Abstract
The type III secretion system (T3SS) encoded by Salmonella pathogenicity island 1 (SPI-1) is important for the invasion of epithelial cells during development of Salmonella-associated enterocolitis. It has been suggested that the level and timing of the expression of the SPI-1 T3SS proteins and effectors dictate the consequences of bacterial infection and pathogenesis. However, the expression of these proteins has not been extensively studied in vivo, especially during the later stages of salmonellosis when the infection is established. We have constructed recombinant Salmonella strains that contain a FLAG epitope inserted in-frame to genes invJ, prgJ, sipC, sipD, sopA and sopB, and investigated the expression of the tagged proteins both in vitro and in vivo during murine salmonellosis. Mice were inoculated intraperitoneally or intragastrically with the tagged Salmonella strains. At different time points post-infection, bacteria were recovered from various organs, and the expression of the tagged proteins was determined. Our results provide direct evidence that PrgJ and SipD are expressed in Salmonella colonizing the liver and ileum of infected animals at both the early and late stages of infection. Furthermore, our study has shown that the InvJ protein is expressed preferentially in Salmonella colonizing the ileum but not the liver, while SipC is expressed preferentially in Salmonella colonizing the liver but not the ileum. Thus, Salmonella appears to express different SPI-1 proteins and effectors when colonizing specific tissues. Our results suggest that differential expression of these proteins may be important for tissue-specific aspects of bacterial pathogenesis such as gastroenterititis in the ileum and systemic infection in the liver.
INTRODUCTION
Salmonella (e.g. S. enterica serovar Typhimurium) is the leading cause of food-borne diseases in the United States, causing diverse diseases ranging from mild, self-limiting gastroenteritis to life-threatening systemic infection (Olsen et al., 2000). As a facultative intracellular pathogen, Salmonella can invade non-phagocytic cells such as intestinal epithelial cells and it survives in phagocytes during systemic infection. Two hallmarks of Salmonella pathogenesis, i.e. host invasion and intracellular proliferation, correlate with the genes in Salmonella pathogenicity islands (SPIs), which are large horizontally transferred regions containing genes that have been shown to be important for pathogenesis (Schmidt & Hensel, 2004). Salmonella pathogenicity island 1 (SPI-1) contains invasion genes, while Salmonella pathogenicity island 2 (SPI-2) contains genes required for intracellular survival and replication, and has a critical role in systemic infection (Abrahams & Hensel, 2006; Galan, 2001; Waterman & Holden, 2003). Both SPI-1 and SPI-2 encode type III secretion systems (T3SSs), which are specialized organelles that deliver effector proteins to host cell membranes and the cytosol (Galan & Wolf-Watz, 2006; Hueck, 1998). The T3SS apparatus is a needle-like structure that spans the inner and outer membranes of the bacterial envelope and secretes translocon and effector proteins. Translocon proteins allow delivery of effector proteins to eukaryotic cells (Galan & Wolf-Watz, 2006; Hueck, 1998), leading to modulation of host cells and immunity, and promotion of bacterial pathogenesis (Abrahams & Hensel, 2006; Galan, 2001; Waterman & Holden, 2003). In addition to the well-characterized SPI-1 and SPI-2, many other SPIs have been described in Salmonella but their roles have not yet been fully investigated (Blanc-Potard et al., 1999; Hensel, 2004; Kiss et al., 2007). Characterization of the expression patterns of the SPI-1 proteins and effectors and of those of other SPIs should provide insight into the roles of these factors in Salmonella infection.
The modulation of expression of genes coding for SPI-1 proteins and its effectors is remarkably complex and is yet to be fully characterized (Ellermeier & Slauch, 2007; Waterman & Holden, 2003). For example, the expression of some genes coding for SPI-1 proteins and effectors has been shown to be induced upon invasion of both macrophages and epithelial cells and several SPI-1 factors are essential for intracellular replication (Giacomodonato et al., 2007; Pfeifer et al., 1999; Steele-Mortimer et al., 2002). Furthermore, SPI-1-encoded proteins and effectors, PrgI, SipA, SipB, SpaO, SptP, SopA, SopB, SopD, and SopE2, were found to be expressed by Salmonella in the spleens of infected animals at the late stages of infection (Giacomodonato et al., 2007; Gong et al., 2009). These results suggest that in addition to their generally recognized roles in invasion, the SPI-1 factors may play an important role post-invasion. Hence, the role of the SPI-1 factors in bacterial pathogenesis, especially during the late stages of salmonellosis, needs further characterization and their expression in vivo needs to be studied.
Extensive studies have been carried out to investigate the expression of SPI-1 factors under different culture conditions in vitro (Ellermeier & Slauch, 2007; Lober et al., 2006). However, most of these studies were performed by examining the transcription levels of these genes using either microarrays or a reporter system (Gantois et al., 2006; Huang et al., 2007; Lober et al., 2006); protein expression controlled by the native promoters for these T3SS factors has not been extensively investigated. In addition, the expression of many of these factors in vivo, especially during the established phase of infection, is poorly characterized. In this study, we have generated Salmonella strains that contained a FLAG epitope sequence inserted in-frame into the carboxyl terminus of Salmonella genes invJ, prgJ, sipC, sipD, sopA and sopB. InvJ, PrgJ, SipC and SipD are SPI-1-encoded proteins while SopA and SopB are SPI-1 effectors (Abrahams & Hensel, 2006; Galan, 2001; Waterman & Holden, 2003). We examined the expression of the tagged proteins in vitro and in vivo during murine salmonellosis. Our results suggest that Salmonella expressed different SPI-1 proteins and effectors when colonizing specific tissues and that differential expression of these proteins may be important for bacterial pathogenesis in specific tissues.
METHODS
Generation of plasmids and tagged mutants.
Table 1 lists the bacterial strains and plasmid constructs used in the study. Construct pUC-H1PF1 contains the sequence coding for the FLAG epitope and the kanamycin-resistance cassette and was used as the template to amplify the DNA fragments for homologous targeting in Salmonella strain ST14028s (Su et al., 2009). The FLAG epitope is an octapeptide tag (N-DYKDDDDK-C) that has been widely used for tagging proteins, which in turn can be detected and studied using an anti-FLAG antibody (Chubet & Brizzard, 1996). Table 2 lists the primers used to construct the tagged mutants. For each tagged mutant, a pair of primers was designed to amplify the FLAG-epitope coding sequence and kanamycin-resistance gene using pUC-H1PF1 as the template (Su et al., 2009). The resulting PCR products were transformed into Salmonella ST14028s carrying plasmid pKD46. The tagged mutants were constructed using the λ Red recombinase method (Datsenko & Wanner, 2000), following the procedures as described previously (Gong et al., 2009; Lu et al., 2002, 2003). The non-polar strains were selected for their sensitivity to kanamycin and further confirmed using PCR. The regions for the tagged ORFs in all the generated tagged strains (i.e. the homologous recombinant mutants, P22-transduced mutants, and non-polar mutants) were sequenced to confirm that no other mutations were present in these regions (Gong et al., 2009).
Table 1.
Strains and plasmid constructs used in the study
| Strain or plasmid | Description | Reference/source |
|---|---|---|
| Salmonella typhimurium | ||
| ST14028s | Wild-type and parental strain | Lu et al. (1999) |
| T-InvJ | invJ : : 1xFLAG | This study |
| T-PrgJ | prgJ : : 1xFLAG | This study |
| T-SipC | sipC : : 1xFLAG | This study |
| T-SipD | sipD : : 1xFLAG | This study |
| T-SopA | sopA : : 1xFLAG | This study |
| T-SopB | sopB : : 1xFLAG | This study |
| Escherichia coli | ||
| DH5α | F−φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17( |
Invitrogen |
| Plasmids | ||
| pUC-H1PF1 | Apr Kanr, template plasmid for 1xFLAG epitope tag | Su et al. (2009) |
| pKD46 | Apr, containing the Red recombinase of λ phage | Datsenko & Wanner (2000) |
| pCP20 | Containing the expression cassette of flippase, which can remove the kanamycin-resistance cassette from the mutant strains | Datsenko & Wanner (2000) |
Table 2.
Primers used to construct the tagged strains
| ORFs | Upstream primer | Downstream primer |
|---|---|---|
| invJ | 5′-GCTGGCACCTGACGCGAGACGATC AACAAAATCCGCAGCAGCAACAGCA CAGACAGCAATCTGGCGAGGAGGAT GACGCCGATTACAAGGATGACGACGA-3′ | 5′-CCGGCCATGGCGCTGGCATTCTG TCGCGGTTTGCGCCAATAGCCATT CGCGACGATCAATCTGTCTCACAC GCAATGACACATATGAATATCCTC CTTAGTT-3′ |
| prgJ | 5′-CAGATTATAACCTGTATGTTTCTA TGGTCAGTACCCTTACTCGTAAAGG AGTCGGGGCTGTTGAAACGCTATTA CGCTCAGATTACAAGGATGACGACG A-3′ | 5′-GTCCAGTCCTTTTAAAAGATCCT TATCCTTACAGCCGGCAAGGGTCA TTACCAGCAGAAAAGTATATAGAT ATCGACGAACATATGAATATCCTCC TTAGTT-3′ |
| sipC | 5′-AGGAAATGCTGAAAACAATGGAG AGCATTAACCAGTCGAAAGCATCC GCACTCGCTGCTATCGCAGGCAATA TTCGCGCTGATTACAAGGATGACGA CGA-3′ | 5′-TATTAAGCATAATATCCCCAGTTC GCCATCAGGAGCGCGATTAAATCA CACCCATGATGGCGTATAGATGACC TTTCAGACATATGAATATCCTCCTTA GTT-3′ |
| sipD | 5′-CATTGTACGACAACCTGGTAAAA GTGCTGAGCAGTACGATAAGTAGC AGCCTGGAAACCGCCAAAAGCTTC CTGCAAGGAGATTACAAGGATGAC GACGA-3′ | 5′-TTGATCTCGGTCTGCATACCTGGC ATTATGACGGGGGGCTGAGTCCTTA CACTTGTAACCATTATTAATATCCTC TTCTGCATATGAATATCCTCCTTAGTT-3′ |
| sopA | 5′-TTGTGGCGGATAGTATGCAACGT CATGCCAGAAAATATTTCCCGAGT GTTCTGTCATCCATCCTGCCACTGG CCTGGGCGGATTACAAGGATGACG ACGA-3′ | 5′-AAAATGCATGGACTAAAACGGGT CTATGTACAGAGGGACACAACGCTG TGTCCCTTAATTCCATGCGGGTTGAG GCTGGACATATGAATATCCTCCTTAG TT-3′ |
| sopB | 5′-TCAATCTTTCCTATCAAAAACGA GTTGGGGATGAAAATATTTGGCAG TCAGTAAAAGGCATTTCTTCATTA ATCACATCTGATTACAAGGATGAC GACGA-3′ | 5′-GTGGCTCATCTTCTGGCGCATCCA GGCCTAACGCGTCATATAAACGATT TAATAGACTTTCCATATAGTTACCTC AAGACCATATGAATATCCTCCTTAGT T-3′ |
Animal studies.
Female BALB/c and SCID mice (6–8 weeks old) were obtained from Jackson Laboratory. Overnight bacterial cultures were serially diluted to suitable densities (c.f.u. ml−1) in PBS for infection. To assess the virulence of the tested strains, mice (five animals per group) were inoculated either intragastrically with 5×106 c.f.u. per BALB/c mouse and 1×103 c.f.u. per SCID mouse or intraperitoneally with 1×102 c.f.u. per BALB/c mouse and 1×101 c.f.u. per SCID mouse. For organ colonization and in vivo protein expression experiments, mice (five animals per group) were inoculated intraperitoneally with 1×105 or 1×107 c.f.u. of the bacterial strains per BALB/c mouse or 1×102 or 1×104 c.f.u. per SCID mouse, and were euthanized at 5 days or 18 h after inoculation, respectively. Mice (five animals per group) were also inoculated intragastrically with 1×105 or 1×108 c.f.u. of the bacterial strains per BALB/c mouse or 1×102 or 1×104 c.f.u. per SCID mouse and were euthanized at 7 days or 24 h after inoculation, respectively. The liver was collected from infected mice, and the ileum was dissected from the small intestine. All organs were homogenized in cold PBS, and bacterial c.f.u. were determined by plating of serially diluted organ homogenates. Protein extracts for Western blot analyses were prepared by a modification of a procedure described previously (Gong et al., 2009; Lu et al., 2002, 1999). Briefly, the homogenates of the liver and ileum samples were centrifuged at 9000 g at 4 °C for 10 min. Pellets were incubated in lysis buffer (120 mM NaCl, 4 mM MgCl2, 20 mM Tris/HCl, pH 7.5, 1 % Triton X-100) at 4 °C for 1 h, and released bacteria were collected by centrifugation at 18 000 g for 10 min. Harvested bacteria were then resuspended in PBS, and adjusted for the bacterial c.f.u. before being used for Western blot analysis for in vivo expression of Salmonella proteins. Level of tagged proteins from the bacterial strains recovered from the animals were determined. In experiments with intraperitoneally infected mice, we assayed the percentage level of the tagged proteins in the bacteria recovered from the liver and ileum, relative to the level of tagged SipD protein in strain T-SipD recovered from the liver and ileum, respectively, at 5 days post-inoculation. In experiments with intragastrically infected mice, we assayed the percentage level of the tagged proteins in the bacteria recovered from the organs, relative to the level of tagged SipD protein in strain T-SipD recovered from the livers at 7 days post-inoculation. The values are the means from triplicate experiments.
Determination of c.f.u. counts.
We used an aliquot of tissue homogenate or bacterial culture to determine its c.f.u. ml−1 by serial dilution with PBS and plating on LB agar plates (Gong et al., 2009; Lu et al., 2003). Bacteria were enumerated after overnight incubation. Each sample was analysed in triplicate and the analysis was repeated at least three times; the c.f.u. of the sample were expressed as the mean of the values obtained. The concentrations of bacteria were recorded as c.f.u. per ml of organ homogenate or culture. The limit of bacteria detection in the organ homogenates was 10 c.f.u. ml−1. Those samples that were negative at a 10−1 dilution were designated a value of 10 (101) c.f.u. ml−1.
In vitro growth analysis of Salmonella.
Growth analysis of bacteria in LB broth was carried out by first inoculating a single colony in 2 ml LB broth and culturing at 37 °C with shaking at 250 r.p.m. overnight (about 16 h) (Gong et al., 2009). The overnight culture was diluted and 30 μl of the culture was then inoculated into 3 ml fresh LB broth and cultured at 37 °C and 250 r.p.m. At time points of 0, 2, 4, 6, 8, 10, 12, 14, 16 and 24 h after inoculation, 100 μl bacterial culture was collected and used for analysis by limiting dilution in sterile 96-well plates, and then plated on LB agar plates to determine the c.f.u. ml−1. Each sample was analysed in triplicate and the analysis was repeated at least three times. The mean value of c.f.u. ml−1 was used to generate the growth curve (Gong et al., 2009).
Expression of the tagged proteins in vitro.
To study the effect of different culture conditions on the protein expression in vitro, 20 μl overnight bacterial cultures was inoculated into 1 ml antibiotic-free LB broth and shaken at 250 r.p.m. and 37 °C for 4 h. The bacterial cultures were centrifuged at 5000 g for 5 min. To study the effect of pH, the pelleted bacteria were resuspended in 1 ml fresh LB broth (control, pH 7.0) or 1 ml LB broth at pH 3.0, 5.0, 7.2 and 8.4, respectively, shaken at 250 r.p.m. and 37 °C for an additional 6 h, and then collected. To study the effect of osmolarity, the pelleted bacteria were resuspended in 1 ml NaCl-free LB broth supplemented with 0, 42.5, 85, 170, 340 and 680 mM NaCl, respectively, shaken at 250 r.p.m. and 37 °C for an additional 6 h, and collected. Regular LB broth, which contained 170 mM NaCl, was used as the control. To study the effect of oxygen ventilation, the pelleted bacteria were resuspended in 1.5 ml fresh LB broth. One group of bacteria was shaken at 250 r.p.m. and 37 °C for an additional 6 h with good aeration (control) while another group of bacteria was transferred into 1.5 ml microcentrifuge tubes with their covers closed tightly, and incubated at 37 °C without shaking for an additional 6 h. To study the effect of butyrate, the pelleted bacteria were resuspended in 1 ml fresh LB broth (control) or 1 ml LB broth containing 10 mM sodium butyrate, shaken at 250 r.p.m. and 37 °C for an additional 6 h, and then collected. Protein extract samples for Western blot analysis were prepared from the pellets and supernatants of bacterial cultures, following the procedure described previously (Gong et al., 2009; Lu et al., 2002, 1999). The analyses were repeated at least three times.
Western blot analyses.
Polypeptides from bacterial lysates were separated on SDS-containing 10–12 % polyacrylamide gels, transferred to nitrocellulose membranes, and reacted with anti-mouse IgG conjugated with alkaline phosphatase in addition to the antibodies against the FLAG sequence (Sigma) and Salmonella DnaK protein, following the procedure described previously (Gong et al., 2009; Lu et al., 2003). The membranes were subsequently stained with a chemiluminescent substrate with the aid of a Western chemiluminescent substrate kit (Amersham, GE Healthcare) and quantified with a STORM840 phosphorimager. Normalization of samples was also carried out by loading total proteins extracted from the same number of c.f.u. (5×107 c.f.u.) of bacteria in each lane. Quantification was performed in the linear range of protein detection.
RESULTS
Characterization of the growth of tagged strains in culture and in mice
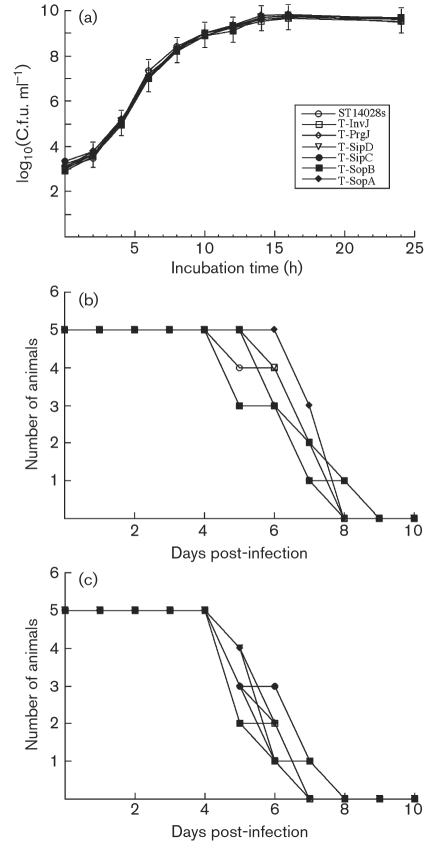
We inserted the FLAG epitope tag sequence into Salmonella ORFs invJ, prgJ, sipC, sipD, sopA and sopB of the wild-type Salmonella strain (ST14028s) to generate strains T-InvJ, T-PrgJ, T-SipC, T-SipD, T-SopA and T-SopB, encoding the respective tagged proteins (Table 1). In order to use the expression of the tagged proteins as a model to monitor the corresponding proteins during Salmonella infection, it was necessary to determine whether the tagged strains retain the growth and virulence properties of the parental (wild-type) ST14028s strain both in vitro and in vivo. All the tagged strains grew as well as ST14028s in LB broth (Fig. 1a), suggesting that the insertion of the tag sequence did not significantly affect bacterial growth in vitro (Giacomodonato et al., 2007).
Fig. 1.
Growth analysis of bacterial strains in LB broth (a) and mortality of BALB/c (b) and SCID mice (c) infected with different strains. BALB/c mice (b) and CB17 SCID mice (c) (5 animals per group) were infected intragastrically with 5×106 and 1×103 c.f.u., respectively, of each bacterial strain. Means±sd are plotted.
We used both immunocompetent BALB/c and immunodeficient CB17 SCID mice to investigate the pathogenesis and virulence of the constructed Salmonella strains. We inoculated the animals intraperitoneally to assess the ability of the bacteria to cause systemic infection. Furthermore, we infected the mice via intragastric inoculation, which represents a natural route of infection. Following infection, we determined the colonization of spleen, liver and ileum by the tagged Salmonella strains at different time points after infection. We found no significant differences in the colonization of the internal organs between the parental (wild-type) ST14028s strain and the tagged strains, regardless of the route of inoculation (Table 3). We also determined the survival rates of the infected animals in order to assess the virulence of the tagged strains. When intragastrically infected with 5×106 c.f.u. of the tagged or the wild-type strains, all BALB/c mice died within 9 days post-infection (Fig. 1b). When SCID mice were infected intragastrically with 1×103 c.f.u. bacteria, all animals died within 8 days post-infection (Fig. 1c). In either strain of mice, no significant difference was observed between the wild-type and tagged strains (Fig. 1b, c). Similar results were also observed when animals were intraperitoneally infected with the strains (data not shown). These results suggest that tagging of the target ORF does not impair the invasiveness, growth or virulence of the bacteria, and that the tagged strains can be used as model strains to study the infection of Salmonella in vitro and in vivo, including the expression of SPI-1 proteins and effectors.
Table 3.
Numbers of bacteria (c.f.u.) in different organs from animals
Mice were infected either intraperitoneally (i.p.) or intragastrically (i.g.) with 1×105 c.f.u. for BALB/c mice or 1×102 c.f.u. for SCID mice. A group of five mice was infected and the organs were harvested at 5 days (for i.p. infection) or 7 days (for i.g. inoculation) post-infection. Each sample was analysed in triplicate and the analysis was repeated at least three times. The concentrations of bacteria were recorded as c.f.u. per ml organ homogenate. The c.f.u. counts of the sample were expressed as the mean±sd of the values obtained. The limit of bacterial detection in the organ homogenates was 10 c.f.u. ml−1.
| Salmonella strain | Colonization (i.p.) (log c.f.u. per organ) | Colonization (i.g.) (log c.f.u. per organ) | ||
|---|---|---|---|---|
| Liver | Spleen | Liver | Ileum | |
| BALB/c mice | ||||
| ST14028s | 8.1±0.5 | 7.0±0.5 | 7.8±0.5 | 7.0±0.5 |
| T-InvJ | 8.3±0.5 | 7.3±0.5 | 7.9±0.5 | 7.0±0.5 |
| T-PrgJ | 8.8±0.5 | 7.7±0.5 | 7.8±0.5 | 7.1±0.5 |
| T-SipC | 8.5±0.5 | 7.8±0.5 | 8.1±0.5 | 7.5±0.5 |
| T-SipD | 8.0±0.5 | 7.5±0.5 | 8.0±0.5 | 7.1±0.5 |
| T-SopA | 7.9±0.5 | 7.2±0.5 | 7.9±0.5 | 6.8±0.5 |
| T-SopB | 8.1±0.5 | 7.2±0.5 | 7.7±0.5 | 6.9±0.5 |
| SCID mice | ||||
| ST14028s | 8.2±0.5 | 7.3±0.5 | 7.9±0.5 | 8.1±0.5 |
| T-InvJ | 8.0±0.5 | 7.2±0.5 | 7.8±0.5 | 8.2±0.5 |
| T-PrgJ | 8.1±0.5 | 7.2±0.5 | 8.2±0.5 | 8.3±0.5 |
| T-SipC | 8.4±0.5 | 7.9±0.5 | 7.9±0.5 | 8.0±0.5 |
| T-SipD2 | 8.3±0.5 | 7.8±0.5 | 7.8±0.5 | 8.0±0.5 |
| T-SopA | 8.1±0.5 | 7.6±0.5 | 8.0±0.7 | 8.7±0.5 |
| T-SopB | 8.0±0.5 | 7.6±0.5 | 8.1±0.5 | 8.3±0.5 |
Tagged protein expression under various culture conditions
Salmonella encounters a series of extreme environmental changes in the gut in vivo in order to initiate infection, and expression of its genes, such as those coding for SPI-1 proteins and effectors, is expected to be regulated to allow the bacteria to adapt to new environments in support of infection (Arricau et al., 1998; Galan & Curtiss, 1990; Tartera & Metcalf, 1993). To investigate the synthesis and secretion of the SPI-1 proteins and effectors, we grew each of the tagged strains under five different conditions that resembled the early stages of its natural infection, and examined the expression of the tagged proteins.
Expression in LB broth.
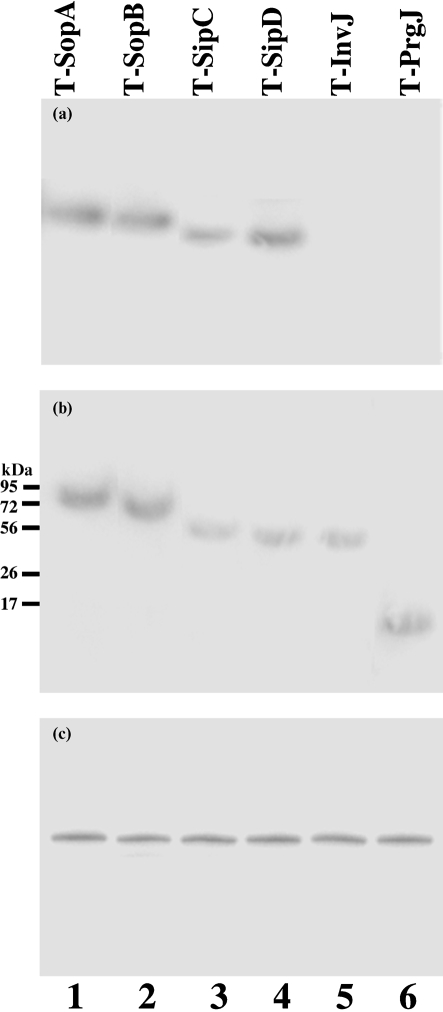
The expression of the tagged proteins was examined with an anti-FLAG antibody (Fig. 2a, b), using the expression of bacterial DnaK protein as the internal control (Fig. 2c). Normalization of samples was also carried out by loading total proteins extracted from the same number of c.f.u. (5×107 c.f.u.) of bacteria in each lane. InvJ, PrgJ, SipC, SipD, SopA and SopB were detected in Salmonella cultured in LB broth (Fig. 2b). Furthermore, SipC, SipD, SopA and SopB, but not InvJ or PrgJ, were detected in the culture supernatant (Fig. 2a), consistent with the previous observations that InvJ and PrgJ are the structural components of the needle complex (Galan & Wolf-Watz, 2006).
Fig. 2.
Western blot analyses of the synthesis (b; cell extracts) and secretion (a; supernatants) of the tagged proteins from strains T-SopA (89 kDa) (lane 1), T-SopB (64 kDa) (lane 2), T-SipC (45 kDa) (lane 3), T-SipD (40 kDa) (lane 4), T-InvJ (39 kDa) (lane 5) and T-PrgJ (13 kDa) (lane 6). The expression of DnaK in bacterial cells was used as the internal control (c). Protein samples were separated in SDS-polyacrylamide gels and reacted with antibodies against the FLAG sequence (a, b) and DnaK (c). The molecular masses of some of the proteins in the PageRuler protein size markers (Fermentas) are shown. Each lane was loaded with lysates prepared from 5×107 c.f.u. bacteria.
Effect of pH.
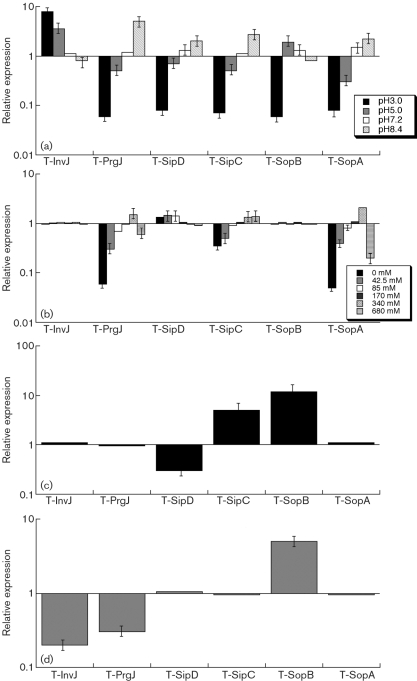
During its enteric infection, Salmonella needs to survive in the stomach, which is acidic, before establishing colonization in the intestine, which is relatively basic. Few SPI-1 proteins and effectors are expressed in the stomach and the expression of these proteins is induced with increasing pH levels from acidic conditions in the stomach to the conditions in the intestines (Ellermeier & Slauch, 2007; Galan & Wolf-Watz, 2006). We grew bacterial strains under different pH conditions (pH 3.0, 5.0, 7.2 and 8.4) and determined the effect of pH values on the expression of the tagged ORFs (Fig. 3a). The expression of the tagged proteins, except InvJ and SopB, was downregulated at low pH (pH 3.0 and pH 5.0) and induced at neutral and basic conditions (pH 7.2 and pH 8.4). In contrast, SopB had the highest expression at pH 5.0, and InvJ had the highest expression at pH 3.0 (Fig. 3a), suggesting that this protein may be expressed at a considerable level as early as in the stomach during Salmonella infection in vivo.
Fig. 3.
Effect of pH (a), osmolarity (b), limitation of oxygen (c), and the presence of butyrate (d) on the expression of the tagged proteins. The tagged strains were grown in culture media at different pH (a), at various concentrations of NaCl (b), in the presence and limitation of oxygen (c), or in the absence and presence of 10 mM butyrate (d) as described in Methods. The values of the relative expression, which are the means±sd from triplicate experiments, represent the ratios of the level of the tagged protein under the experimental conditions to the control pH 7.0 condition (a), to the control condition of 170 mM NaCl of regular LB broth (b), to the control condition of the presence of oxygen (c), or to the control condition of the absence of butyrate (d). The analyses were repeated at least three times.
Effect of osmolarity.
High osmolarity is an environmental stress that bacteria encounter in the intestines, and is believed to promote Salmonella adhesion and invasion to intestinal epithelial cells, contributing to its virulence (Tartera & Metcalf, 1993). Recent studies using a Salmonella nucleotide microarray showed that the transcription levels of SPI-1 genes sipB, sipC and sipD increased significantly in high-osmolarity conditions (e.g. 300 mM NaCl) (Arricau et al., 1998; Huang et al., 2007). However, little is known about the effect of the osmolarity on the protein expression of SPI-1 factors (Mizusaki et al., 2008).
We grew our bacterial strains in the presence of different concentrations of NaCl to investigate the effect of osmolarity on the protein levels of SPI-1 factors. Fig. 3(b) shows the results of the expression of the tagged proteins determined by Western blot analyses. Osmolarity appeared to have no significant impact on the expression of InvJ and SopB. Higher osmolarity of up to 340 mM NaCl favoured the expression of PrgJ and SopA. Consistent with the microarray study (Arricau et al., 1998; Huang et al., 2007), increased osmolarity induced SipC protein expression. However, in contrast to the findings of the microarray study, the expression of SipD protein was higher at low concentrations of NaCl (i.e. 0, 42.5 and 85 mM) than at high concentrations (i.e. 170, 340 and 680 mM) (Fig. 3b).
Effect of oxygen limitation.
Oxygen limitation, an important characteristic of the environment in the intestines, can induce Salmonella invasiveness of intestinal mucosa, whereas aerobic conditions render Salmonella less invasive (Jones & Falkow, 1994; Lee & Falkow, 1990). The expression of the transcripts of several SPI-1 genes can be regulated by oxygen, osmolarity, pH, PhoP/Q and HilA (Bajaj et al., 1996). In our experiments, oxygen limitation had no significant impact on the protein expression of InvJ, PrgJ and SopA (Fig. 3c). Decreased levels of oxygen appeared to induce the protein expression of SipC and SopB, but inhibited the expression of SipD.
Effect of butyrate.
Under the anaerobic environment in the intestines, bacterial fermentation occurs, leading to the accumulation of three types of organic acids: acetate, propionate and butyrate (Cummings et al., 1987). Limitation of butyrate could lead to intestinal inflammation, and administration of butyrate could alleviate the severity of Salmonella infection of intestinal epithelium (Van Immerseel et al., 2004a, b). The transcription levels of 17 SPI-1 genes have been shown to be downregulated at least twofold after exposure of Salmonella to 10 mM butyrate (Gantois et al., 2006). However, little is known about the effects of butyrate on protein levels of these factors. Our results showed that, in the presence of butyrate, the protein levels of InvJ and PrgJ decreased while that of SopB increased (Fig. 3d). In contrast, incubation with 10 mM butyrate did not significantly affect the protein levels of SipC, SipD or SopA.
Tagged protein expression in vivo during intraperitoneal infection
BALB/c mice were infected intraperitoneally in order to study SPI-1 protein and effector expression during systemic bacterial infection. At different time points post-infection, the liver and ileum were harvested. Western blot analysis with an anti-FLAG antibody was carried out to determine the expression of the tagged proteins in Salmonella isolated from the liver and ileum of infected mice, using the expression of the bacterial protein DnaK as the internal control (Fig. 4a, b). Normalization of samples was also carried out by loading total proteins extracted from the same number of c.f.u. (5×107 c.f.u.) of bacteria in each lane. A similar amount of the DnaK protein was detected from 5×107 c.f.u. of each bacterial strain regardless of the infection route (intraperitoneal or intragastric) or time point post-infection (12–24 h or 5–7 days) (data not shown), suggesting that the protein level of DnaK was not significantly different in bacteria from the liver and ileum.
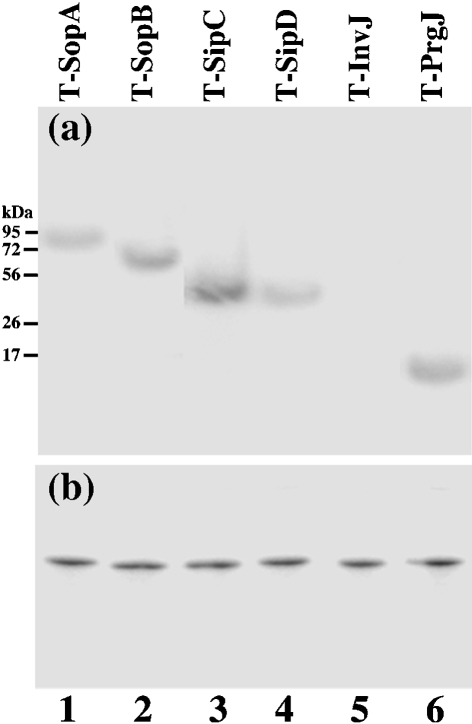
Fig. 4.
Western blot analyses of the expression of the tagged proteins from strains T-SopA (lane 1), T-SopB (lane 2), T-SipC (lane 3), T-SipD (lane 4), T-InvJ (lane 5) and T-PrgJ (lane 6) recovered from the livers of infected mice. BALB/c mice were intraperitoneally infected with 1×105 c.f.u. of the tagged strains, and bacteria were recovered from the livers at 5 days post-inoculation. Each lane was loaded with material from 5×107 c.f.u. bacteria. Protein samples were reacted with antibodies against the FLAG sequence (a) and DnaK (b).
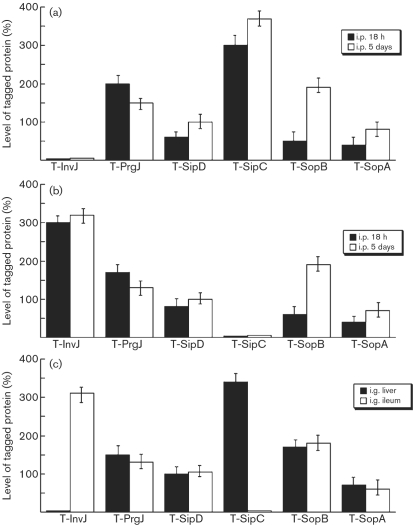
Salmonella isolated from both the liver and ileum at 18 h post-infection were found to express PrgJ, SipD, SopA and SopB. In contrast, a substantial level of InvJ was detected in Salmonella isolated from the ileum but not the liver, while a substantial level of SipC was observed in Salmonella recovered from the liver but not the ileum (Fig. 5a, b). To rule out the residual expression of the proteins from in vitro bacterial growth, BALB/c mice were intraperitoneally infected with 1×105 c.f.u. bacteria for a longer period and tissues were harvested at 5 days post-infection, prior to the onset of severe disease associated with infection. We again found that proteins PrgJ, SipD, SopB and SopA were expressed in Salmonella isolated from both the liver and ileum, and that InvJ and SipC were preferentially expressed by Salmonella recovered from the ileum and liver, respectively (Fig. 5a, b). Indeed, the expression of the InvJ protein was more than 25-fold higher than that of SipC in Salmonella isolated from the ileum, while the SipC protein was expressed at least 35 times more than InvJ in Salmonella isolated from the liver (Fig. 5a, b).
Fig. 5.
Level of tagged proteins from the bacterial strains recovered from the liver and ileum of infected mice. In (a) and (b), BALB/c mice were intraperitoneally (i.p.) infected with 1×107 or 1×105 c.f.u. of the tagged strains, and bacteria were recovered from the organs at 18 h or 5 days post-inoculation, respectively. The values, which are the means±sd from triplicate experiments, represent the relative percentage of the level of the tagged proteins in the bacteria recovered from the liver (a) and ileum (b), relative to the level of tagged SipD in strain T-SipD recovered from the liver and ileum, respectively, at 5 days post-inoculation (taken as 100 %). In (c), BALB/c mice were intragastrically (i.g.) infected with 1×105 c.f.u. of the tagged strains, and bacteria were recovered from the liver and ileum at 7 days post-inoculation. The values, which are the means±sd from triplicate experiments, represent the level of the tagged proteins in the bacteria recovered from the organs relative to the level of tagged SipD in strain T-SipD recovered from the liver at 7 days post-inoculation.
Tagged protein expression in vivo during oral infection
BALB/c mice were infected intragastrically with 1×105 c.f.u. bacteria in order to study whether the tagged proteins are expressed during Salmonella infection acquired by the natural route. Livers and ileums were collected and the bacteria were recovered at day 7 post-infection, prior to the onset of severe disease associated with infection. The level of DnaK protein did not appear to be significantly different in bacteria recovered from the liver and ileum (data not shown). PrgJ, SipD, SopA and SopB were detected in Salmonella isolated from both the liver and ileum, while InvJ and SipC were expressed preferentially in Salmonella isolated from the ileum and liver, respectively (Fig. 5c). These results provide direct evidence that the InvJ and SipC proteins are differentially expressed during oral infection of mice.
DISCUSSION
In this study, we have characterized the expression of InvJ, PrgJ, SipC, SipD, SopA and SopB in vitro and in vivo. To our knowledge, our results provide the first direct evidence that InvJ and SipC are differentially expressed in Salmonella colonizing the ileum and liver, respectively. Furthermore, this study demonstrates for the first time that PrgJ and SipD are expressed in vivo in the liver and ileum in both the early and late stages of Salmonella infection. These results suggest that specific SPI-1 proteins and effectors are expressed by Salmonella in different tissues and that differential expression of these proteins may be important for bacterial pathogenesis in certain tissues, such as gastroenterititis in the intestine and typhoid fever during systemic infection in the liver.
Previous studies have shown that the SopA and SopB proteins are expressed in Salmonella isolated from the spleen (Giacomodonato et al., 2007). Our results are consistent with these previous observations, and further demonstrate that these proteins are also expressed in Salmonella isolated from the liver and ileum. Our results also provide direct evidence that PrgJ and SipD proteins are expressed in vivo. PrgJ is a component of the needle complex or ‘injectisome’ that is responsible for transport of the proteins of the type III secretion pathway (Galan & Wolf-Watz, 2006; Kubori et al., 2000). Within the complex, PrgJ makes up the inner rod substructure, which connects to the needle structure that is composed of the PrgI protein. Recent evidence has suggested that the stoichiometry of PrgI and PrgJ, which is dictated by their protein expression levels, affects the length of the needle complex formed, and consequently the ability of the bacteria to enter epithelial cells and induce cytotoxicity in macrophages (Galan & Wolf-Watz, 2006; Kubori et al., 2000; Marlovits et al., 2006). The effector protein SipD is a Salmonella invasion protein (Sip) that functions as a translocase to deploy effector proteins during the initiation of the bacterial entry process (Galan & Wolf-Watz, 2006; Hueck, 1998). SipD is located at the tip of the needle complex prior to the contact with the host cells, and has been recently shown to be essential for initial contact of Salmonella to non-phagocytic cells (Lara-Tejero & Galan, 2009). Our results showing the presence of PrgJ and SipD in bacteria isolated from animals suggest that these two proteins may be needed for bacterial pathogenesis in the liver and ileum during both the early and late stages of Salmonella infection.
Our results demonstrate that SipC and InvJ are differentially expressed in vivo by Salmonella when it colonizes the liver and ileum, respectively. SipC interacts with SipB to form an extracellular complex following their secretion through the SPI-1 T3SS, and they are thought to assemble into a plasma membrane-integral structure (translocon) that mediates effector delivery and facilitates effector transport (Hayward & Koronakis, 1999; Ly & Casanova, 2007; Scherer et al., 2000). In addition to its role as a component of the translocon, SipC is an actin-binding protein that nucleates actin filament formation in vitro and contributes to Salmonella-induced inflammation in vivo (Chang et al., 2007). Its central amino acid region (residues 201–220) is essential for its actin nucleation activity and the C-terminal amino acid region (residues 321–409) is required for translocation of effectors (Chang et al., 2005). While the expression of SipC has been extensively studied in vitro, its expression in vivo in the liver and ileum has not been extensively investigated. The preferential expression of SipC by Salmonella colonizing the liver but not the ileum suggests that the level of this protein is highly regulated in vivo and that appropriate levels of expression may contribute to different consequences of pathogenesis. This is consistent with the recent observations that the translocase activity of SipC is important for delivery of effector proteins and attachment of Salmonella to non-phagocytic cells; however, in the context of systemic infection, its actin-binding activity may facilitate bacterial infection of phagocytes (Chang et al., 2007; Galan & Wolf-Watz, 2006; Lara-Tejero & Galan, 2009). Thus, examination of the expression of SipC and other SPI-1 factors in vivo in the context of infection, as reported in our study, is crucial to ultimately understand the actual function and action of these factors.
Previous studies have indicated that the InvJ protein is essential for needle complex assembly and protein secretion critical for bacterial entry (Galan & Wolf-Watz, 2006; Marlovits et al., 2006; Sukhan et al., 2001). However, its expression in vivo in the liver and ileum has not been reported. Our results showed that InvJ is preferentially expressed by Salmonella colonizing the ileum but not the liver. This suggests that efficient expression of this protein may not be needed by Salmonella in the liver, possibly because bacterial entry can be accomplished by phagocytosis during systemic infection. It is also conceivable that tissue-specific differential regulation of the expression of InvJ, a protein essential for the formation of the needle complex, may provide another mechanism in vivo for the regulation of the amounts of effector proteins to be secreted into host cells. Recent studies have revealed hierarchical transport of different effectors during Salmonella entry and extensive ordered synergistic and antagonist relationships between these effectors following their delivery into the host cells (Cain et al., 2008; Galan & Wolf-Watz, 2006; Winnen et al., 2008). Thus, differential expression of InvJ may dictate the amounts of needle complexes available during bacterial entry, resulting in hierarchical transport of specific effectors and specific functional interplay (synergistic or antagonist relationships) among these proteins in the host cells, and leading to specific pathological consequences in different tissues.
It is possible that the observed expression of the tagged ORFs is due to adventitious mutations introduced during the construction and growth of the mutants in vitro and in animals. Furthermore, the function and expression of the ORFs may be affected by insertion of an epitope tag, which may have an impact on the function of other genes adjacent to the insertion region, and therefore possibly affect the expression of the tagged ORF. However, several lines of evidence suggest that this is not likely. First, the tagged mutants grew as well as the wild-type ST14028s strain in LB broth and in both BALB/c and SCID mice (Fig. 1a, Table 3). Second, the mutants exhibited similar virulence as the ST14028s strain in vivo (Fig. 1b, c). These results suggest that the FLAG tag insertion neither affected the function of the tagged ORF nor caused any adventitious mutations that may have affected bacterial virulence and pathogenicity. Thus, we believe that the observed expression of the tagged proteins represent the expression of the wild-type SPI-1 proteins and effectors in vitro and in vivo.
Some of the protein expression results in our study may not be consistent with the results from the expression of the transcripts of the genes coding for SPI-1 proteins and effectors that have been recently published (Gantois et al., 2006; Huang et al., 2007) as the expression of these factors can be independently controlled transcriptionally and post-transcriptionally (Ellermeier & Slauch, 2007). Furthermore, the amounts of proteins expressed from these genes in vivo may be in a delicate balance as there is hierarchical transport of different effectors during Salmonella entry and extensively ordered synergistic and antagonist relationships between these effectors following their delivery into the host cells. Thus, our results of the SPI-1 protein and effector expression in vivo may not necessarily correlate with previous observations in vitro. It is possible that the ability of the bacteria to establish successful infection and cause pathogenesis in specific tissues may be significantly influenced by a balance of the amounts of these factors available during infection. Our results complement and further extend previous findings of the expression of these SPI-1 factors, and demonstrate the importance of examining protein expression in vivo in the context of infection. It will be interesting to determine whether factors of other SPIs are also differentially expressed in specific tissues. More studies on the expression of the factors of SPI-1 as well as of other SPIs in different organs in vivo are needed to understand the exact roles of these proteins in Salmonella infection and pathogenesis in specific tissues.
Acknowledgments
We thank Gerry Abenes, Paul Rider, Hongwei Gu and Huiyuan Jiang for suggestions and excellent technical assistance. G.-P. V. was a recipient of an Undergraduate Research Appentice Program Summer Scholarship (UC-Berkeley). E. Y. and Y. B. were partially supported by a Block Grant Predoctoral Fellowship (UC-Berkeley). The research has been supported by grants from USDA (CALR-2005-01892) and NIH (RO1-AI050468 and RO1-DE014145).
Abbreviations
SPI, Salmonella pathogenicity island
T3SS, type III secretion system
References
- Abrahams, G. L. & Hensel, M. (2006). Manipulating cellular transport and immune responses: dynamic interactions between intracellular Salmonella enterica and its host cells. Cell Microbiol 8, 728–737. [DOI] [PubMed] [Google Scholar]
- Arricau, N., Hermant, D., Waxin, H., Ecobichon, C., Duffey, P. S. & Popoff, M. Y. (1998). The RcsB-RcsC regulatory system of Salmonella typhi differentially modulates the expression of invasion proteins, flagellin and Vi antigen in response to osmolarity. Mol Microbiol 29, 835–850. [DOI] [PubMed] [Google Scholar]
- Bajaj, V., Lucas, R. L., Hwang, C. & Lee, C. A. (1996). Co-ordinate regulation of Salmonella typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol Microbiol 22, 703–714. [DOI] [PubMed] [Google Scholar]
- Blanc-Potard, A. B., Solomon, F., Kayser, J. & Groisman, E. A. (1999). The SPI-3 pathogenicity island of Salmonella enterica. J Bacteriol 181, 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain, R. J., Hayward, R. D. & Koronakis, V. (2008). Deciphering interplay between Salmonella invasion effectors. PLoS Pathog 4, e1000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, J., Chen, J. & Zhou, D. (2005). Delineation and characterization of the actin nucleation and effector translocation activities of Salmonella SipC. Mol Microbiol 55, 1379–1389. [DOI] [PubMed] [Google Scholar]
- Chang, J., Myeni, S. K., Lin, T. L., Wu, C. C., Staiger, C. J. & Zhou, D. (2007). SipC multimerization promotes actin nucleation and contributes to Salmonella-induced inflammation. Mol Microbiol 66, 1548–1556. [DOI] [PubMed] [Google Scholar]
- Chubet, R. G. & Brizzard, B. L. (1996). Vectors for expression and secretion of FLAG epitope-tagged proteins in mammalian cells. Biotechniques 20, 136–141. [DOI] [PubMed] [Google Scholar]
- Cummings, J. H., Pomare, E. W., Branch, W. J., Naylor, C. P. & Macfarlane, G. T. (1987). Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28, 1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko, K. A. & Wanner, B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97, 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellermeier, J. R. & Slauch, J. M. (2007). Adaptation to the host environment: regulation of the SPI1 type III secretion system in Salmonella enterica serovar Typhimurium. Curr Opin Microbiol 10, 24–29. [DOI] [PubMed] [Google Scholar]
- Galan, J. E. (2001). Salmonella interactions with host cells: type III secretion at work. Annu Rev Cell Dev Biol 17, 53–86. [DOI] [PubMed] [Google Scholar]
- Galan, J. E. & Curtiss, R., III (1990). Expression of Salmonella typhimurium genes required for invasion is regulated by changes in DNA supercoiling. Infect Immun 58, 1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan, J. E. & Wolf-Watz, H. (2006). Protein delivery into eukaryotic cells by type III secretion machines. Nature 444, 567–573. [DOI] [PubMed] [Google Scholar]
- Gantois, I., Ducatelle, R., Pasmans, F., Haesebrouck, F., Hautefort, I., Thompson, A., Hinton, J. C. & Van Immerseel, F. (2006). Butyrate specifically down-regulates salmonella pathogenicity island 1 gene expression. Appl Environ Microbiol 72, 946–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomodonato, M. N., Uzzau, S., Bacciu, D., Caccuri, R., Sarnacki, S. H., Rubino, S. & Cerquetti, M. C. (2007). SipA, SopA, SopB, SopD and SopE2 effector proteins of Salmonella enterica serovar Typhimurium are synthesized at late stages of infection in mice. Microbiology 153, 1221–1228. [DOI] [PubMed] [Google Scholar]
- Gong, H., Su, J., Bai, Y., Miao, L., Kim, K., Yang, Y., Liu, F. & Lu, S. (2009). Characterization of the expression of Salmonella Type III secretion system factor PrgI, SipA, SipB, SopE2, SpaO, and SptP in cultures and in mice. BMC Microbiol 9, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward, R. D. & Koronakis, V. (1999). Direct nucleation and bundling of actin by the SipC protein of invasive Salmonella. EMBO J 18, 4926–4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel, M. (2004). Evolution of pathogenicity islands of Salmonella enterica. Int J Med Microbiol 294, 95–102. [DOI] [PubMed] [Google Scholar]
- Huang, X., Xu, H., Sun, X., Ohkusu, K., Kawamura, Y. & Ezaki, T. (2007). Genome-wide scan of the gene expression kinetics of Salmonella enterica serovar typhi during hyperosmotic stress. Int J Mol Sci 8, 116–135. [Google Scholar]
- Hueck, C. J. (1998). Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev 62, 379–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, B. D. & Falkow, S. (1994). Identification and characterization of a Salmonella typhimurium oxygen-regulated gene required for bacterial internalization. Infect Immun 62, 3745–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss, T., Morgan, E. & Nagy, G. (2007). Contribution of SPI-4 genes to the virulence of Salmonella enterica. FEMS Microbiol Lett 275, 153–159. [DOI] [PubMed] [Google Scholar]
- Kubori, T., Sukhan, A., Aizawa, S. I. & Galan, J. E. (2000). Molecular characterization and assembly of the needle complex of the Salmonella typhimurium type III protein secretion system. Proc Natl Acad Sci U S A 97, 10225–10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Tejero, M. & Galan, J. E. (2009). Salmonella enterica serovar Typhimurium pathogenicity island 1-encoded type III secretion system translocases mediate intimate attachment to nonphagocytic cells. Infect Immun 77, 2635–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C. A. & Falkow, S. (1990). The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci U S A 87, 4304–4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lober, S., Jackel, D., Kaiser, N. & Hensel, M. (2006). Regulation of Salmonella pathogenicity island 2 genes by independent environmental signals. Int J Med Microbiol 296, 435–447. [DOI] [PubMed] [Google Scholar]
- Lu, S., Manges, A. R., Xu, Y., Fang, F. C. & Riley, L. W. (1999). Analysis of virulence of clinical isolates of Salmonella enteritidis in vivo and in vitro. Infect Immun 67, 5651–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, S., Killoran, P. B., Fang, F. C. & Riley, L. W. (2002). The global regulator ArcA controls resistance to reactive nitrogen and oxygen intermediates in Salmonella enterica serovar Enteritidis. Infect Immun 70, 451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, S., Killoran, P. B. & Riley, L. W. (2003). Association of Salmonella enterica serovar Enteritidis yafD with resistance to chicken egg albumen. Infect Immun 71, 6734–6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly, K. T. & Casanova, J. E. (2007). Mechanisms of Salmonella entry into host cells. Cell Microbiol 9, 2103–2111. [DOI] [PubMed] [Google Scholar]
- Marlovits, T. C., Kubori, T., Lara-Tejero, M., Thomas, D., Unger, V. M. & Galan, J. E. (2006). Assembly of the inner rod determines needle length in the type III secretion injectisome. Nature 441, 637–640. [DOI] [PubMed] [Google Scholar]
- Mizusaki, H., Takaya, A., Yamamoto, T. & Aizawa, S. (2008). Signal pathway in salt-activated expression of the Salmonella pathogenicity island 1 type III secretion system in Salmonella enterica serovar Typhimurium. J Bacteriol 190, 4624–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen, S. J., MacKinnon, L. C., Goulding, J. S., Bean, N. H. & Slutsker, L. (2000). Surveillance for foodborne-disease outbreaks – United States, 1993–1997. MMWR CDC Surveill Summ 49, 1–62. [PubMed] [Google Scholar]
- Pfeifer, C. G., Marcus, S. L., Steele-Mortimer, O., Knodler, L. A. & Finlay, B. B. (1999). Salmonella typhimurium virulence genes are induced upon bacterial invasion into phagocytic and nonphagocytic cells. Infect Immun 67, 5690–5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer, C. A., Cooper, E. & Miller, S. I. (2000). The Salmonella type III secretion translocon protein SspC is inserted into the epithelial cell plasma membrane upon infection. Mol Microbiol 37, 1133–1145. [DOI] [PubMed] [Google Scholar]
- Schmidt, H. & Hensel, M. (2004). Pathogenicity islands in bacterial pathogenesis. Clin Microbiol Rev 17, 14–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele-Mortimer, O., Brumell, J. H., Knodler, L. A., Meresse, S., Lopez, A. & Finlay, B. B. (2002). The invasion-associated type III secretion system of Salmonella enterica serovar Typhimurium is necessary for intracellular proliferation and vacuole biogenesis in epithelial cells. Cell Microbiol 4, 43–54. [DOI] [PubMed] [Google Scholar]
- Su, J., Gong, H., Lai, J., Main, A. & Lu, S. (2009). The potassium transporter Trk and external potassium modulate Salmonella enterica protein secretion and virulence. Infect Immun 77, 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhan, A., Kubori, T., Wilson, J. & Galan, J. E. (2001). Genetic analysis of assembly of the Salmonella enterica serovar Typhimurium type III secretion-associated needle complex. J Bacteriol 183, 1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartera, C. & Metcalf, E. S. (1993). Osmolarity and growth phase overlap in regulation of Salmonella typhi adherence to and invasion of human intestinal cells. Infect Immun 61, 3084–3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Immerseel, F., De Buck, J., De Smet, I., Pasmans, F., Haesebrouck, F. & Ducatelle, R. (2004a). Interactions of butyric acid- and acetic acid-treated Salmonella with chicken primary cecal epithelial cells in vitro. Avian Dis 48, 384–391. [DOI] [PubMed] [Google Scholar]
- Van Immerseel, F., Fievez, V., de Buck, J., Pasmans, F., Martel, A., Haesebrouck, F. & Ducatelle, R. (2004b). Microencapsulated short-chain fatty acids in feed modify colonization and invasion early after infection with Salmonella enteritidis in young chickens. Poult Sci 83, 69–74. [DOI] [PubMed] [Google Scholar]
- Waterman, S. R. & Holden, D. W. (2003). Functions and effectors of the Salmonella pathogenicity island 2 type III secretion system. Cell Microbiol 5, 501–511. [DOI] [PubMed] [Google Scholar]
- Winnen, B., Schlumberger, M. C., Sturm, A., Schupbach, K., Siebenmann, S., Jenny, P. & Hardt, W. D. (2008). Hierarchical effector protein transport by the Salmonella Typhimurium SPI-1 type III secretion system. PLoS One 3, e2178. [DOI] [PMC free article] [PubMed] [Google Scholar]