Abstract
Immunohistochemistry is a ubiquitous technique in histology. Often, the goal of such studies is the quantification of some parameter associated with a particular antigen. When used correctly, the optical disector offers a statistically relevant approach to achieve this goal without bias from cell size, shape, or orientation. This three-dimensional counting probe is virtually embedded within the depth of the tissue section, thus avoiding sampling near the cut surfaces of the section, where cells are often lost during the cutting and subsequent processing steps. It follows that the probability that a cell could be immunolabeled should be equal throughout the section depth to correctly employ the optical disector. In this report, we demonstrate that parameters commonly used in immunohistochemistry often leave the middle of the section unlabeled. Furthermore, the degree of incomplete penetration varies among antibodies but can be overcome in some cases by extending the incubation time of the secondary antibody. The detection of this phenomenon in immunofluorescence preparations and the implications of these findings for quantitative stereology using the optical disector are discussed. (J Histochem Cytochem 58:577–584, 2010)
Keywords: stereology, optical disector, penetration, immunohistochemistry, antibody
Immunohistochemistry (IHC) is a technique widely used to identify cells of a specific phenotype within tissue sections. In many cases, the primary conclusions of such studies are based on the quantification of some aspect of labeled cells as the dependent variable, such as cell number of the total length of a feature. It is therefore imperative that the labeling and the subsequent quantification schemes be optimized to allow valid, unbiased conclusions to be reached.
Design-based stereological methods permit efficient quantification of IHC-labeled cells without bias from cell size, shape, orientation, or distribution (West 1999). When the optical disector is used as a counting probe, a subfraction of the total depth of the tissue section (known as the height sampling fraction) is used for counting (West et al. 1991). Embedding the disector within the central portion of a relatively thick section's depth, with guard zones above and below the disector in which no quantification is performed, ensures that estimates of cell number are not biased by cells lost from the surface during the cutting and tissue-processing steps, a phenomenon known as “lost caps” (Hedreen 1998). Thus, the truly quantitative data in IHC preparations are in the central portion of the section depth.
In order for the optical disector to provide unbiased estimates of cell number from IHC-labeled tissue, the target signal must be present throughout the depth of the tissue section. However, in practice, this is often a problem with thicker sections. Indeed, antibody penetration problems have often prevented the quantification of parameters of interest (Stewart and Maxwell 2003; Mack et al. 2004).
The current study reveals that some (and in our experience, most) antibodies used under standard fluorescence IHC conditions (short, 1- to 2-hr incubation times for secondary antibodies) do not penetrate fully through relatively thick (i.e., 40-μm) brain sections. Use of the optical disector counting probe is most suitable for sections with a postprocessing thickness of at least 25 μm (Lyck et al. 2006). This thickness allows a sufficient depth to accommodate the disector, complete with guard zones above and below it to eliminate bias from lost caps and to provide microscopic resolution in the z-plane sufficient to identify immunolabeled targets. Though previous studies have reported incomplete signal penetration using enzyme-linked IHC (Lyck et al. 2006; Torres et al. 2006), we are unaware of any report examining this phenomenon in detail that uses immunofluorescent preparations.
The purpose of this report is to raise awareness of this phenomenon in fluorescent IHC. It is our contention that the lack of knowledge of this issue has serious quantitative implications for many studies that base their primary conclusions on data obtained from immunolabeled tissue sections. We present representative data, using several antibodies against widely expressed and commonly used brain antigens to demonstrate that frequently used incubation times for fluorochrome-linked secondary antibodies (1–2 hr, which we will refer to as the “standard conditions” in this report) are generally insufficient to allow complete penetration through frozen sections of rat brain. Finally, we demonstrate that a prolonged incubation time with secondary antibody can solve the penetration problem for one antigen but not for others. Therefore, we caution against the use of standard IHC methods for unbiased quantitative purposes and strongly suggest that reagent penetration be carefully confirmed and optimized to prevent systematic underestimation of estimated parameters such as cell number.
Materials and Methods
All procedures were conducted in accordance with University of Lethbridge and Canadian Council on Animal Care animal welfare committee guidelines. Long-Evans hooded rats (3–4 months of age) were anesthetized with a lethal injection of sodium pentobarbital (150 mg/ml). Rats underwent transcardial perfusion with 150 ml of 0.1 M PBS (pH 7.4), followed by 200 ml of 4% paraformaldehyde (PFA) in 0.1 M PBS. Brains were subsequently postfixed in 4% PFA in PBS for 24 hr at 4C. This solution was then replaced by 30% sucrose in PBS containing 0.02% sodium azide as a preservative. When the brains had sunk, they were cut into 40-μm slices with a freezing sliding microtome (model 860; American Optical, Buffalo, NY) and collected in centrifuge tubes containing PBS with 0.02% sodium azide. Data presented were replicated in at least three individual animals in each case.
IHC was conducted with free-floating sections at room temperature, using 0.1 M PBS with 0.3% Triton X-100 as the diluent in all cases. The following primary antibodies were used in this study: mouse anti-NeuN (catalog no. MAB377; Chemicon, Temecula, CA), rabbit anti-S100β (catalog no. 37; Swant, Bellinzona, Switzerland), mouse anti-microtubule-associated protein 2 (MAP2) (catalog no. M1406; Sigma, Saint Louis, MO), and rabbit anti-fibroblast growth factor receptor 2 (FGFR2) (catalog no. F0300; Sigma). Each section was double-labeled with NeuN (1:500 dilution) and S100β (1:5000 dilution) or MAP2 (1:500 dilution) and FGFR2 (1:400 dilution) to eliminate any variability due to between-section differences. After a 16-hr incubation at room temperature, sections were rinsed three times with PBS, after which secondary antibodies were applied for either 1 hr or 24 hr at room temperature. For both time points, sections were processed from the same animal to eliminate potential inter-animal differences. NeuN was detected using a donkey anti-mouse antibody conjugated to Alexa 488 (product no. A-21202) at 1:500 dilution (Invitrogen; Carlsbad, CA); S100β and FGFR2 were detected with a donkey anti-rabbit antibody conjugated to Cy3 (product no. 711-165-152) at 1:500 dilution (Jackson ImmunoResearch; West Grove, PA); and MAP2 was detected by using a donkey anti-mouse antibody conjugated to Cy2 (Jackson ImmunoResearch) at a dilution of 1:1000. To help determine the top and bottom of the section and, as a general permeability control check, all sections were also stained with the nuclear dye DAPI (product no. D8417; Sigma) for 10 min before they were mounted. Sections were dried on the slide for ∼2 min and cover-slipped with a glycerol-based aqueous mounting medium to maintain as much of the original section thickness as possible.
To qualitatively determine the depth of reagent penetration, a series of z-plane stacks were captured using a 60× water immersion objective (1.2 numerical aperture) on a Nikon C1 Eclipse confocal microscope (Melville, NY) or a 63× water immersion lens (1.2 numerical aperture) on a Zeiss LSM510 confocal microscope (Jena, Germany). Stacks of images were collected with a z-plane step size of 2 μm throughout the section depth. The gain settings were set using the 1-hr secondary antibody condition by using the pseudocolor mode of the software, bringing the strongest signal in the imaged field just into saturation. The same settings were used to capture image stacks from the 24-hr antibody in the secondary conditions. This was done to provide a truer representation of the relative intensity of labeling between different secondary incubation times and to avoid underexposing the 1-hr incubation condition. Quantification of mean optical intensity by z-plane section was accomplished by using the plot z-axis-profile function in the public source ImageJ software (http://rsbweb.nih.gov/ij/).
Epifluorescence images were collected for incubation times beyond 24 hr because, at the antibody dilutions used, the background signal often became too high to suitably demonstrate incomplete signal penetration via confocal microscopy. This had the added benefit of demonstrating incomplete penetration without specialized equipment. These images are presented in epifluorescence figures as the top, middle, and bottom portions of the section depth, using a digital microcator from Stereoinvestigator software (Microbrightfield Bioscience; Williston, VT).
Results
To determine the permeability of immunofluorescent reagents under standard IHC conditions, we processed sections of a commonly used thickness of 40 μm (Chung et al. 2009; Lu et al. 2009; Marchant et al. 2009). Several sections were processed from at least three different animals, with qualitatively similar results in each case.
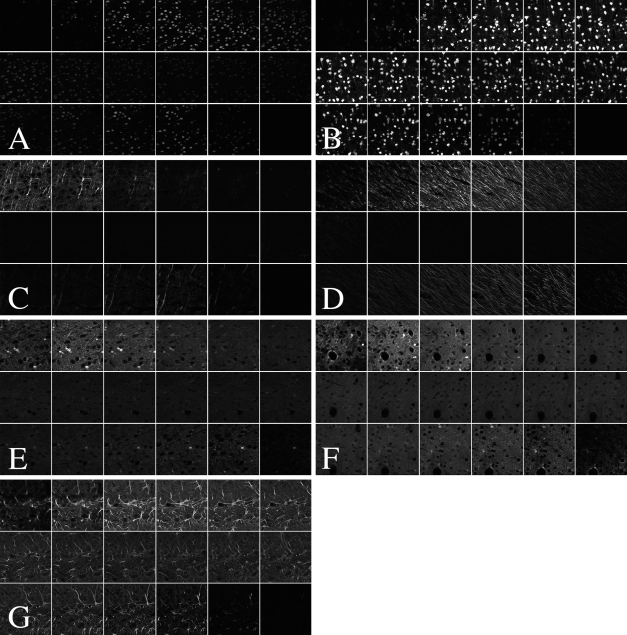
To detect the presence of the mature neuronal phenotype marker NeuN, we used a donkey anti-mouse secondary antibody conjugated to Alexa 488. We compared samples from a standard incubation time of 1 hr in secondary antibody with tissue from a prolonged secondary incubation time of 24 hr. After a 1-hr incubation, the signal was very weak toward the middle portion of the section (Figure 1A), a situation that would lead to an underestimation of the neuronal number, using the optical disector and live epifluorescence-based counting. By contrast, another section from the same animal with a 24-hr incubation in the secondary antibody resulted in complete signal penetration with homogenous signal strength through the section depth (Figure 1B). This outcome is ideal for live epifluorescence microscopy counting.
Figure 1.
Confocal z-plane stacks at 2-μm intervals (top left of each image panel is the section top; bottom right of each image panel is the section bottom) illustrate incomplete antibody penetration. NeuN signal penetration is incomplete under standard 1-hr secondary antibody incubation times (A) but can be pushed to completion in the 24-hr secondary incubation condition (B). MAP2 does not achieve complete penetration at either 1 hr in secondary antibody (C) or 24-hr incubation (D). The same is true of S100β at 1 hr (E) and at 24 hr in secondary antibody (F). By contrast, labeling is complete for FGFR2 after only 1 hr in secondary antibody (G). Importantly, the same secondary antibody was used to detect S100β and FGFR2.
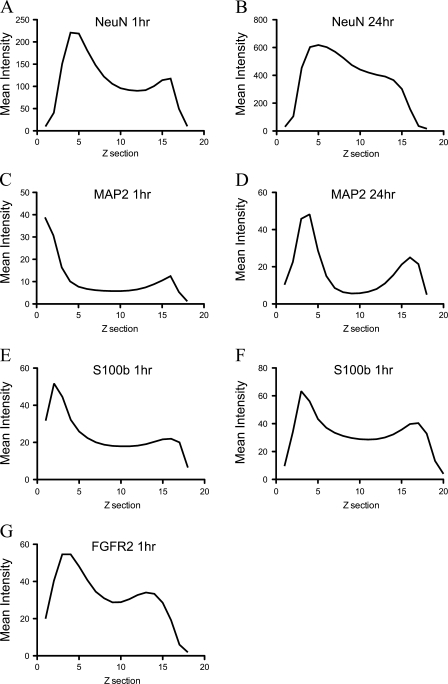
We performed similar experiments using several other antibodies. MAP2, a marker of neuronal dendrites, shows profoundly incomplete penetration after 1 hr in secondary antibody (Figure 1C). Signal penetration depth was improved slightly by incubation for 24 hr in secondary antibody but was still not present throughout the section depth (Figure 1D). We also processed sections for the mature glial marker S100β. With a 1-hr secondary incubation, labeling was incomplete (Figure 1E). An extended incubation time in secondary antibody did not sufficiently improve penetration (Figure 1F). Interestingly, the signal from the antibody to FGFR2 did achieve complete penetration under standard incubation conditions [1 hr in secondary antibody (Figure 1G)]. Critically, the secondary antibody used to detect this antigen was the same used to detect S100β, which did not show complete penetration, even with extended incubation times (Figure 1F). This implies that in contrast to the situation with NeuN, the secondary antibody is not at fault in this case. The general pattern of penetration was quantified by providing the z-axis profiles of mean signal intensity through the stack depth (Figures 2A–2G).
Figure 2.
Quantification of penetration with prolonged incubation times in secondary antibody from samples shown in Figure 1. Graphs in A and B represent the signal intensity by z-plane section for NeuN for 1 hr in secondary antibody (A) and for 24 hr (B). Penetration results for 1 hr in secondary antibody (C and E) and 24 hr in secondary antibody (D and F) are shown for MAP2 and S100β, respectively. A 1-hr incubation time in secondary antibody to detect FGFR2 is shown in G.
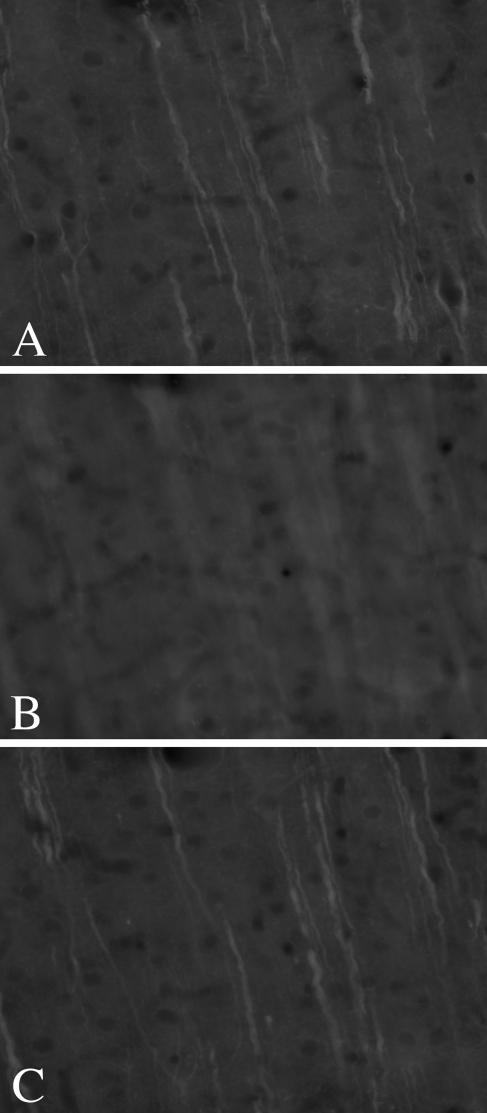
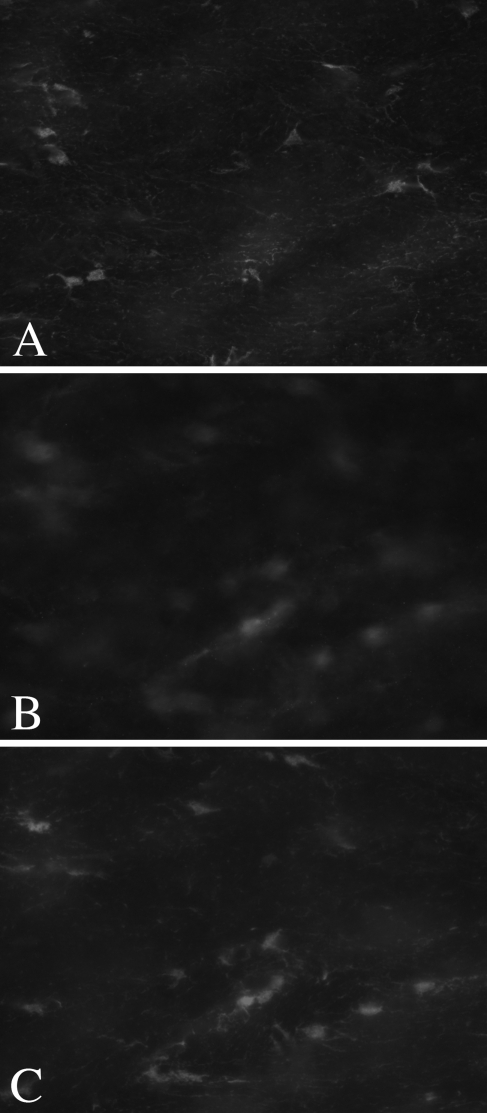
One potential reason for the lack of penetration of MAP2 and S100β after a 24-hr incubation in secondary antibody is that even these extended times were insufficient. We therefore sought to repeat these experiments with very long incubation times. However, even after 5 days in each primary antibody, followed by 5 days in secondary antibody, neither signal achieved complete penetration. Figure 3 shows images of MAP2 from the top (Figure 3A), middle (Figure 3B), and bottom (Figure 3C) of the section, using epifluorescence microscopy. Note that the incomplete penetration can be easily detected under these conditions as an out-of-focus “haze” in the central portion of the section. Similar results were seen for S100β (Figures 4A–4C).
Figure 3.
Epifluorescence reveals incomplete penetration of MAP2 IHC after 5 days in primary and 5 days in secondary antibodies. Images from the section top (A), middle (B), and bottom (C) demonstrate that labeling is present on either side of the tissue section but not in the middle. The incomplete penetration is readily identified as an out-of-focus area in the center of the section.
Figure 4.
Five days in primary antibody followed by 5 days in secondary antibody are insufficient to obtain complete signal penetration for S100β. Epifluorescent images from the section top (A), middle (B), and bottom (C) indicate that labeling is not present in the center of the section.
Discussion
These data illustrate that several antibodies fail to fully penetrate relatively thick brain tissue sections, with commonly used incubation times (Cina et al. 2009; Guyon et al. 2009; Tanaka et al. 2009) and section thicknesses. In addition, the degree of permeability between different antibodies was variable. However, a prolonged incubation time in fluorochrome-labeled secondary antibody did result in complete penetration for one antibody but had little effect on the others.
In an attempt to discover the reasons for this variability, we explored several parameters. The degree of immunolabeling through the depth of sections appeared to be independent of whether the antibody was monoclonal or polyclonal, of the species donating the primary or secondary antibody, or of the fluorophore used. We have also found that other variables commonly suggested to increase penetration, such as increased incubation temperatures (37C), varying amounts and types of detergent (up to 1% Triton X-100 or Tween-20), and different amounts of fixation (4% or 1% PFA), had no effect on reagent penetration (data not shown).
The implications of these data are profound for IHC-dependent quantitative analysis. When the optical disector method is used, it is best to place the disector into the central portion of thicker sections, thus avoiding the problem of lost caps, which will underestimate cell number. The degree of lost caps can be due to several factors, such as the harshness of tissue processing, the sharpness of the microtome blade (Hedreen 1998), and the density of cells in the region of interest. This necessarily implies that the staining must be present through the section and, ideally, with uniform intensity throughout (Lyck et al. 2006) to permit the identification of cells in the center of the section with a probability proportional to their number and not their staining intensity.
It is easy to appreciate the fact that incomplete antibody penetration will result in a systematic underestimation of cell number when the optical disector is used. This situation can lead to grossly distorted data, skewing the conclusions of a study. This situation could be particularly misleading in experiments designed to colocalize multiple antigens that have differential tissue penetration signals. Given the fact that many studies base their conclusions on the numbers of cells present in a given structure normally or after various treatments, we believe this issue to be of critical importance.
Though we have not used intermediate secondary antibody incubation times in the present study, it is certainly possible that there may be penetration differences across times of several hours. Thus, researchers should be careful to not leave sections in secondary antibodies for various amounts of time, as this could influence quantitative measures. In cases where incomplete penetration has not yet been achieved, differences in cell number could result solely from differences in antibody penetration.
The inability to notice the lack of complete penetration of IHC reagents is likely very common in practice. Maintaining section thickness is critical in this regard. Many studies that dehydrate sections as part of the processing procedures can exhibit substantial collapse in the z-plane. In fact, postprocessing collapses of between 60% (Long et al. 1998) and more than 80% (Coggeshall 2001) are not atypical. Under these conditions, it would be difficult to notice a central unlabeled zone, even using confocal microscopy. In addition, heterogeneously expressed antigens could easily go unnoticed. With the tissue preparation procedures used here, we were able to maintain ∼80% of the original section thickness, which allowed us to easily identify incomplete penetration.
As we demonstrate using epifluorescence microscopy, incomplete penetration is detected as an out-of-focus “haze” in the center of the section. To assess this, it is important to maintain as much of the original tissue thickness as possible. A useful approach in this regard is to wet-mount some IHC-labeled sections on an unsubbed slide with a few drops of buffer. Gently lay a cover slip on the sections and focus through the section depth; this method will allow an assessment of signal penetration. The slide can then be immersed in a dish of buffer and the sections retrieved for permanent mounting.
For antibodies that seem to be resistant to complete penetration, simply cutting thinner sections may seem like a reasonable option (Lemmens et al. 2008). However, as thinner sections are cut, the quantitative disadvantages become increasingly apparent. To use the optical disector, it is advantageous to work with relatively thick sections to provide enough height in which to embed the optical disector and to have sufficient guard zones at the cut surfaces to eliminate bias from lost caps. Thus, the ideal approach is to use thicker sections and to strive for complete reagent penetration.
Though some authors choose to restrict the quantification of cell number to the tissue section surface where antibody permeability may be complete (Eisch et al. 2000), this method should be generally discouraged. Because the lost-caps phenomenon is dependent on several factors such as blade sharpness, harshness of tissue processing, and the density of cells in the region of interest, quantifying cells near the section surfaces could be differentially biased. In the context of the optical disector, some researchers (Peterson 2004) have suggested that in cases of incomplete reagent penetration, the disector height could simply be adjusted to sample within the penetrated region. For the reasons mentioned above, however, this is not an ideal situation, and proper reagent penetration should be a primary goal.
It is important to note that the current study used only immunofluorescence preparations. A previous study by Torres (Torres et al. 2006) examined antibody penetration in enzyme-linked, biotinylated IHC in great detail. In an effort to increase penetration, they used a variety of manipulations, including modifications of the diaminobenzidine substrate dilution and incubation times, extensions of secondary and tertiary reagent incubation times, post-sectioning cell membrane disruptions, free-floating versus slide-mounted processing, and differential fixation. The authors identified PFA-based fixation as a major determinant of antibody penetration: a NeuN signal was present throughout the section depth in tissue fixed with 1% or 2% PFA but not in 4% PFA-fixed tissue.
The study by Torres (Torres et al. 2006) differs from ours in several ways. First, they used enzyme-linked IHC with subsequent diaminobenzidine-based development. In addition, they used streptavidin-biotin complex IHC. They found that extending the incubation time in secondary antibody as we have done here does not result in increased penetration. However, we find that with the same primary antibody used in their work (NeuN), we were able to achieve complete penetration with extended, fluorochrome-linked secondary antibody incubation times. In addition, we have tested the degree of penetration of both NeuN and S100β in tissue fixed with 4% or 1% PFA and have observed no differences under any of these conditions. These data suggest that methodological changes that work for one type of IHC processing are likely not applicable to all types. This diversity of outcomes underscores the importance of assessing reagent penetration under all conditions before proceeding with quantification.
This report provides an important caveat for researchers wishing to apply quantitative measures to immunolabeled materials. Our work underscores the importance of careful histological protocol development to ensure adequate IHC labeling quality for subsequent quantification. Based on these data, it is our contention that many studies that use standard IHC conditions currently in the literature contain variable underestimates of the parameter quantified solely as a function of antibody penetration depth. Depending on the final stages of tissue preparation, incomplete penetration may often go unnoticed. We strongly advise against a “one protocol fits all” approach to quantitative IHC and suggest that others carefully confirm the full penetration of IHC reagents before undertaking experiments whose conclusions will be based on quantitative data from IHC-processed tissue.
Acknowledgments
We thank Doug Bray for insightful comments on this work.
This article is a JHC article of the month. This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication with the exception of the JHC articles of the month which are immediately released for public access.
This work was funded by Alberta Heritage Foundation for Medical Research, Canadian Institutes of Health Research, and Natural Sciences and Engineering Research Council of Canada.
References
- Chung CY, Koprich JB, Siddiqi H, Isacson O (2009) Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. J Neurosci 29:3365–3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cina C, Maass K, Theis M, Willecke K, Bechberger JF, Naus CC (2009) Involvement of the cytoplasmic C-terminal domain of connexin43 in neuronal migration. J Neurosci 29:2009–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall RE (2001) Commentary of the paper by Benes and Lange. Trends Neurosci 24:376–377 [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ (2000) Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci USA 97:7579–7584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon A, Tardy MP, Rovere C, Nahon JL, Barhanin J, Lesage F (2009) Glucose inhibition persists in hypothalamic neurons lacking tandem-pore K+ channels. J Neurosci 29:2528–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedreen JC (1998) Lost caps in histological counting methods. Anat Rec 250:366–372 [DOI] [PubMed] [Google Scholar]
- Lemmens EM, Schijns OE, Beuls EA, Hoogland G (2008) Cytogenesis in the dentate gyrus after neonatal hyperthermia-induced seizures: what becomes of surviving cells? Epilepsia 49:853–860 [DOI] [PubMed] [Google Scholar]
- Long JM, Kalehua AN, Muth NJ, Hengemihle JM, Jucker M, Calhoun ME, Ingram DK, et al. (1998) Stereological estimation of total microglia number in mouse hippocampus. J Neurosci Methods 84:101–108 [DOI] [PubMed] [Google Scholar]
- Lu XH, Fleming SM, Meurers B, Ackerson LC, Mortazavi F, Lo V, Hernandez D, et al. (2009) Bacterial artificial chromosome transgenic mice expressing a truncated mutant parkin exhibit age-dependent hypokinetic motor deficits, dopaminergic neuron degeneration, and accumulation of proteinase K-resistant alpha-synuclein. J Neurosci 29:1962–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyck L, Jelsing J, Jensen PS, Lambertsen KL, Pakkenberg B, Finsen B (2006) Immunohistochemical visualization of neurons and specific glial cells for stereological application in the porcine neocortex. J Neurosci Methods 152:229–242 [DOI] [PubMed] [Google Scholar]
- Mack AF, Sussmann C, Hirt B, Wagner HJ (2004) Displaced amacrine cells disappear from the ganglion cell layer in the central retina of adult fish during growth. Invest Ophthalmol Vis Sci 45:3749–3755 [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Hamlin AS, McNally GP (2009) Lateral hypothalamus is required for context-induced reinstatement of extinguished reward seeking. J Neurosci 29:1331–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DA (2004) The use of fluorescent probes in cell-counting procedures. In Evans SM, Janson AM, Nyengaard JR, eds. Quantitative Methods in Neuroscience. New York, Oxford University Press, 85–114
- Stewart W, Maxwell DJ (2003) Distribution of and organisation of dorsal horn neuronal cell bodies that possess the muscarinic m2 acetylcholine receptor. Neuroscience 119:121–135 [DOI] [PubMed] [Google Scholar]
- Tanaka DH, Yanagida M, Zhu Y, Mikami S, Nagasawa T, Miyazaki J, Yanagawa Y, et al. (2009) Random walk behavior of migrating cortical interneurons in the marginal zone: time-lapse analysis in flat-mount cortex. J Neurosci 29:1300–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres EM, Meldrum A, Kirik D, Dunnett SB (2006) An investigation of the problem of two-layered immunohistochemical staining in paraformaldehyde fixed sections. J Neurosci Methods 158:64–74 [DOI] [PubMed] [Google Scholar]
- West MJ (1999) Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci 22:51–61 [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ (1991) Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec 231:482–497 [DOI] [PubMed] [Google Scholar]