Abstract
Germ line acquisition of ecotropic proviruses occurs at a high frequency in the progeny of SWR/J-RF/J hybrid mice carrying two genetically linked RF/J ecotropic proviral loci, Emv-16 and Emv-17 (N. A. Jenkins and N. G. Copeland, Cell 43:811-819, 1985). To determine if genetic background affects proviral integration frequency, I analyzed a series of crosses in which the two RF/J proviral loci were transferred onto different provirus-negative background strains. Unlike SWR/J-RF/J hybrid progeny, few CBA/CaJ-RF/J hybrid mice were identified that carried new germ line proviral loci. These results indicate that genetic factors other than the linked RF/J proviral loci contribute to the increased frequency of germ line provirus integration seen in the SWR/J-RF/J hybrids. The frequency of proviral acquisition appeared to increase when females carrying Emv-16, Emv-17, and at least one new proviral locus were further backcrossed, suggesting that integration frequency can be increased by genetic manipulation. The breeding data are consistent with the hypothesis that virus from the mother infects the egg or the early embryo. Analysis of the transmission frequency and cosegregation patterns of new proviral loci indicated that viral integration occurs after the first round of DNA replication and before the germ line is set aside during embryogenesis, with a majority of viral integrations occurring at the two-cell stage of development, and independent viral integrations can occur in the same or in different cells of the embryo.
Full text
PDF


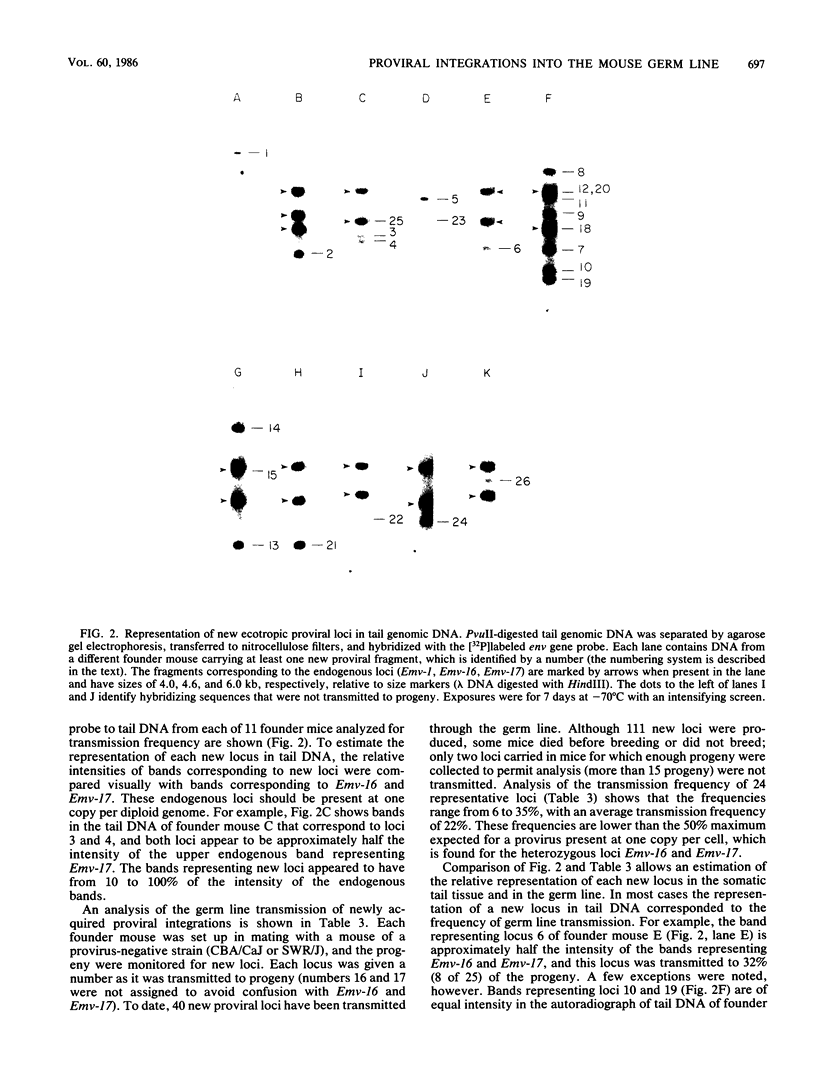




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckler C. E., Staal S. P., Rowe W. P., Martin M. A. Variation in the number of copies and in the genomic organization of ecotropic murine leukemia virus proviral sequences in sublines of AKR mice. J Virol. 1982 Aug;43(2):629–640. doi: 10.1128/jvi.43.2.629-640.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Struuck F. D., Duran-Reynals M. L., Lilly F. Genetic and nongenetic factors in expression of infectious murine leukemia viruses in mice of the DBA/2 x RF cross. Cell. 1980 Oct;21(3):849–855. doi: 10.1016/0092-8674(80)90448-1. [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Hutchison K. W., Jenkins N. A. Excision of the DBA ecotropic provirus in dilute coat-color revertants of mice occurs by homologous recombination involving the viral LTRs. Cell. 1983 Jun;33(2):379–387. doi: 10.1016/0092-8674(83)90419-1. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Frankel W., Potter T. A., Rosenberg N., Lenz J., Rajan T. V. Retroviral insertional mutagenesis of a target allele in a heterozygous murine cell line. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6600–6604. doi: 10.1073/pnas.82.19.6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W., Gilbert W. Germ-line MuLV reintegrations in AKR/J mice. Nature. 1982 Apr 29;296(5860):865–868. doi: 10.1038/296865a0. [DOI] [PubMed] [Google Scholar]
- Herr W., Gilbert W. Somatically acquired recombinant murine leukemia proviruses in thymic leukemias of AKR/J mice. J Virol. 1983 Apr;46(1):70–82. doi: 10.1128/jvi.46.1.70-82.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R., Harbers K., Schnieke A., Löhler J., Chumakov I., Jähner D., Grotkopp D., Hoffmann E. Germline integration of moloney murine leukemia virus at the Mov13 locus leads to recessive lethal mutation and early embryonic death. Cell. 1983 Jan;32(1):209–216. doi: 10.1016/0092-8674(83)90511-1. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G. High frequency germline acquisition of ecotropic MuLV proviruses in SWR/J-RF/J hybrid mice. Cell. 1985 Dec;43(3 Pt 2):811–819. doi: 10.1016/0092-8674(85)90254-5. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus. J Virol. 1982 Jul;43(1):26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P. The Fv-1 gene of the mouse and its control of murine leukemia virus replication. Curr Top Microbiol Immunol. 1979;86:67–122. doi: 10.1007/978-3-642-67341-2_3. [DOI] [PubMed] [Google Scholar]
- King W., Patel M. D., Lobel L. I., Goff S. P., Nguyen-Huu M. C. Insertion mutagenesis of embryonal carcinoma cells by retroviruses. Science. 1985 May 3;228(4699):554–558. doi: 10.1126/science.3838595. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Chattopadhyay S. K., Teich N. M., Rowe W. P., Levine A. S. AKR murine leukemia virus genome: frequency of sequences in DNA of high-, low-, and non-virus-yielding mouse strains. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3555–3559. doi: 10.1073/pnas.71.9.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark W. H., Signorelli K., Lacy E. An insertional mutation in a transgenic mouse line results in developmental arrest at day 5 of gestation. Cold Spring Harb Symp Quant Biol. 1985;50:453–463. doi: 10.1101/sqb.1985.050.01.057. [DOI] [PubMed] [Google Scholar]
- Mayer A., Duran-Reynals M. L., Lilly F. Fv-1 regulation of lymphoma development and of thymic ecotropic and xenotropic MuLV expression in mice of the AKR/J x RF/J cross. Cell. 1978 Oct;15(2):429–435. doi: 10.1016/0092-8674(78)90012-0. [DOI] [PubMed] [Google Scholar]
- Mayer A., Struuck F. D., Duran-Reynals M. L., Lilly F. Maternally transmitted resistance to lymphoma development in mice of reciprocal crosses of the RF/J and AKR/J strains. Cell. 1980 Feb;19(2):431–436. doi: 10.1016/0092-8674(80)90517-6. [DOI] [PubMed] [Google Scholar]
- McCubrey J., Horowitz J. M., Risser R. Structure and expression of endogenous ecotropic murine leukemia viruses in RF/J mice. J Exp Med. 1982 Nov 1;156(5):1461–1474. doi: 10.1084/jem.156.5.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren A. Reproduction in single-sex chimeras. Basic Life Sci. 1978;12:125–134. doi: 10.1007/978-1-4684-3390-6_10. [DOI] [PubMed] [Google Scholar]
- Melamedoff M., Lilly F., Duran-Reynals M. L. Suppression of endogenous murine leukemia virus by maternal resistance factor. J Exp Med. 1983 Aug 1;158(2):506–514. doi: 10.1084/jem.158.2.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. L., Chan H. W. Identification of ecotropic proviral sequences in high- and low-ecotropic-virus-producing mouse strains. J Virol. 1982 Sep;43(3):1038–1045. doi: 10.1128/jvi.43.3.1038-1045.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Wilkie T. M., Chen H. Y., Brinster R. L. Transmission distortion and mosaicism in an unusual transgenic mouse pedigree. Cell. 1984 Apr;36(4):869–877. doi: 10.1016/0092-8674(84)90036-9. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Bishop J. M., Varmus H. E. Multiple arrangements of viral DNA and an activated host oncogene in bursal lymphomas. Nature. 1982 Jan 21;295(5846):209–214. doi: 10.1038/295209a0. [DOI] [PubMed] [Google Scholar]
- Quint W., Quax W., van der Putten H., Berns A. Characterization of AKR murine leukemia virus sequences in AKR mouse substrains and structure of integrated recombinant genomes in tumor tissues. J Virol. 1981 Jul;39(1):1–10. doi: 10.1128/jvi.39.1.1-10.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rowe W. P. Genetic factors in the natural history of murine leukemia virus infection: G. H. A. Clowes Memorial Lecture. Cancer Res. 1973 Dec;33(12):3061–3068. [PubMed] [Google Scholar]
- Rowe W. P., Kozak C. A. Germ-line reinsertions of AKR murine leukemia virus genomes in Akv-1 congenic mice. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4871–4874. doi: 10.1073/pnas.77.8.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pincus T. Quantitative studies of naturally occurring murine leukemia virus infection of AKR mice. J Exp Med. 1972 Feb 1;135(2):429–436. doi: 10.1084/jem.135.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki W., Baranska W., Koprowski H. Susceptibility of unfertilized and fertilized mouse eggs to simian virus 40 and Moloney sarcoma virus. J Natl Cancer Inst. 1971 Nov;47(5):1045–1051. [PubMed] [Google Scholar]
- Searle A. G. Evidence from mutable genes concerning the origin of the germ line. Basic Life Sci. 1978;12:209–224. doi: 10.1007/978-1-4684-3390-6_16. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steffen D. L., Taylor B. A., Weinberg R. A. Continuing germ line integration of AKV proviruses during the breeding of AKR mice and derivative recombinant inbred strains. J Virol. 1982 Apr;42(1):165–175. doi: 10.1128/jvi.42.1.165-175.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephension J. R., Reynolds R. K., Tronick S. R., Aaronson S. A. Distribution of three classes of endogenous type-C RNA viruses among inbred strains of mice. Virology. 1975 Oct;67(2):404–414. doi: 10.1016/0042-6822(75)90442-0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E. Form and function of retroviral proviruses. Science. 1982 May 21;216(4548):812–820. doi: 10.1126/science.6177038. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Ortiz S. Retroviruses as mutagens: insertion and excision of a nontransforming provirus alter expression of a resident transforming provirus. Cell. 1981 Jul;25(1):23–36. doi: 10.1016/0092-8674(81)90228-2. [DOI] [PubMed] [Google Scholar]
- Wagner E. F., Covarrubias L., Stewart T. A., Mintz B. Prenatal lethalities in mice homozygous for human growth hormone gene sequences integrated in the germ line. Cell. 1983 Dec;35(3 Pt 2):647–655. doi: 10.1016/0092-8674(83)90097-1. [DOI] [PubMed] [Google Scholar]
- Wolf D., Rotter V. Inactivation of p53 gene expression by an insertion of Moloney murine leukemia virus-like DNA sequences. Mol Cell Biol. 1984 Jul;4(7):1402–1410. doi: 10.1128/mcb.4.7.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woychik R. P., Stewart T. A., Davis L. G., D'Eustachio P., Leder P. An inherited limb deformity created by insertional mutagenesis in a transgenic mouse. Nature. 1985 Nov 7;318(6041):36–40. doi: 10.1038/318036a0. [DOI] [PubMed] [Google Scholar]