet MA, LeDouarin NM (1990) A new approach to the development of the cerebellum provided by the quail-chick marker system. Development 108:19–31 [DOI] [PubMed] [Google Scholar]
- Hidalgo-Sánchez M, Millet S, Bloch-Gallego E, Alvarado-Mallart RM (2005) Specification of the meso-isthmo-cerebellar region: the Otx2/Gbx2 boundary. Brain Res Brain Res Rev 49:134–149 [DOI] [PubMed] [Google Scholar]
- Kimura J, Suda Y, Kurokawa D, Hossain ZM, Nakamura M, Takahashi M, Hara A, et al. (2005) Emx2 and Pax6 function in cooperation with Otx2 and Otx1 to develop caudal forebrain primordium that includes future archipallium. J Neurosci 25:5097–5108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhar SG, Feng L, Vidan S, Ross ME, Hatten ME, Heintz N (1993) Changing patterns of gene expression define four stages of cerebellar granule neuron differentiation. Development 117:97–104 [DOI] [PubMed] [Google Scholar]
- Lamparello P, Baybis M, Pollard J, Hol EM, Eisenstat DD, Aronica E, Crino PB (2007) Developmental lineage of cell types in cortical dysplasia with balloon cells. Brain 130:2267–2276 [DOI] [PubMed] [Google Scholar]
- Larsen KB, Lutterodt M, Rath MF, Møller M (2009) Expression of the homeobox genes PAX6, OTX2, and OTX1 in the early human fetal retina. Int J Dev Neurosci 27:485–492 [DOI] [PubMed] [Google Scholar]
- Lutterodt MC, Rosendahl M, Andersen CY, Skouby SO, Byskov AG (2009) Age determination enhanced by embryonic foot bud and foot plate measurements in relation to Carnegie stages, and the influence of maternal cigarette smoking. Hum Reprod 24:1825–1833 [DOI] [PubMed] [Google Scholar]
- Mallamaci A, Di Blas E, Briata P, Boncinelli E, Corte G (1996) OTX2 homeoprotein in the developing central nervous system and migratory cells of the olfactory area. Mech Dev 58:165–178 [DOI] [PubMed] [Google Scholar]
- Martinez S (2001) The isthmic organizer and brain regionalization. Int J Dev Biol 45:367–371 [PubMed] [Google Scholar]
- Martinez-Morales JR, Signore M, Acampora D, Simeone A, Bovolenta P (2001) Otx genes are required for tissue specification in the developing eye. Development 128:2019–2030 [DOI] [PubMed] [Google Scholar]
- Matsuo I, Kuratani S, Kimura C, Takeda N, Aizawa S (1995) Mouse Otx2 functions in the formation and patterning of rostral head. Genes Dev 9:2646–2658 [DOI] [PubMed] [Google Scholar]
- Michiels EM, Oussoren E, Van Groenigen M, Pauws E, Bossuyt PM, Voûte PA, Baas F (1999) Genes differentially expressed in medulloblastoma and fetal brain. Physiol Genomics 1:83–91 [DOI] [PubMed] [Google Scholar]
- Millet S, Campbell K, Epstein DJ, Losos K, Harris E, Joyner AL (1999) A role for Gbx2 in repression of Otx2 and positioning the mid/hindbrain organizer. Nature 401:161–164 [DOI] [PubMed] [Google Scholar]
- Nishida A, Furukawa A, Koike C, Tano Y, Aizawa S, Matsuo I, Furukawa T (2003) Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat Neurosci 6:1255–1263 [DOI] [PubMed] [Google Scholar]
- Omodei D, Acampora D, Mancuso P, Prakash N, Di Giovannantonio LG, Wurst W, Simeone A (2008) Anterior-posterior graded response to Otx2 controls proliferation and differentiation of dopaminergic progenitors in the ventral mesencephalon. Development 135:3459–3470 [DOI] [PubMed] [Google Scholar]
- O'Rahilly R (1979) Early human development and the chief sources of information on staged human embryos. Eur J Obstet Gynecol Reprod Biol 9:273–280 [DOI] [PubMed] [Google Scholar]
- Pantò MR, Zappalà A, Tuorto F, Cicirata F (2004) Role of the Otx1 gene in cell differentiation of mammalian cortex. Eur J Neurosci 19:2893–2902 [DOI] [PubMed] [Google Scholar]
- Puelles E, Acampora D, Gogoi R, Tuorto F, Papalia A, Guillemot F, Ang SL, et al. (2006) Otx2 controls identity and fate of glutamatergic progenitors of the thalamus by repressing GABAergic differentiation. J Neurosci 26:5955–5964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles E, Acampora D, Lacroix E, Signore M, Annino A, Tuorto F, Filosa S, et al. (2003) Otx dose-dependent integrated control of antero-posterior and dorso-ventral patterning of midbrain. Nat Neurosci 6:453–460 [DOI] [PubMed] [Google Scholar]
- Puelles E, Annino A, Tuorto F, Usiello A, Acampora D, Czerny T, Brodski C, et al. (2004) Otx2 regulates the extent, identity and fate of neuronal progenitor domains in the ventral midbrain. Development 131:2037–2048 [DOI] [PubMed] [Google Scholar]
- Ragge NK, Brown AG, Poloschek CM, Lorenz B, Henderson RA, Clarke MP, Russell-Eggitt I, et al. (2005) Heterozygous mutations of OTX2 cause severe ocular malformations. Am J Hum Genet 76:1008–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P (1971) Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electron microscopic study in Macacus rhesus. J Comp Neurol 141:283–312 [DOI] [PubMed] [Google Scholar]
- Rakic P, Ayoub AE, Breuning JJ, Dominguez MH (2009) Decision by division: making cortical maps. Trends Neurosci 32:291–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath MF, Morin F, Shi Q, Klein DC, Møller M (2007) Ontogenetic expression of the Otx2 and Crx homeobox genes in the retina of the rat. Exp Eye Res 85:65–73 [DOI] [PubMed] [Google Scholar]
- Rath MF, Muñoz E, Ganguly S, Morin F, Shi Q, Klein DC, Møller M (2006) Expression of the Otx2 homeobox gene in the developing mammalian brain: embryonic and adult expression in the pineal gland. J Neurochem 97:556–566 [DOI] [PubMed] [Google Scholar]
- Rhinn M, Dierich A, Shawlot W, Behringer RR, Le Meur M, Ang SL (1998) Sequential roles for Otx2 in visceral endoderm and neuroectoderm for forebrain and midbrain induction and specification. Development 125:845–856 [DOI] [PubMed] [Google Scholar]
- Scholpp S, Foucher I, Staudt N, Peukert D, Lumsden A, Houart C (2007) Otx1l, Otx2 and Irx1b establish and position the ZLI in the diencephalon. Development 134:3167–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone A, Acampora D, Gulisano M, Stornaiuolo A, Boncinelli E (1992) Nested expression domains of four homeobox genes in developing rostral brain. Nature 358:687–690 [DOI] [PubMed] [Google Scholar]
- Simeone A, Acampora D, Mallamaci A, Stornaiuolo A, D'Apice MR, Nigro V, Boncinelli E (1993) A vertebrate gene related to orthodenticle contains a homeodomain of the bicoid class and demarcates anterior neuroectoderm in the gastrulating mouse embryo. EMBO J 12:2735–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagaard Janas M, Nowakowski RS, Møllgård K (1991) Glial cell differentiation in neuron-free and neuron-rich regions. II. Early appearance of S-100 protein positive astrocytes in human fetal hippocampus. Anat Embryol (Berl) 184:559–569 [DOI] [PubMed] [Google Scholar]
- Suda Y, Matsuo I, Kuratani S, Aizawa S (1996) Otx1 function overlaps with Otx2 in development of mouse forebrain and midbrain. Genes Cells 1:1031–1044 [DOI] [PubMed] [Google Scholar]
- Suda Y, Nakabayashi J, Matsuo I, Aizawa S (1999) Functional equivalency between Otx2 and Otx1 in development of the rostral head. Development 126:743–757 [DOI] [PubMed] [Google Scholar]
- Theiler K (1972) The House Mouse. Berlin, Springer-Verlag
- Weimann JM, Zhang YA, Levin ME, Devine WP, Brûlet P, McConnell SK (1999) Cortical neurons require Otx1 for the refinement of exuberant axonal projections to subcortical targets. Neuron 24:819–831 [DOI] [PubMed] [Google Scholar]
- Zhang YA, Okada A, Lew CH, McConnell SK (2002) Regulated nuclear trafficking of the homeodomain protein otx1 in cortical neurons. Mol Cell Neurosci 19:430–446 [DOI] [PubMed] [Google Scholar]