Abstract
The depletion of l-tryptophan (L-Trp) has been associated with the inhibition of growth of micro-organisms and also has profound effects on T cell proliferation and immune tolerance. The enzyme indoleamine 2,3-dioxygenase (IDO) catalyses the rate-limiting step in the catabolic pathway of L-Trp. Gene expression analysis has shown upregulation of genes involved in L-Trp catabolism in in vitro models of dengue virus (DENV) infection. To understand the role of IDO during DENV infection, we measured IDO activity in sera from control and DENV-infected patients. We found increased IDO activity, lower levels of L-Trp and higher levels of l-kynurenine in sera from DENV-infected patients during the febrile days of the disease compared with patients with other febrile illnesses and healthy donors. Furthermore, we confirmed upregulation of IDO mRNA expression in response to DENV infection in vitro, using a dendritic cell (DC) model of DENV infection. We found that the antiviral effect of gamma interferon (IFN-γ) in DENV-infected DCs in vitro was partially dependent on IDO activity. Our results demonstrate that IDO plays an important role in the antiviral effect of IFN-γ against DENV infection in vitro and suggest that it has a role in the immune response to DENV infections in vivo.
INTRODUCTION
The enzyme indoleamine 2,3-dioxygenase (IDO) has been implicated in antimicrobial defence mechanisms and in immune regulation. IDO (reviewed by King & Thomas, 2007) catalyses the rate-limiting step in the kynurenine pathway, which is the major degradation route for l-tryptophan (L-Trp) in mammals. IDO can be induced in response to various stimuli. The most potent inducer is gamma interferon (IFN-γ), although other inflammatory stimuli, including type-I interferons (IFNs) (IFN-α/β) and bacterial lipopolysaccharide (LPS), and some viruses and intracellular pathogens, can induce IDO expression (King & Thomas, 2007; Puccetti, 2007). The IDO promoter contains a single IFN-γ activation site specific for IFN-γ, as well as two non-specific IFN-stimulated response elements, which can respond to IFN-α and IFN-β as well as IFN-γ (Puccetti, 2007).
It has been reported that IDO can inhibit the growth of parasites, such as Toxoplasma spp. (Thomas et al., 1993), viruses, including herpes simplex virus (Adams et al., 2004), cytomegalovirus (Bodaghi et al., 1999) and vaccinia virus (Terajima & Leporati, 2005), and bacteria, such as Chlamydia spp. (Rapoza et al., 1991; Thomas et al., 1993), streptococci, enterococci (MacKenzie et al., 1999) and staphylococci (Schroten et al., 2001). The antimicrobial effect of IDO is reported to be dependent on depletion of L-Trp (MacKenzie et al., 2007). On the other hand, IDO-dependent immune regulation may be a consequence of the accumulation of immune-active or toxic metabolites or other signalling events (MacKenzie et al., 2007). IDO-dependent T cell suppression by dendritic cells (DCs) suggests that biochemical changes due to L-Trp catabolism have profound effects on T cell proliferation, differentiation and effector functions (Mellor & Munn, 2004) and promote immune tolerance (King & Thomas, 2007).
Dengue virus (DENV) is a single-stranded RNA flavivirus that exists as four different serotypes: DENV1, DENV2, DENV3 and DENV4 (Monath, 1994). It is transmitted to humans by mosquitoes and there are a broad spectrum of clinical manifestations (Chaturvedi et al., 2006). Clinical symptoms include high fever, headache, myalgia, skin rash, thrombocytopenia, coagulation alterations, hepatic inflammation and haemorrhagic manifestations. The severe form of the disease, dengue haemorrhagic fever (DHF), is associated with increased vascular permeability that results in vascular leakage (Rothman & Ennis, 1999).
Previous data from our laboratory using gene expression analysis (Affymetrix GeneChips, HG-U133A) have shown the specific upregulation of IDO gene expression in response to DENV infection of human umbilical vein endothelial cells (HUVECs) in vitro (data not shown). To investigate the role of IDO during DENV infection, first we measured IDO activity in serum from healthy donors, DENV-infected patients and patients with other febrile illness. We found increased IDO activity in serum from DENV-infected patients compared with other controls. Then, we studied the role of IDO in the control of the infection in vitro and in our model, we found that the IFN-γ-mediated antiviral effect against DENV is partially dependent on IDO activity.
METHODS
Patients and laboratory analysis.
Patients were enrolled in a study protocol conducted by the University of Massachusetts Medical School (UMMS), Worcester, MA, USA, and Banco Municipal de Sangre del Distrito Capital (BMS), Caracas, Venezuela (Becerra et al., 2008). Written informed consent was obtained from all subjects. Febrile patients with no evidence of other defined infections were enrolled. Patients attended the clinic daily until 2 days after resolution of the fever. A convalescence consultation was performed within 2 weeks of the onset of symptoms. A complete clinical examination and routine laboratory tests were performed as described previously (Becerra et al., 2008). Based on corporal temperature, we defined ‘fever day zero (0)’ as the day of defervescence. The febrile period included days before defervescence and the post-febrile period included day 0 and days after defervescence. We included a group of healthy or normal donors (n=8) from BMS as controls to establish the normal values for our analysis.
Dengue case definition.
Dengue cases were defined following the World Health Organization (WHO) guidelines (WHO, 2003). DENV serotype-specific RT-PCR and anti-DENV antibody detection were performed as described previously (Becerra et al., 2008). Patients were classified as DENV-infected based on the positive detection of DENV RNA, presence of IgM antibodies and/or at least a fourfold increase in the haemagglutination inhibition (HI) titre in convalescent compared with enrolment sample; febrile patients with no evidence of bacterial infection and a negative result for DENV RNA and IgM antibody tests were classified as having other febrile illness (OFI). Appropriate medical examination and laboratory testing accompanied the diagnostic outcome of OFI cases. The HI levels were used to further classify DENV-infected patients as having a primary infection (HI titre ≤1 : 1280) or secondary infection (HI titre >1 : 1280) (WHO, 2003).
DC preparation, culture and DENV infection.
Blood was obtained from healthy volunteers at UMMS. DCs were prepared from blood-derived peripheral blood mononuclear cells (PBMCs) isolated using a density-gradient centrifugation over Ficoll-Paque Plus, 1.077 g dl−1 (GE Healthcare). Positive selection of CD14 cells was performed using the CD14-positive selection magnetic cell sorting kit (Miltenyi Biotec). Monocytes were incubated in RPMI 1640 supplemented with 10 % fetal calf serum, 500 U interleukin (IL)-4 ml−1 (Pepro-Tech) and 800 U ml−1 granulocyte-macrophage colony stimulation factor (Pepro-Tech). After 7 days in culture, DCs were pre-treated with 500 ng IFN-γ ml−1 (R&D Systems) in the absence or presence of 1 mM 1-methyl-tryptophan (1-MT) (Sigma-Aldrich) overnight. DENV type 2-New Guinea C at an m.o.i. of 0.1–0.2 was used for DENV infections. After 2 h, cells were washed and fresh medium containing 500 ng IFN-γ ml−1 with or without 1 mM 1-MT was added. Cells were cultured for 48 h, collected and stored at −80 °C until analysis.
Flow cytometry.
Immature DC conversion was assessed by flow cytometry. Cells were surface-stained at day 6 using monoclonal antibodies: anti-CD14-pacific blue (PB), anti-CD1a-allophycocyanin (APC) and anti-CD83-phycoerythrin (PE) (all from BD). Intracellular detection of DENV antigens was performed in fixed and permeabilized cells using the Cytofix/Cytoperm kit (BD) and stained with anti-DENV complex antibody conjugated to fluorescein isothiocyanate (FITC) (Chemicon). Samples were analysed using the FACSAria flow cytometer and Flow-Jo software (BD).
RNA preparation and RT-PCR.
Total cellular RNA was prepared using the RNeasy kit (Qiagen). The reverse transcription reaction was performed in triplicate with 50–100 ng total cellular RNA (Taqman Reverse Transcription kit, Applied Biosystems), using oligo-dT as primer. The reactions were performed at 25 °C for 10 min, 48 °C for 30 min and 95 °C for 5 min. The Taqman PCRs were performed using the universal PCR Master Mix (2×) and specific primers and probes (Applied Biosystems). PCR was performed in the 7300 Taqman PCR System (Applied Biosystems) at 50 °C for 2 min, 94.5 °C for 10 min and 40 cycles of 95 °C for 15 s and 60 °C for 1 min. β-Actin was used as an endogenous control; DENV and IDO mRNA were quantified using relative quantification (Rq) and the 2−ΔΔCt method (Livak & Schmittgen, 2001).
Serum sample preparation for L-Trp and l-kynurenine (L-Kyn) quantification.
Serum samples from patients were analysed using high performance liquid chromatography (HPLC) coupled to mass spectrometry (MS). A standard solution containing L-Trp and L-Kyn (both Sigma-Aldrich) was prepared from 1 mg ml−1 stock solutions in 0.1 % formic acid (Fluka Analytical). Four serial dilutions (fourfold) of the mixed standards (100 μg L-Trp ml−1, 10 μg L-Kyn ml−1) were prepared to construct a four point calibration curve. Serum samples (50 μl) and standards were mixed with 5 μl 2.4 M perchloric acid (Fisher Scientific) containing 75 μg ml−1 of the internal standard, L-Trp-(indole-d5) (d5-Trp) (Cambridge Isotope Laboratories). All samples and standards were adjusted to a final concentration of 1 % trichloroacetic acid (Sigma-Aldrich) and centrifuged and the supernatant was analysed.
Liquid chromatography-MS.
Samples were injected onto a PRISM RP C12, 100×1 mm, 0.5 μm column (Thermo Scientific), with a Rheos 2000 pumping system (LEAP Technologies) to deliver the mobile phase. The mobile phase was held at 2 % acetonitrile in 2 % aqueous formic acid for 3.5 min and then programmed to 40 % acetonitrile in 2 % aqueous formic acid for 7 min at a flow rate of 50 μl min−1 for the separation. The eluent from the column was directed to the electrospray ion source (capillary 3 kV, cone 20 V, desolvation 300 °C, source 150 °C) of a Waters Quattro II triple quadrupole mass spectrometer. Data were acquired in the multiple reaction monitoring mode with the argon collision gas at 2.0×10−3 mbar. For measurement of L-Trp, the m/z 206 MH+ precursor ion was selected and fragmented (collision energy 10 eV) and the m/z 189 product ion was monitored. For measurement of L-Kyn, the m/z 209.1 MH+ precursor ion was selected and fragmented (collision energy 11 eV) and the m/z 192.2 product ion was monitored. For measurement of d5-Trp (internal standard) the m/z 210 MH+ precursor ion was selected and fragmented (collision energy 11 eV) and the m/z 192.1 product ion monitored. The complete cycle time was 18 ms. The concentration of analytes in the samples was calculated by measuring the peak area ratios of the analytes to the internal standard, with reference to a mean of the bracketing external calibration curves that were acquired before and after every group of six samples.
Statistical analysis.
The Mann–Whitney U test and non-parametric ANOVA were used for comparisons between groups for continuous variables not normally distributed. χ2 was used to compare categorical data. Statistical analysis was performed using the software SPSS 15.0 for Windows.
RESULTS
Characterization of patients
We studied a total of 21 febrile patients, of which 15 were classified as DENV-infected and six as OFI. OFI patients were characterized by the presence of a febrile illness not associated with a defined infection; these patients did meet the criteria required for enrolment stated in the protocol guidelines, even though negative results for the specific DENV diagnostic tests (DENV RNA and IgM) were obtained. Among the DENV-infected patients, five were classified as having primary infections and ten as having secondary infections. The characteristics of each group of patients are summarized in Table 1. Significant differences in the minimum white blood cell (WBC) count (P=0.024), minimum platelet count (P=0.002), maximum aspartate aminotransferase (AST) (P=0.001), maximum alanine transaminase (ALT) (P=0.006) and maximum prolonged thrombin time difference (ΔTT) (P=0.014) were observed between OFI and DENV-infected patients. When we compared primary and secondary infections, we found differences in the minimum platelet count (P=0.002) and maximum ALT (P=0.014). Out of 15 DENV-infected patients, nine had thrombocytopenia (platelet counts lower than 100 000 μl−1), 13 had haemorrhagic manifestations (petechiae, epistaxis, gum bleeding or haematoma), two had more than 20 % haemoconcentration and four had evidence of vascular leakage (pleural effusion, ascites or increased gall bladder wall thickness). Two of the DENV-infected patients were classified as having DHF according to the WHO classification guidelines (WHO, 2003).
Table 1.
Characterization of patients
Patients were classified as OFI or DENV-infected according to DENV RNA and IgM detection. DENV-infected patients were positive for both DENV RNA and IgM; OFI patients were negative for both parameters. Primary patients had HI titres ≤1 : 1280 and secondary patients had HI titres >1280.
| OFI | DENV-infected | |||
|---|---|---|---|---|
| Total | Primary | Secondary | ||
| No. | 6 | 15 | 5 | 10 |
| Age* | 28 (13–48) | 22 (9–40) | 24 (9–28) | 22 (11–40) |
| Sex (female : male) | 4 : 2 | 4 : 11 | 3 : 2 | 1 : 9 |
| Laboratory parameters† | ||||
| Minimum WBC (×103 μl−1) | 5.2±0.8 | 3.0±0.3 | 2.3±0.5 | 3.3±0.4 |
| Minimum platelet count (×103 μl−1) | 198±13 | 91±13 | 137±18 | 68±14 |
| Maximum ΔTT (s) | 3.1±0.4 | 11.5±3.6 | 4.7±0.8 | 14.8±5.1 |
| Maximum AST (U ml−1) | 26.5±5.2 | 202.1±44.7 | 95.2±18.9 | 255.5±60.3 |
| Maximum ALT (U ml−1) | 35.8±16.5 | 164.3±32.8 | 67.0±8.8 | 213.0±41.2 |
| Clinical signs/symptoms‡ | ||||
| Haemorrhagic manifestations§ | 2 | 13 | 4 | 9 |
| Vascular leakage|| | 0 | 4 | 0 | 4 |
*Age in years (median and range per group).
†Laboratory parameters expressed as mean±se.
‡Frequency of signs/symptoms in each group during the acute stage of the disease.
§Types of haemorrhage: petechiae (spontaneous and/or positive tourniquet test), epistaxis, gum bleeding and haematoma.
||Evidence of vascular leakage (ultrasound study): pleural effusion, ascites and increased gall bladder wall thickness.
L-Trp and L-Kyn levels in patient sera
We assayed sera from healthy donors, OFI and DENV-infected patients for L-Trp and L-Kyn using HPLC-MS/MS. Serum levels of L-Trp and L-Kyn in OFI and DENV-infected patients during the febrile, post-febrile and convalescence stages of the disease are shown in Fig. 1. Median levels (range given in parentheses) measured in serum from healthy donors (n=8) were 11.8 μg ml−1 (8.5 –15.9) for L-Trp and 0.66 μg ml−1 (0.35–0.97) for L-Kyn.
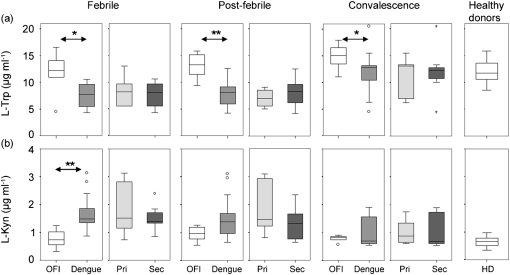
Fig. 1.
Levels of L-Trp and L-Kyn in patient sera measured using HPLC-MS/MS. L-Trp (a) and L-Kyn (b) were measured in patients at the febrile, post-febrile and convalescence stages of the disease and in healthy donors. Box-and-whisker plots show comparisons between OFI (white boxes) and DENV-infected (grey boxes) patients and between primary (Pri; light grey boxes) and secondary (Sec; dark grey boxes) DENV infections. Mann–Whitney analysis showed statistically significant differences in L-Trp and L-Kyn levels at the febrile stage (*P=0.026 and **P=0.002), and in L-Trp levels at post-febrile (**P=0.002) and convalescence (*P=0.035) stages, between OFI and DENV-infected patients. No significant differences were found between primary and secondary DENV patients at any stage.
We found lower levels of L-Trp in sera from DENV-infected patients compared with OFI patients and healthy donors (Fig. 1a). The decrease was statistically significant at the febrile (P=0.026) and post-febrile (P=0.002) stages of the disease when comparing DENV and OFI patients. Levels of L-Trp in DENV-infected patients were lower during the febrile and post-febrile stages compared with the convalescence day (P<0.05 and P<0.01, respectively); L-Trp levels increased to normal values at the convalescence day. Levels of L-Trp were not significantly different in OFI patients between febrile, post-febrile and convalescence stages. We did not find statistically significant differences in levels of L-Trp between primary and secondary DENV infections at any stage of the disease. We also did not find statistically significant differences in L-Trp levels between healthy donors and OFI patients at any stage of the disease.
DENV-infected patients had higher levels of L-Kyn in sera than OFI patients and healthy donors (Fig. 1b). The increase was statistically significant at the febrile stage of the disease (P=0.002) when comparing DENV and OFI patients. Levels of L-Kyn in DENV-infected patients were higher during the febrile stage of the disease than the post-febrile and convalescence stages (P<0.05). Levels of L-Kyn were not significantly different in OFI patients between febrile, post-febrile and convalescence stages. Levels of L-Kyn in primary and secondary DENV infections were not statistically significantly different at any stage of the disease. We did not find statistically significant differences in L-Kyn levels between healthy donors and OFI patients at any stage of the disease.
IDO activity in patient sera
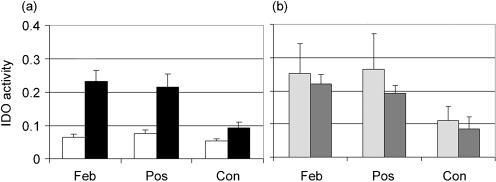
IDO activity was calculated as the ratio of the level of L-Kyn and level of L-Trp (Fig. 2). The IDO activity for healthy donors (n=8) was 0.055±0.006. We found higher IDO activity in sera from DENV-infected patients (Fig. 2a) at the febrile (P=0.001) and post-febrile (P=0.002) stages of the disease, compared with OFI. The activity increased by about 3.5 times at the febrile stage and 3 times at the post-febrile stage. We found higher IDO activity in sera from DENV-infected patients when comparing the febrile and post-febrile stages to the convalescence stage (P<0.01 and P<0.001, respectively). IDO activity in sera from DENV-infected patients decreased by the day of convalescence, but was still above normal values. We compared IDO activity in sera from primary and secondary DENV infections and found no statistically significant differences at any stage of the disease (Fig. 2b). We did not find differences in IDO activity in sera from OFI donors between febrile, post-febrile and convalescence stages. We did not find statistically significant differences between IDO activity in sera from OFI and healthy donors.
Fig. 2.
IDO activity in patient sera. IDO activity was calculated as a ratio by dividing L-Kyn (μg ml−1) by L-Trp (μg ml−1) for each disease stage: febrile (Feb), post-febrile (Pos) and convalescence (Con). Means±se are shown. (a) IDO activity for OFI (white bars) and DENV-infected (black bars) patients; Mann–Whitney analysis showed significant differences between OFI and DENV-infected patients at febrile (P=0.001), post-febrile (P=0.002), and convalescence (P=0.045) stages of infection. (b) IDO activity for patients with primary (light grey bars) and secondary (dark grey bars) infections; Mann–Whitney analysis showed no significant differences.
Expression of IDO in DCs in response to DENV infection in vitro
We studied the expression of the IDO gene in DCs in response to DENV infection. We measured IDO mRNA levels in uninfected and DENV-infected DCs at 12, 24 and 48 h post-infection, using the Rq/2−ΔΔCt method (Livak & Schmittgen, 2001). The results are summarized in Table 2. We found higher levels of IDO mRNA in DENV-infected DCs compared with uninfected DCs in three different donors at both 24 and 48 h. At 24 and 48 h, IDO mRNA in DENV-infected DCs compared with uninfected DCs (IDO mRNA levels in uninfected DCs set at 1) was induced fourfold and >100-fold, respectively. The treatment of DCs with IFN-γ plus DENV results in an increased expression of the IDO mRNA compared with the expression of IDO after the infection or treatment alone (data not shown).
Table 2.
IDO expression in DCs in response to DENV infection
| Time post-infection (h) | DENV* |
|---|---|
| 12 | 1±1 (n=3) |
| 24 | 4±3 (n=3) |
| 48 | 113±97 (n=3) |
*Relative IDO mRNA levels determined using the Rq/2−ΔΔCt method in DENV-infected DCs compared with uninfected DCs. Mean±se and number of independent experiments (in parentheses) at each time point are shown.
In vitro IFN-γ inhibition of DENV infection of DCs is partially dependent on IDO activity
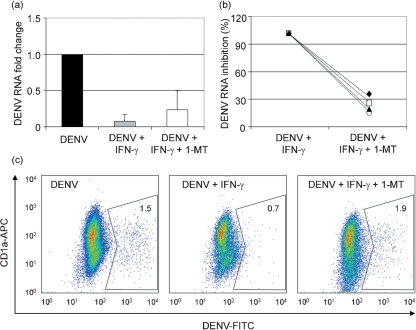
IFN-γ has a potent antiviral effect against DENV in vitro and it also upregulates the expression of IDO. We investigated whether the antiviral effect of IFN-γ against DENV is dependent on IDO activity, using 1-MT, a competitive inhibitor of L-Trp. Fig. 3 shows the effect of treatment with IFN-γ in the absence or presence of 1-MT in DENV-infected DCs. DENV RNA levels (Fig. 3a and b) were quantified using the Rq/2−ΔΔCt method (Livak & Schmittgen, 2001). β-Actin was used as an endogenous control and DENV-infected DCs as the calibrator sample. Our results show that DCs treated with IFN-γ and infected with DENV for 48 h had lower levels of DENV RNA compared with untreated DCs infected with DENV. When we added 1-MT the inhibitory effect of IFN-γ on DENV RNA levels was partially reversed (Fig. 3a). The percentage of the IFN-γ effect that was reversed in samples incubated with 1-MT was 75.2±3.7 % (mean±se, n=4; Fig. 3b). Fig. 3(c) shows the percentage of DENV-positive DCs, determined using flow cytometry. Treatment with IFN-γ in the absence of 1-MT reduced the percentage of DENV-infected DCs compared with untreated DCs, while the addition of 1-MT reversed the antiviral effect of IFN-γ.
Fig. 3.
Effect of IFN-γ and 1-MT treatment in DENV-infected DCs. (a) Fold change in DENV RNA levels in DCs infected with DENV, DCs infected with DENV in the presence of 500 μg IFN-γ ml−1 and DCs infected with DENV in the presence of 500 μg IFN-γ ml−1 and 1 mM 1-MT. Means±se from four independent experiments are shown. (b) Antiviral effect of IFN-γ treatment compared with IFN-γ+1-MT treatment of DENV-infected DCs. Percentage inhibition of DENV RNA after IFN-γ treatment (100 %) and percentage inhibition of DENV RNA after IFN-γ+1-MT treatment are shown. DENV RNA was quantified using the Rq/2−ΔΔCt method; β-actin was used as an endogenous control and DENV-infected DCs as the calibrator. Four independent experiments are shown. (c) Percentage of DENV-infected DCs determined by flow cytometry. Dot-plot analysis (CD1a-APC vs DENV antigen-FITC) of the live cell population was carried out. The percentage of FITC-positive cells for each treatment is shown (n=1).
DISCUSSION
Using an experimental approach based on gene expression analysis, the expression of IDO was found to be upregulated in HUVECs infected with DENV in vitro compared with mock-infected cells (data not shown). Due to the fact that IDO catalyses one of the reactions in the kynurenine pathway, the major route for L-Trp degradation in mammals, we investigated this metabolic pathway in in vivo DENV infections.
In this work, we present evidence of increased IDO activity in DENV-infected patients. IDO catalyses the rate-limiting step in the kynurenine pathway: the oxidation of L-Trp to N-formyl-kynurenine. We found significantly lower levels of L-Trp during the febrile and post-febrile stages of the disease and higher levels of L-Kyn during the febrile stage of the disease in DENV-infected patients compared with OFI patients and healthy donors. We also found that DENV-infected patients had increased IDO activity compared with OFI patients and healthy donors; the increase in IDO activity was significant during all three of the disease stages studied. It is possible that the mechanisms triggered in response to the infection continued to be active when the convalescence samples were collected 2 weeks after the onset of the disease. The relative increase in IDO activity in primary versus secondary infected patients was not statistically significant. It is possible that due to our limited number of patients with primary infections (young adult cohort), we could not establish a strong comparative analysis between patients with primary and secondary infections. In addition, most of our patients were classified as having dengue fever (DF) with two haemorrhagic cases (DHF). For this reason, we could not analyse our results according to the severity of the disease. Further work should be directed to explore L-Trp and L-Kyn levels and IDO activity in mild versus severe dengue cases, and between dengue and other viral infections.
The results obtained from comparisons of OFI and DENV-infected patients led us to hypothesize that increased IDO activity during DENV infections is part of the immune system response that is triggered to control the infection. The depletion of L-Trp and the formation of kynurenine pathway metabolites are the processes that characterize the effect of IDO in the innate immune response against pathogens and also as an immune regulator (King & Thomas, 2007). One of the mechanisms proposed to explain IDO-mediated biological effects is the depletion of L-Trp. When the amount of available L-Trp is decreased, the production of proteins is altered. It has been reported that the depletion of L-Trp provokes a ‘starvation state’ that results in inhibition of the growth of micro-organisms in vitro (Carlin et al., 1989).
We used the DENV-infected DC in vitro model to study IDO expression in response to DENV infection. Our results showed upregulation of IDO mRNA in response to DENV infection in DCs. We also observed a synergistic effect between DENV and IFN-γ in the induction of the IDO gene. Next, we used the DENV-infected DC model to study the role of IDO in the antiviral mechanism mediated by IFN-γ. It has been reported that IFN-γ exerts a potent antiviral effect against DENV (Libraty et al., 2001). Our results confirmed this observation, as treatment of DCs with IFN-γ reduced the amount of DENV RNA compared with untreated DENV-infected DCs. Moreover, when we treated DCs with IFN-γ in the presence of 1-MT [an analogue of L-Trp that has been shown to be a competitive inhibitor of IDO activity (Cady & Sono, 1991)], we found that the antiviral effect of IFN-γ against DENV was inhibited by up to 70 %. Recently, Heseler et al. (2008) also used 1-MT to inhibit the anti-parasitic effect of IFN-γ and IL-1 in an in vitro model of Toxoplasma gondii infection. Our results suggest that in the model of DENV-infected DCs in vitro, the antiviral effect of IFN-γ is partially dependent on IDO activity. The activation of different antiviral mechanisms can lead to successful control of the infection in different cell types or in cases where other pathways are blocked by the virus.
A fully functional IFN-γ pathway in dengue infections leads to a protective immune response. Some authors have found lower serum levels of IFN-γ in patients with DHF compared with patients with DF (Chaturvedi et al., 2000), while others found higher IFN-γ levels in DHF patients (Libraty et al., 2002). Both type-I and type-II IFNs have an antiviral effect against DENV even though the in vitro antiviral effect of IFN-α is achieved only when cells are pre-treated with the cytokine and then infected with the virus, while IFN-γ exerts its antiviral effect when added either before or after the infection (Ho et al., 2005). It also has been shown that DENV is able to block the effect of IFN-α (Jones et al., 2005) and that IFN-α is a weak inducer of IDO (Hassanain et al., 1993). Among the antiviral mechanisms induced by IFN-γ are the upregulation of IDO, studied in the present work, and the production of nitric oxide (NO). Levels of NO in sera from dengue patients classified as having DF are higher than levels in DHF patients (Valero et al., 2002). Additionally, the inducible form of the nitric oxide synthase (iNOS) has been detected in monocytes isolated from dengue patients classified as having DF (Neves-Souza et al., 2005). The association of higher NO levels with the mild manifestation of the disease suggests a protective role of NO in DENV infections in vivo. A cross-regulation between iNOS and IDO has been previously reported. It is known that NO is able to inhibit IDO activity in vitro (Thomas et al., 2007). A recent report found that IFN-γ-induced upregulation of iNOS/NO seems to be dependent on an intact kynurenine pathway, and blocking IDO with 1-MT diminishes both iNOS expression and NO production (Kujundzić & Lowenthal, 2008); on the other hand, other kynurenine pathway intermediate metabolites seem to inhibit iNOS expression and activity (Sekkai et al., 1997). Further work needs to be done to understand the relationship between iNOS and IDO.
IDO activity or L-Trp depletion could have other biological effects that we did not explore in this model. It has been reported that depletion of L-Trp is associated with inhibition of the proliferation of immune cells and induction of tolerance (reviewed by Mellor & Munn, 2004). IDO could be considered to be a double-edged sword in the immune response against pathogens. Immune cells, including antigen-presenting cells like DCs, are able to express IDO in response to various stimuli. On one hand, the inhibition of virus/parasite replication/growth is a desirable effect of IDO. On the other hand, the suppression of proliferation and induction of immune tolerance could be seen to be detrimental effects of IDO. A major role of DCs in the immune system is the activation of the cellular immune response, presenting antigens to T cells in the context of major histocompatibility complex antigens. Specific T cell clones become activated and undergo clonal expansion. The effect of IDO in this process could be to suppress specific immune responses directed at the elimination of the virus. In particular, the induction of the inhibitory molecule CTLA-4 and the transcription factor Foxp3 under low L-Trp and high L-Kyn conditions has been reported in T cells (Fallarino et al., 2006). The possible role of IDO-mediated suppression of the immune response in DENV infection deserves further investigation.
Acknowledgments
This study was funded by NIAID grants nos U01 AI070484, U01 AI45440, and U19 AI057319. We thank Fundasangre and Fondo Nacional de Ciencia y Tecnología (FONACIT F-2005000241), and the personnel at Banco Municipal de Sangre, Caracas, Venezuela, for the clinical evaluation of the patients and laboratory tests and the Instituto Nacional de Higiene ‘Rafael Rangel’ for serological testing of the samples.
References
- Adams, O., Besken, K., Oberdörfer, C., MacKenzie, C. R., Takikawa, O. & Däubener, W. (2004). Role of indoleamine-2,3-dioxygenase in alpha/beta and gamma interferon-mediated antiviral effects against herpes simplex virus infections. J Virol 78, 2632–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra, A., Warke, R. V., de Bosch, N., Rothman, A. L. & Bosch, I. (2008). Elevated levels of soluble ST2 protein in dengue virus infected patients. Cytokine 41, 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodaghi, B., Goureau, O., Zipeto, D., Laurent, L., Virelizier, J. L. & Michelson, S. (1999). Role of IFN-γ-induced indoleamine 2,3 dioxygenase and inducible nitric oxide synthase in the replication of human cytomegalovirus in retinal pigment epithelial cells. J Immunol 162, 957–964. [PubMed] [Google Scholar]
- Cady, S. G. & Sono, M. (1991). 1-Methyl-dl-tryptophan, β-(3-benzofuranyl)-dl-alanine (the oxygen analog of tryptophan), and β-[3-benzo(b)thienyl]-dl-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase. Arch Biochem Biophys 291, 326–333. [DOI] [PubMed] [Google Scholar]
- Carlin, J. M., Ozaki, Y., Byrne, G. I., Brown, R. R. & Borden, E. C. (1989). Interferons and indoleamine 2,3-dioxygenase: role in antimicrobial and antitumor effects. Experientia 45, 535–541. [DOI] [PubMed] [Google Scholar]
- Chaturvedi, U. C., Agarwal, R., Elbishbishi, E. A. & Mustafa, A. S. (2000). Cytokine cascade in dengue hemorrhagic fever: implications for pathogenesis. FEMS Immunol Med Microbiol 28, 183–188. [DOI] [PubMed] [Google Scholar]
- Chaturvedi, U., Nagar, R. & Shrivastava, R. (2006). Dengue and dengue haemorrhagic fever: implications of host genetics. FEMS Immunol Med Microbiol 47, 155–166. [DOI] [PubMed] [Google Scholar]
- Fallarino, F., Grohmann, U., You, S., McGrath, B. C., Cavener, D. R., Vacca, C., Orabona, C., Bianchi, R., Belladonna, M. L. & other authors (2006). The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol 176, 6752–6761. [DOI] [PubMed] [Google Scholar]
- Hassanain, H. H., Chon, S. Y. & Gupta, S. L. (1993). Differential regulation of human indoleamine 2,3-dioxygenase gene expression by interferons-γ and -α. Analysis of the regulatory region of the gene and identification of an interferon-gamma-inducible DNA-binding factor. J Biol Chem 268, 5077–5084. [PubMed] [Google Scholar]
- Heseler, K., Spekker, K., Schmidt, S. K., Mackenzie, C. R. & Daubener, W. (2008). Antimicrobial and immunoregulatory effects mediated by human lung cells: role of IFN-γ-induced tryptophan degradation. FEMS Immunol Med Microbiol 52, 273–281. [DOI] [PubMed] [Google Scholar]
- Ho, L. J., Hung, L. F., Weng, C. Y., Wu, W. L., Chou, P., Lin, Y. L., Chang, D. M., Tai, T. Y. & Lai, J. H. (2005). Dengue virus type 2 antagonizes IFN-α but not IFN-γ antiviral effect via down-regulating Tyk2-STAT signaling in the human dendritic cell. J Immunol 174, 8163–8172. [DOI] [PubMed] [Google Scholar]
- Jones, M., Davidson, A., Hibbert, L., Gruenwald, P., Schlaak, J., Ball, S., Foster, G. R. & Jacobs, M. (2005). Dengue virus inhibits alpha interferon signaling by reducing STAT2 expression. J Virol 79, 5414–5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, N. J. & Thomas, S. R. (2007). Molecules in focus: indoleamine 2,3-dioxygenase. Int J Biochem Cell Biol 39, 2167–2172. [DOI] [PubMed] [Google Scholar]
- Kujundzić, R. N. & Lowenthal, J. W. (2008). The role of tryptophan metabolism in iNOS transcription and nitric oxide production by chicken macrophage cells upon treatment with interferon gamma. Immunol Lett 115, 153–159. [DOI] [PubMed] [Google Scholar]
- Libraty, D. H., Pichyangkul, S., Ajariyakhajorn, C., Endy, T. P. & Ennis, F. A. (2001). Human dendritic cells are activated by dengue virus infection: enhancement by gamma interferon and implications for disease pathogenesis. J Virol 75, 3501–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libraty, D. H., Endy, T. P., Houng, H. S., Green, S., Kalayanarooj, S., Suntayakorn, S., Chansiriwongs, W., Vaughn, D. W., Nisalak, A. & other authors (2002). Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J Infect Dis 185, 1213–1221. [DOI] [PubMed] [Google Scholar]
- Livak, K. J. & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- MacKenzie, C. R., Hucke, C., Müller, D., Seidel, K., Takikawa, O. & Däubener, W. (1999). Growth inhibition of multiresistant enterococci by interferon-γ-activated human uro-epithelial cells. J Med Microbiol 48, 935–941. [DOI] [PubMed] [Google Scholar]
- MacKenzie, C. R., Heseler, K., Müller, A. & Däubener, W. (2007). Role of indoleamine 2,3-dioxygenase in antimicrobial defence and immuno-regulation: tryptophan depletion versus production of toxic kynurenines. Curr Drug Metab 8, 237–244. [DOI] [PubMed] [Google Scholar]
- Mellor, A. L. & Munn, D. H. (2004). IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 4, 762–774. [DOI] [PubMed] [Google Scholar]
- Monath, T. P. (1994). Dengue: the risk to developed and developing countries. Proc Natl Acad Sci U S A 91, 2395–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves-Souza, P. C., Azeredo, E. L., Zagne, S. M., Valls-de-Souza, R., Reis, S. R., Cerqueira, D. I., Nogueira, R. M. & Kubelka, C. F. (2005). Inducible nitric oxide synthase (iNOS) expression in monocytes during acute Dengue Fever in patients and during in vitro infection. BMC Infect Dis 5, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puccetti, P. (2007). On watching the watchers: IDO and type I/II IFN. Eur J Immunol 37, 876–879. [DOI] [PubMed] [Google Scholar]
- Rapoza, P. A., Tahija, S. G., Carlin, J. P., Miller, S. L., Padilla, M. L. & Byrne, G. I. (1991). Effect of interferon on a primary conjunctival epithelial cell model of trachoma. Invest Ophthalmol Vis Sci 32, 2919–2923. [PubMed] [Google Scholar]
- Rothman, A. L. & Ennis, F. A. (1999). Immunopathogenesis of Dengue hemorrhagic fever. Virology 257, 1–6. [DOI] [PubMed] [Google Scholar]
- Schroten, H., Spors, B., Hucke, C., Stins, M., Kim, K. S., Adam, R. & Däubener, W. (2001). Potential role of human brain microvascular endothelial cells in the pathogenesis of brain abscess: inhibition of Staphylococcus aureus by activation of indoleamine 2,3-dioxygenase. Neuropediatrics 32, 206–210. [DOI] [PubMed] [Google Scholar]
- Sekkai, D., Guittet, O., Lemaire, G., Tenu, J. P. & Lepoivre, M. (1997). Inhibition of nitric oxide synthase expression and activity in macrophages by 3-hydroxyanthranilic acid, a tryptophan metabolite. Arch Biochem Biophys 340, 117–123. [DOI] [PubMed] [Google Scholar]
- Terajima, M. & Leporati, A. M. (2005). Role of indoleamine 2,3-dioxygenase in antiviral activity of interferon-γ against vaccinia virus. Viral Immunol 18, 722–729. [DOI] [PubMed] [Google Scholar]
- Thomas, S. M., Garrity, L. F., Brandt, C. R., Schobert, C. S., Feng, G. S., Taylor, M. W., Carlin, J. M. & Byrne, G. I. (1993). IFN-gamma-mediated antimicrobial response. Indoleamine 2,3-dioxygenase-deficient mutant host cells no longer inhibit intracellular Chlamydia spp. or Toxoplasma growth. J Immunol 150, 5529–5534. [PubMed] [Google Scholar]
- Thomas, S. R., Terentis, A. C., Cai, H., Takikawa, O., Levina, A., Lay, P. A., Freewan, M. & Stocker, R. (2007). Post-translational regulation of human indoleamine 2,3-dioxygenase activity by nitric oxide. J Biol Chem 282, 23778–23787. [DOI] [PubMed] [Google Scholar]
- Valero, N., Espina, L. M., Anez, G., Torres, E. & Mosquera, J. A. (2002). Short report: increased level of serum nitric oxide in patients with dengue. Am J Trop Med Hyg 66, 762–764. [DOI] [PubMed] [Google Scholar]
- WHO (2003). Joint WHO HQ/SEAROP/WPRO meeting on DengueNet implementation in South-East Asia and the Western Pacific, Kuala Lumpur, 11–13 December 2003. Wkly Epidemiol Rec 78, 346–347. [PubMed] [Google Scholar]